Validation of a New Duplex Real-Time Polymerase Chain Reaction for Chlamydia trachomatis DNA Detection in Ocular Swab Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. qPCR Conditions
2.4. Direct Immunofluorescence Detection (DFA)
2.5. Statistical Analysis
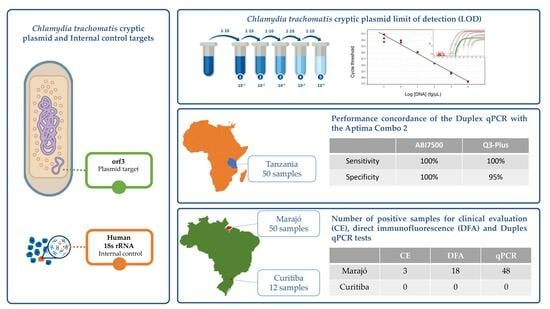
3. Results
3.1. qPCR Optimization and Analytical Parameters
3.2. Performance on Clinical Samples
3.3. Application on a Portable qPCR Instrument
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brazil, Ministry of Health. Guia Prático para Operacionalização da Campanha Nacional de Hanseníase, Verminoses, Tracoma e Esquistossomose; Ministério da Saúde: Brasilia, Brasil, 2016. [Google Scholar]
- Wright, H.R.; Turner, A.; Taylor, H.R. Trachoma. Lancet 2008, 371, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W.; Liesegang, T.J.; Schwam, B.L. Chlamydia Conjunctivitis and Central Retinal Vein Occlusion. Am. J. Ophthalmol. 2005, 140, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Guzey, M.; Ozardali, I.; Basar, E.; Aslan, G.; Satici, A.; Karadede, S. A survey of trachoma: The histopathology and the mechanism of progressive cicatrization of eyelid tissues. Ophthalmologica 2000, 214, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lucena, A.; Akaishi, P.M.S.; de Rodrigues, M.L.V.; Cruz, A.A.V. Upper eyelid entropion and dry eye in cicatricial trachoma without trichiasis. Arq. Bras. Oftalmol. 2012, 75, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Rajak, S.N.; Habtamu, E.; Weiss, H.A.; Bedri, A.; Gebre, T.; Bailey, R.L.; Mabey, D.C.W.; Khaw, P.T.; Gilbert, C.E.; Emerson, P.M.; et al. The clinical phenotype of trachomatous trichiasis in Ethiopia: Not all trichiasis is due to entropion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Seadi, C.F.; Oravec, R.; Poser B von Cantarelli, V.V.; Rossetti, M.L. Diagnóstico laboratorial da infecção pela Chlamydia trachomatis: Vantagens e desvantagens das técnicas. J. Bras. Patol. Med. Lab. 2002, 38, 125–133. [Google Scholar] [CrossRef]
- Papp, J.R.; Schachter, J.; Gaydos, C.A.; Van Der Pol, B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2014, 63, 1–19. [Google Scholar]
- Nishiwaki-Dantas, M.C.; de Abreu, M.T.; de Melo, C.M.; Romero, I.L.; Neto, R.B.M.; Dantas, P.E.C. Direct fluorescent antibody assay and polymerase chain reaction for the detection of Chlamydia trachomatis in patients with vernal keratoconjunctivitis. Clinics 2011, 66, 2013–2018. [Google Scholar] [CrossRef]
- Neinstein, L.S.; Rabinovitz, S. Detection of Chlamydia trachomatis. A study of the direct immunofluorescence technique and a review diagnostic limitation. J. Adolesc. Health Care 1989, 10, 10–15. [Google Scholar] [CrossRef]
- Fredlund, H.; Falk, L.; Jurstrand, M.; Unemo, M. Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: Impact on epidemiological surveillance and interventions. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2004, 112, 771–784. [Google Scholar] [CrossRef]
- Wang, S. The Microimmunofluorescence Test for Chlamydia pneumoniae Infection: Technique and Interpretation. J. Infect. Dis. 2000, 181, S421–S425. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Hampton, T.J.; Hayes, L.J.; Ward, M.E.; Whittle, H.C.; Mabey, D.C. Polymerase chain reaction for the detection of ocular chlamydial infection in trachoma-endemic communities. J. Infect. Dis. 1994, 170, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Sugita, S.; Tomaru, Y.; Hono, A.; Nakamuro, T.; Kubota, T.; Takase, H.; Mochizuki, M.; Takahashi, M.; Shimizu, N. Establishment of multiplex solid-phase strip PCR test for detection of 24 ocular infectious disease pathogens. