Soluble Human Epidermal Growth Factor Receptor 2 (sHER2) as a Potential Risk Assessment, Screening, and Diagnostic Biomarker of Lung Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. sHER2 ELISA
2.3. Statistical Analysis
3. Results
3.1. sHER2 Concentrations Are Associated with Age in Healthy Men
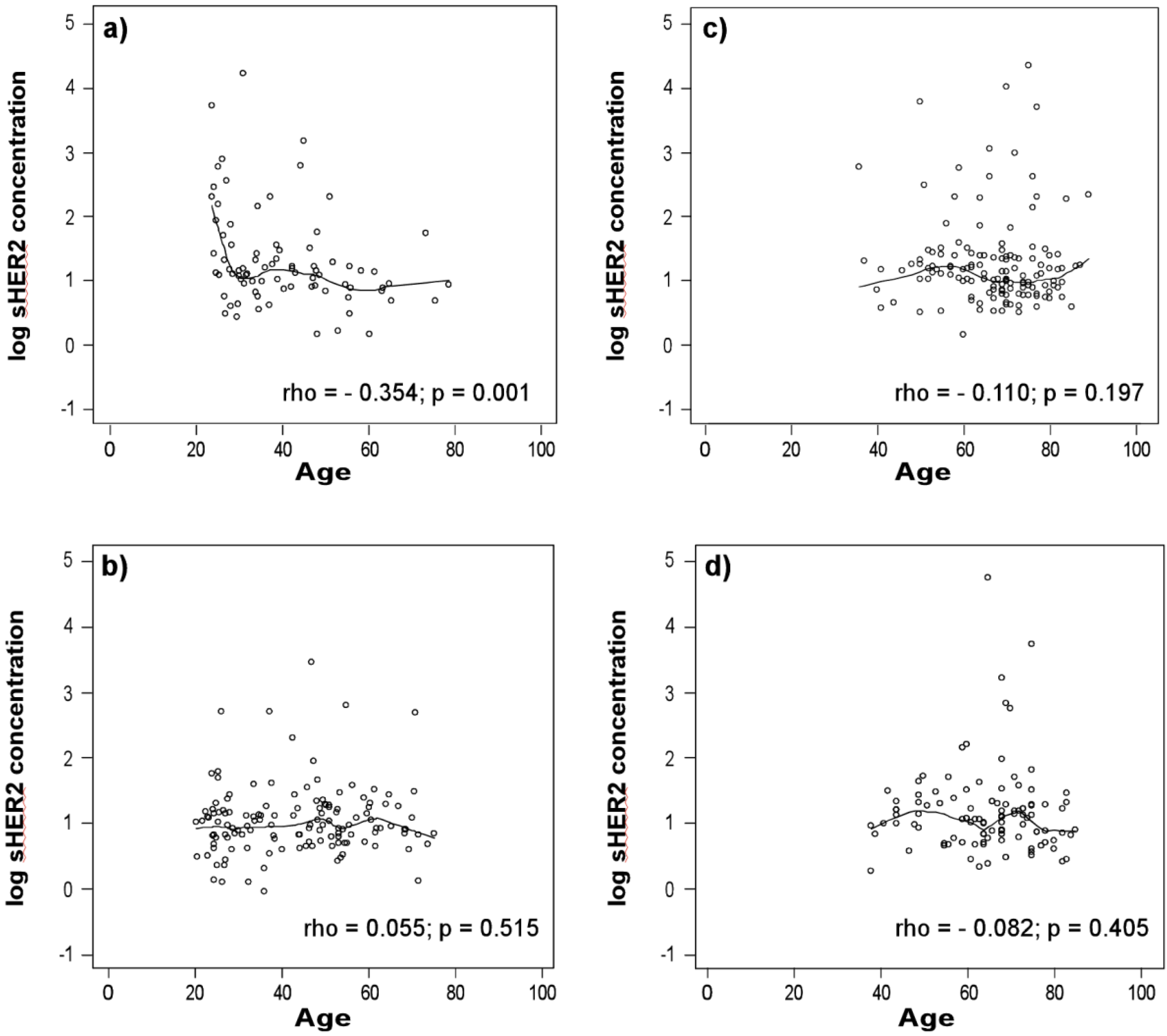
3.2. sHER2 Concentrations are Higher in Patients with Adenocarcinoma

| Quintiles of Serum sHER2 | OR (95% CI) for Women | OR (95% CI) for Men |
|---|---|---|
| ≤1.85 ng/mL | referent | 1.01 (0.57, 1.78) |
| 2.40 ng/mL | 1.48 (1.09, 2.02) | 1.49 (0.79, 2.82) |
| 3.00 ng/mL | 2.06 (1.16, 3.66) | 2.08 (0.94, 4.62) |
| 3.65 ng/mL | 2.77 (1.23, 6.22) | 2.79 (1.05, 7.74) |
| ≥6.60 ng/mL | 6.74 (1.48, 30.67) | 6.78 (1.36, 33.79) |