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.H.; Last, A.; Molina-Gonzalez, S.; Cassama, E.; Butcher, R.; Nabicassa, M.; McCarthy, E.; Burr, S.E.; Mabey, D.C.; Bailey, R.L.; et al. Development and Evaluation of a Next-Generation Digital PCR Diagnostic Assay for Ocular Chlamydia trachomatis Infections. J. Clin. Microbiol. 2013, 51, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zou, S.; Yang, X.; Yang, D.; Chen, X. Development of multiplex real-time quantitative PCR for simultaneous detection of Chlamydia trachomatis and Ureaplasma parvum. Clin. Biochem. 2012, 45, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Twin, J.; Fairley, C.K.; Donovan, B.; Tan, S.E.; Yu, J.; Garland, S.M.; Tabrizi, S.N. Development and evaluation of an ompA quantitative real-time PCR assay for Chlamydia trachomatis serovar determination. J. Clin. Microbiol. 2010, 48, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Hong, K.C.; Schachter, J.; Moncada, J.; Lekew, T.; House, J.I.; Zhou, Z.; Neuwelt, M.D.; Rutar, T.; Halfpenny, C.; et al. Detection of Chlamydia trachomatis ocular infection in trachoma-endemic communities by rRNA amplification. Investig. Ophthalmol. Vis. Sci. 2009, 50, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Pickering, H.; Holland, M.J.; Last, A.R.; Burton, M.J.; Burr, S.E. Evaluation of a Chlamydia trachomatis-specific, commercial, real-time PCR for use with ocular swabs. Parasites Vectors 2018, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stare, D.; Harding-Esch, E.; Munoz, B.; Bailey, R.; Mabey, D.; Holland, M.; PRET Study Group. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: The partnership for rapid elimination of trachoma (PRET) in Tanzania and the Gambia. Ophthalm. Epidemiol. 2011, 18, 20–29. [Google Scholar] [CrossRef]
- Jenson, A.; Dize, L.; Mkocha, H.; Munoz, B.; Lee, J.; Gaydos, C.; Quinn, T.; West, S.K. Field Evaluation of the Cepheid GeneXpert Chlamydia trachomatis Assay for Detection of Infection in a Trachoma Endemic Community in Tanzania. PLoS Negl. Trop. Dis. 2013, 7, e2265. [Google Scholar] [CrossRef]
- Kowalski, R.P.; Thompson, P.P.; Kinchington, P.R.; Gordon, J.Y. Evaluation of the SmartCycler II System for Real-Time Detection of Viruses and Chlamydia from Ocular Specimens. Arch. Ophthalmol. 2006, 124, 1135–1139. [Google Scholar] [CrossRef]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- and Middle-Income Countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed]
- Dize, L.; Gaydos, C.A.; Quinn, T.C.; West, S.K. Stability of Chlamydia trachomatis on storage of dry swabs for accurate detection by nucleic acid amplification tests. J. Clin. Microbiol. 2015, 53, 1046–1047. [Google Scholar] [CrossRef]
- Dize, L.; West, S.; Quinn, T.C.; Gaydos, C.A. Pooling ocular swab specimens from Tanzania for testing by Roche Amplicor and Aptima Combo 2 assays for the detection of Chlamydia trachomatis: Accuracy and cost-savings. Diagn. Microbiol. Infect. Dis. 2013, 77, 289–291. [Google Scholar] [CrossRef]
- Tian, L.; Wang, N.-L. Trachoma control: The SAFE strategy. Int. J. Ophthalmol. 2018, 11, 1887–1888. [Google Scholar]
- Dize, L.; West, S.; Williams, J.A.; Van Der Pol, B.; Quinn, T.C.; Gaydos, C.A. Comparison of the Abbott m2000 RealTime CT Assay and the Cepheid GeneXpert CT/NG Assay to the Roche Amplicor CT Assay for Detection of Chlamydia trachomatis in Ocular Samples from Tanzania. J. Clin. Microbiol. 2013, 51, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.; Hardick, J. Point of care diagnostics for sexually transmitted infections: Perspectives and advances. Expert Rev. Anti-Infect. Ther. 2014, 12, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T. Diagnostic Procedures to Detect Chlamydia trachomatis Infections. Microorganisms 2016, 4, 25. [Google Scholar] [CrossRef]
- Herbst de Cortina, S.; Bristow, C.C.; Joseph Davey, D.; Klausner, J.D. A Systematic Review of Point of Care Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect. Dis. Obstet. Gynecol. 2016, 2016, 4386127. [Google Scholar] [CrossRef]
- Cristillo, A.D.; Bristow, C.C.; Peeling, R.; Van Der Pol, B.; de Cortina, S.H.; Dimov, I.K.; Pai, N.P.; Shin, D.J.; Chiu, R.Y.T.; Klapperich, C.; et al. Point-of-Care Sexually Transmitted Infection Diagnostics: Proceedings of the STAR Sexually Transmitted Infection-Clinical Trial Group Programmatic Meeting. Sex. Transm. Dis. 2017, 44, 211–218. [Google Scholar] [CrossRef]
- Hislop, J.; Quayyum, Z.; Flett, G.; Boachie, C.; Fraser, C.; Mowatt, G. Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men. Health Technol. Assess. 2010, 14. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.-E.C.; Roper, K.G.; Divena, M.A.; Lee, H.H.; Taylor, H.R. Correlation of clinical trachoma and infection in Aboriginal communities. PLoS Negl. Trop. Dis. 2011, 5, e986. [Google Scholar] [CrossRef] [PubMed]
- Harding-Esch, E.M.; Holland, M.J.; Schémann, J.-F.; Molina, S.; Sarr, I.; Andreasen, A.A.; Roberts, C.H.; Sillah, A.; Sarr, B.; Harding, E.F.; et al. Diagnostic accuracy of a prototype point-of-care test for ocular Chlamydia trachomatis under field conditions in The Gambia and Senegal. PLoS Negl. Trop. Dis. 2011, 5, e1234. [Google Scholar] [CrossRef]
- Krõlov, K.; Frolova, J.; Tudoran, O.; Suhorutsenko, J.; Lehto, T.; Sibul, H.; Mäger, I.; Laanpere, M.; Tulp, I.; Langel, Ü. Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples. J. Mol. Diagn. JMD 2014, 16, 127–135. [Google Scholar] [CrossRef]
- Turingan, R.S.; Kaplun, L.; Krautz-Peterson, G.; Norsworthy, S.; Zolotova, A.; Joseph, S.J.; Read, T.D.; Dean, D.; Tan, E.; Selden, R.F. Rapid detection and strain typing of Chlamydia trachomatis using a highly multiplexed microfluidic PCR assay. PLoS ONE 2017, 12, e0178653. [Google Scholar] [CrossRef]
- Pearce, D.M.; Shenton, D.P.; Holden, J.; Gaydos, C.A. Evaluation of a Novel Electrochemical Detection Method for Chlamydia trachomatis: Application for Point-of-Care Diagnostics. IEEE Trans. Biomed. Eng. 2011, 58, 755–758. [Google Scholar] [CrossRef]
- Stephens, R.S.; Kalman, S.; Lammel, C.; Fan, J.; Marathe, R.; Aravind, L.; Mitchell, W.; Olinger, L.; Tatusov, R.L.; Zhao, Q.; et al. Genome Sequence of an Obligate Intracellular Pathogen of Humans: Chlamydia trachomatis. Science 1998, 282, 754–759. [Google Scholar] [CrossRef]
- Cereda, M.; Cocci, A.; Cucchi, D.; Raia, L.; Pirola, D.; Bruno, L.; Ferrari, P.; Pavanati, V.; Calisti, G.; Ferrara, F.; et al. Q3: A Compact Device for Quick, High Precision qPCR. Sensors 2018, 18, 2583. [Google Scholar] [CrossRef]
- de Luna, E.J.A.; de Lopes, M.F.C.; Medina, N.H.; Favacho, J.; Cardoso, M.R.A. Prevalence of Trachoma in Schoolchildren in Brazil. Ophthalm. Epidemiol. 2016, 23, 360–365. [Google Scholar] [CrossRef]
- Abreu Caligaris, L.S.; Miyake Morimoto, W.T.; Medina, N.H.; Waldman, E.A. Trachoma prevalence and risk factors among preschool children in a central area of the city of São Paulo, Brazil. Ophthalm. Epidemiol. 2006, 13, 365–370. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Elwell C a Engel, J.N.; Valdivia, R.H. Chlamydial intracellular survival strategies. Cold Spring Harbor Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef]
- Burd, E.M. Validation of laboratory-developed molecular assays for infectious diseases. Clin. Microbiol. Rev. 2010, 23, 550–576. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Comanducci, M.; Ricci, S.; Cevenini, R.; Ratti, G. Diversity of the Chlamydia trachomatis common plasmid in biovars with different pathogenicity. Plasmid 1990, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 239909. [Google Scholar] [CrossRef] [PubMed]
- Hadad, R.; Fredlund, H.; Unemo, M. Evaluation of the new COBAS TaqMan CT test v2.0 and impact on the proportion of new variant Chlamydia trachomatis by the introduction of diagnostics detecting new variant C trachomatis in Orebro county, Sweden. Sex. Transm. Infect. 2009, 85, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Ripa, T.; Nilsson, P.A. A Chlamydia trachomatis strain with a 377-bp deletion in the cryptic plasmid causing false-negative nucleic acid amplification tests. Sex. Transm. Dis. 2007, 34, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Davis, G.S.; Cole, M.J.; Naik, D.; Maru, H.; Woodford, N.; Muir, P.; Horner, P.; Simms, I.; Thickett, G.; et al. Prevalence of new variants of Chlamydia trachomatis escaping detection by the Aptima Combo 2 assay, England, June to August 2019. Eurosurveillance 2019, 24, 1900557. [Google Scholar] [CrossRef]
- Butcher, R.; Houghton, J.; Derrick, T.; Ramadhani, A.; Herrera, B.; Last, A.R.; Massae, P.A.; Burton, M.J.; Holland, M.J.; Roberts, C.H. Reduced-cost Chlamydia trachomatis-specific multiplex real-time PCR diagnostic assay evaluated for ocular swabs and use by trachoma research programmes. J. Microbiol. Methods 2017, 139, 95–102. [Google Scholar] [CrossRef]
- Tang, R.H.; Yang, H.; Choi, J.R.; Gong, Y.; Feng, S.S.; Pingguan-Murphy, B.; Huang, Q.S.; Shi, J.L.; Mei, Q.B.; Xu, F. Advances in paper-based sample pretreatment for point-of-care testing. Crit. Rev. Biotechnol. 2016, 37, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.A.; Quinn, T.C.; Willis, D.; Weissfeld, A.; Hook, E.W.; Martin, D.H.; Ferrero, D.V.; Schachter, J. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J. Clin. Microbiol. 2003, 41, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.W.; Peeling, R.W.; Foster, A.; Mabey, D.C.W. Diagnosis and Assessment of Trachoma. Clin. Microbiol. Rev. 2004, 17, 982–1011. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, A.M.; Derrick, T.; Macleod, D.; Holland, M.J.; Burton, M.J. The Relationship between Active Trachoma and Ocular Chlamydia trachomatis Infection before and after Mass Antibiotic Treatment. PLoS Negl. Trop. Dis. 2016, 10, e0005080. [Google Scholar] [CrossRef]
- Skipp, P.J.S.; Hughes, C.; McKenna, T.; Edwards, R.; Langridge, J.; Thomson, N.R.; Clarke, I.N. Quantitative Proteomics of the Infectious and Replicative Forms of Chlamydia trachomatis. PLoS ONE 2016, 11, e0149011. [Google Scholar] [CrossRef] [PubMed]
- Domeika, M. Diagnosis of infections due to Chlamydia trachomatis. Acta Obstet. Gynecol. Scand. Suppl. 1997, 164, 121–127. [Google Scholar]
- Schachter, J. Which test is best for chlamydia? Curr. Opin. Infect. Dis. 1999, 12, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. Morb. Mortal. Wkly. Report. Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Elnifro, E.M.; Storey, C.C.; Morris, D.J.; Tullo, A.B. Polymerase chain reaction for detection of Chlamydia trachomatis in conjunctival swabs. Br. J. Ophthalmol. 1997, 81, 497–500. [Google Scholar] [CrossRef]
- Burton, M.J.; Holland, M.J.; Faal, N.; Aryee, E.A.N.; Alexander, N.D.E.; Bah, M.; Faal, H.; West, S.K.; Foster, A.; Johnson, G.J.; et al. Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4215–4222. [Google Scholar] [CrossRef]
- Baral, K.; Osaki, S.; Shreshta, B.; Panta, C.R.; Boulter, A.; Pang, F.; Cevallos, V.; Schachter, J.; Lietman, T. Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bull. World Health Organ. 1999, 77, 461–466. [Google Scholar] [PubMed]
- See, C.W.; Alemayehu, W.; Melese, M.; Zhou, Z.; Porco, T.C.; Shiboski, S.; Gaynor, B.D.; Eng, J.; Keenan, J.D.; Lietman, T.M. How reliable are tests for trachoma?—A latent class approach. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6133–6137. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, R.C.; Graziani, A.C.; Leite, K.K.; Surdi, J.A.; Biondo, C.A.; Costa, M.L.; Jacomasso, T.; Cereda, M.; De Fazio, M.; Bianchessi, M.A.; et al. Proof of Concept for a Portable Platform for Molecular Diagnosis of Tropical Diseases. J. Mol. Diagn. 2019, 21, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Tagliazucchi, G.M.; Crocamo, A.; et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Marziliano, N.; Notarangelo, M.F.; Cereda, M.; Caporale, V.; Coppini, L.; Demola, M.A.; Guidorossi, A.; Crocamo, A.; Pigazzani, F.; Boffetti, F.; et al. Rapid and portable, lab-on-chip, point-of-care genotyping for evaluating clopidogrel metabolism. Clin. Chim. Acta 2015, 451, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Q.; Tan, Y.; Wang, R.; Wu, X.; Liu, J.; Liu, R.; Wang, S.; Dong, S. Nanoparticle-Based Lateral Flow Biosensor Integrated with Loop-Mediated Isothermal Amplification for Rapid and Visual Identification of Chlamydia trachomatis for Point-of-Care Use. Front. Microbiol. 2022, 13, 914620. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Q.; Yuan, W.; Shi, Y.; Dong, S.; Luo, X. Visual and rapid identification of Chlamydia trachomatis and Neisseria gonorrhoeae using multiplex loop-mediated isothermal amplification and a gold nanoparticle-based lateral flow biosensor. Front. Cell. Infect. Microbiol. 2023, 13, 1067554. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Li, R.; Wen, Y.; Liang, Y.; Lao, G.; Liu, X.; Zhou, W.; Liu, H.; Xie, J.; et al. Rapid and simultaneous detection of multiple pathogens in the lower reproductive tract during pregnancy based on loop-mediated isothermal amplification-microfluidic chip. BMC Microbiol. 2022, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Derrick, T.R.; Sandetskaya, N.; Pickering, H.; Kölsch, A.; Ramadhani, A.; Mafuru, E.; Massae, P.; Malisa, A.; Mtuy, T.; Burton, M.J.; et al. DjinniChip: Evaluation of a novel molecular rapid diagnostic device for the detection of Chlamydia trachomatis in trachoma-endemic areas. Parasites Vectors 2020, 13, 533. [Google Scholar] [CrossRef]
- Rolando, J.C.; Jue, E.; Barlow, J.T.; Ismagilov, R.F. Real-time kinetics and high-resolution melt curves in single-molecule digital LAMP to differentiate and study specific and non-specific amplification. Nucleic Acids Res. 2020, 48, e42. [Google Scholar] [CrossRef]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex polymerase chain reaction: A practical approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef] [PubMed]
- de Barbeyrac, B.; Goldschmidt, P.; Malembic, S.; Raherison, S.; Clerc, M.; Bodaghi, B.; Bébéar, C.; Chaumeil, C. Quality assessment of conjunctival specimens for detection of Chlamydia trachomatis by PCR in children with active trachoma. Clin. Microbiol. Infect. 2007, 13, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, T.C. Polymerase Chain Reaction: Basic Protocol Plus Troubleshooting and Optimization Strategies. J. Vis. Exp. 2012, 63, e3998. [Google Scholar]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef]
- Tothard, D.R.; Williams, J.A.; Van Der Pol, B.; Jones, R.B. Identification of a Chlamydia trachomatis serovar E urogenital isolate which lacks the cryptic plasmid. Infect. Immun. 1998, 66, 6010–6013. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Radcliffe, G.; Vassallo, R.; Buxton, D.; O’Brien, W.J.; Pelletier, D.A.; Weisburg, W.G.; Klinger, J.D.; Olive, D.M. Infection with a plasmid-free variant Chlamydia related to Chlamydia trachomatis identified by using multiple assays for nucleic acid detection. J. Clin. Microbiol. 1992, 30, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Darville, T.; Chandra-Kuntal, K.; Smith, B.; Andrews, C.W.; O’Connell, C.M. Infectivity acts as in vivo selection for maintenance of the chlamydial cryptic plasmid. Infect. Immun. 2011, 79, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, J.; Hou, S.; Ding, Y.; Yang, Z.; Zeng, H.; Baseman, J.; Zhong, G. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect. Immun. 2014, 82, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Kari, L.; Whitmire, W.M.; Olivares-Zavaleta, N.; Goheen, M.M.; Taylor, L.D.; Carlson, J.H.; Sturdevant, G.L.; Lu, C.; Bakios, L.E.; Randall, L.B.; et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J. Exp. Med. 2011, 208, 2217–2223. [Google Scholar] [CrossRef]
- Zhong, G. Chlamydial Plasmid-Dependent Pathogenicity. Trends Microbiol. 2017, 25, 141–152. [Google Scholar] [CrossRef]
- Dean, D.; Kandel, R.P.; Adhikari, H.K.; Hessel, T. Multiple Chlamydiaceae Species in Trachoma: Implications for Disease Pathogenesis and Control. PLoS Med. 2008, 5, e14. [Google Scholar] [CrossRef] [PubMed]


| Concentration of C. trachomatis DNA (per µL) | Cq (mean ± SD) ABI7500 | Number of Copies of the Synthetic Positive Control (per µL) | Cq (mean ± SD) Q3-Plus |
|---|---|---|---|
| 10 pg | 18.54 ± 0.19 | 100,000 | 20.74 ± 0.93 |
| 1 pg | 22.00 ± 0.29 | 10,000 | 23.99 ± 1.31 |
| 100 fg | 25.99 ± 0.22 | 1000 | 26.74 ± 0.95 |
| 10 fg | 30.14 ± 0.04 | 100 | 31.82 ± 1.17 |
| 1 fg | 34.32 ± 0.65 | 10 | 33.92 ± 0.79 |
| 0.1 fg | 36.76 ± 0.91 | 1 | 36.65 ± 1.63 |
| Sample | Clinical Evaluation (CE) | EBs | DFA Classification | Cq (mean ± SD) | Duplex qPCR Classification | Agreement CE × qPCR | Agreement DFA × qPCR |
|---|---|---|---|---|---|---|---|
| 1 | Negative | 0 | Negative | 36.59 ± 0.59 | Positive | No | No |
| 2 | Negative | 8 | Positive | - | Negative | Yes | No |
| 3 | Negative | 0 | Negative | 36.18 ± 0.54 | Positive | No | No |
| 4 | Negative | 0 | Negative | 41.62 ± 0.00 | Positive | No | No |
| 5 | Negative | 0 | Negative | 38.91 ± 3.36 | Positive | No | No |
| 6 | Negative | 0 | Negative | 35.93 ± 1.49 | Positive | No | No |
| 7 | Negative | 5 | Positive | 36.06 ± 1.73 | Positive | No | Yes |
| 8 | Negative | 2 | Negative | 43.05 ± 1.78 | Positive | No | No |
| 9 | Negative | 3 | Negative | 39.12 ± 2.67 | Positive | No | No |
| 10 | Negative | 0 | Negative | 42.20 ± 4.22 | Positive | No | No |
| 11 | Positive | 7 | Positive | 36.82 ± 0.91 | Positive | Yes | Yes |
| 12 | Negative | 2 | Negative | 38.31 ± 0.00 | Positive | No | No |
| 13 | Negative | 0 | Negative | 37.89 ± 1.43 | Positive | No | No |
| 14 | Negative | 1 | Negative | 33.90 ± 0.49 | Positive | No | No |
| 15 | Positive | 6 | Positive | 36.86 ± 1.49 | Positive | Yes | Yes |
| 16 | Negative | 0 | Negative | 37.07 ± 2.49 | Positive | No | No |
| 17 | Negative | 2 | Negative | 37.83 ± 1.46 | Positive | No | No |
| 18 | Negative | 6 | Positive | 35.08 ± 0.00 | Positive | No | Yes |
| 19 | Negative | 2 | Negative | 37.61 ± 2.79 | Positive | No | No |
| 20 | Negative | 0 | Negative | 37.53 ± 2.81 | Positive | No | No |
| 21 | Negative | 2 | Negative | 33.58 ± 0.89 | Positive | No | No |
| 22 | Negative | 0 | Negative | 34.55 ± 0.37 | Positive | No | No |
| 23 | Negative | 4 | Negative | 35.94 ± 0.21 | Positive | No | No |
| 24 | Negative | 5 | Positive | 34.83 ± 0.16 | Positive | No | Yes |
| 25 | Negative | 0 | Negative | 37.07 ± 1.46 | Positive | No | No |
| 26 | Negative | 6 | Positive | 37.05 ± 1.51 | Positive | No | Yes |
| 27 | Negative | 3 | Negative | 37.78 ± 0.37 | Positive | No | No |
| 28 | Negative | 6 | Positive | 33.10 ± 0.45 | Positive | No | Yes |
| 29 | Negative | 2 | Negative | 36.73 ± 0.59 | Positive | No | No |
| 30 | Negative | 5 | Positive | 37.40 ± 1.19 | Positive | No | Yes |
| 31 | Negative | 6 | Positive | 35.24 ± 0.30 | Positive | No | Yes |
| 32 | Negative | 7 | Positive | 35.26 ± 1.04 | Positive | No | Yes |
| 33 | Negative | 5 | Positive | 34.98 ± 0.76 | Positive | No | Yes |
| 34 | Negative | 6 | Positive | 34.06 ± 0.00 | Positive | No | Yes |
| 35 | Negative | 8 | Positive | 35.23 ± 1.08 | Positive | No | Yes |
| 36 | Positive | 9 | Positive | 38.28 ± 0.50 | Positive | Yes | Yes |
| 37 | Negative | 2 | Negative | 34.23 ± 0.48 | Positive | No | No |
| 38 | Negative | 2 | Negative | 35.84 ± 0.84 | Positive | No | No |
| 39 | Negative | 3 | Negative | 36.93 ± 0.00 | Positive | No | No |
| 40 | Negative | 2 | Negative | 36.59 ± 0.75 | Positive | No | No |
| 41 | Negative | 5 | Positive | - | Negative | Yes | Yes |
| 42 | Negative | 4 | Negative | 35.12 ± 1.11 | Positive | No | No |
| 43 | Negative | 5 | Positive | 40.70 ± 0.00 | Positive | No | Yes |
| 44 | Negative | 7 | Positive | 34.10 ± 0.77 | Positive | No | Yes |
| 45 | Negative | 3 | Negative | 34.50 ± 0.36 | Positive | No | No |
| 46 | Negative | 2 | Negative | 35.23 ± 1.42 | Positive | No | No |
| 47 | Negative | 0 | Negative | 38.71 ± 1.58 | Positive | No | No |
| 48 | Negative | 1 | Negative | 33.72 ± 0.50 | Positive | No | No |
| 49 | Negative | 2 | Negative | 34.53 ± 0.34 | Positive | No | No |
| 50 | Negative | 1 | Negative | 36.38 ± 0.00 | Positive | No | No |
| 51 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 52 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 53 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 54 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 55 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 56 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 57 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 58 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 59 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 60 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 61 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| 62 | Negative | 0 | Negative | - | Negative | Yes | Yes |
| Sample | Pre- Characterization Status (by Aptima Combo 2) | ABI7500 | Q3-Plus | ||||
|---|---|---|---|---|---|---|---|
| Mean Cq for C. trachomatis Target | Detection of Reaction Internal Control | Diagnostic Status | Mean Cq for C. trachomatis Target | Detection of Reaction Internal Control | Diagnostic Status | ||
| 1 | Positive | 22.60 | Yes | Positive | 23.05 | Yes | Positive |
| 2 | Positive | 30.36 | Yes | Positive | 29.75 | Yes | Positive |
| 3 | Positive | 29.65 | Yes | Positive | 28.80 | Yes | Positive |
| 4 | Positive | 25.75 | Yes | Positive | 25.75 | Yes | Positive |
| 5 | Positive | 30.75 | Yes | Positive | 29.40 | Yes | Positive |
| 6 | Positive | 39.28 | Yes | Positive | 36.20 | Yes | Positive |
| 7 | Positive | 38.99 | Yes | Positive | 36.65 | Yes | Positive |
| 8 | Positive | 32.56 | Yes | Positive | 32.45 | Yes | Positive |
| 9 | Positive | 27.45 | Yes | Positive | 26.30 | Yes | Positive |
| 10 | Positive | 25.07 | Yes | Positive | 24.80 | Yes | Positive |
| 11 | Positive | 28.10 | Yes | Positive | 27.15 | Yes | Positive |
| 12 | Positive | 29.83 | Yes | Positive | 33.00 | Yes | Positive |
| 13 | Positive | 37.59 | Yes | Positive | 26.20 | Yes | Positive |
| 14 | Positive | 25.48 | Yes | Positive | 27.10 | Yes | Positive |
| 15 | Positive | 27.43 | Yes | Positive | 32.60 | Yes | Positive |
| 16 | Positive | 35.39 | Yes | Positive | 29.40 | Yes | Positive |
| 17 | Positive | 29.26 | Yes | Positive | 29.30 | Yes | Positive |
| 18 | Positive | 28.82 | Yes | Positive | 36.35 | Yes | Positive |
| 19 | Positive | 37.21 | Yes | Positive | 31.60 | Yes | Positive |
| 20 | Positive | 32.56 | Yes | Positive | 29.55 | Yes | Positive |
| 21 | Positive | 31.50 | Yes | Positive | 33.95 | Yes | Positive |
| 22 | Positive | 40.08 | Yes | Positive | 36.65 | Yes | Positive |
| 23 | Positive | 37.51 | Yes | Positive | 26.45 | Yes | Positive |
| 24 | Positive | 26.01 | Yes | Positive | 27.25 | Yes | Positive |
| 25 | Positive | 29.12 | Yes | Positive | 26.10 | Yes | Positive |
| 26 | Positive | 26.88 | Yes | Positive | 32.30 | Yes | Positive |
| 27 | Positive | 31.41 | Yes | Positive | 29.70 | Yes | Positive |
| 28 | Positive | 30.85 | Yes | Positive | 33.40 | Yes | Positive |
| 29 | Positive | 33.11 | Yes | Positive | 33.95 | Yes | Positive |
| 30 | Positive | 27.99 | Yes | Positive | 27.15 | Yes | Positive |
| 31 | Negative | ND | Yes | Negative | 36.80 | Yes | Positive |
| 32 | Negative | ND | Yes | Negative | 40.25 * | Yes * | Negative |
| 33 | Negative | ND | Yes | Negative | 40.40 * | Yes | Negative |
| 34 | Negative | ND | No | Inconclusive | ND | Yes | Negative |
| 35 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 36 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 37 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 38 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 39 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 40 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 41 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 42 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 43 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 44 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 45 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 46 | Negative | ND | Yes | Negative | 40.30 * | Yes | Negative |
| 47 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 48 | Negative | ND | Yes | Negative | 41.40 * | Yes | Negative |
| 49 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| 50 | Negative | ND | Yes | Negative | ND | Yes | Negative |
| Category | ABI7500 | Q3-Plus | ||||
|---|---|---|---|---|---|---|
| Detections | Total Possible | Rate (%) | Detections | Total Possible | Rate (%) | |
| Positive | 30 | 30 | 100 | 30 | 30 | 100 |
| Negative | 19 | 20 | 95 | 19 | 20 | 95 |
| Inconclusive | 1 | 50 | 2 | 0 | 50 | 0 |
| False-negative | 0 | 30 | 0 | 0 | 0 | 0 |
| False-positive | 0 | 20 | 0 | 1 | 20 | 5 |
| Positive predictive value (PPV) | 100% | 99.45% (CI95% 96.38 to 99.92%) | ||||
| Negative predictive value (NPV) | 99.8% (CI95% 98.9 to 99.9%) | 100% | ||||
| Sensitivity | 100% (CI95% of 88.43% to 100%) | 100% (CI95% of 88.43% to 100%) | ||||
| Specificity | 100% (CI95% of 82.35% to 100%) | 95% (CI95% of 75.13 to 99.87%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favacho, J.d.F.R.; Leite, K.K.; Jacomasso, T.; Farias, A.B.; Franco Filho, L.C.; Gomes, S.T.M.; dos Reis, H.S.; Mota, G.D.; Schluga, P.H.d.C.; Tassi, W.S.; et al. Validation of a New Duplex Real-Time Polymerase Chain Reaction for Chlamydia trachomatis DNA Detection in Ocular Swab Samples. Diagnostics 2024, 14, 892. https://doi.org/10.3390/diagnostics14090892
Favacho JdFR, Leite KK, Jacomasso T, Farias AB, Franco Filho LC, Gomes STM, dos Reis HS, Mota GD, Schluga PHdC, Tassi WS, et al. Validation of a New Duplex Real-Time Polymerase Chain Reaction for Chlamydia trachomatis DNA Detection in Ocular Swab Samples. Diagnostics. 2024; 14(9):892. https://doi.org/10.3390/diagnostics14090892
Chicago/Turabian StyleFavacho, Joana da Felicidade Ribeiro, Keren Kariene Leite, Thiago Jacomasso, Aline Burda Farias, Luciano Chaves Franco Filho, Samara Tatielle Monteiro Gomes, Herald Souza dos Reis, Gardene Dourado Mota, Pedro Henrique de Caires Schluga, Walleyd Sami Tassi, and et al. 2024. "Validation of a New Duplex Real-Time Polymerase Chain Reaction for Chlamydia trachomatis DNA Detection in Ocular Swab Samples" Diagnostics 14, no. 9: 892. https://doi.org/10.3390/diagnostics14090892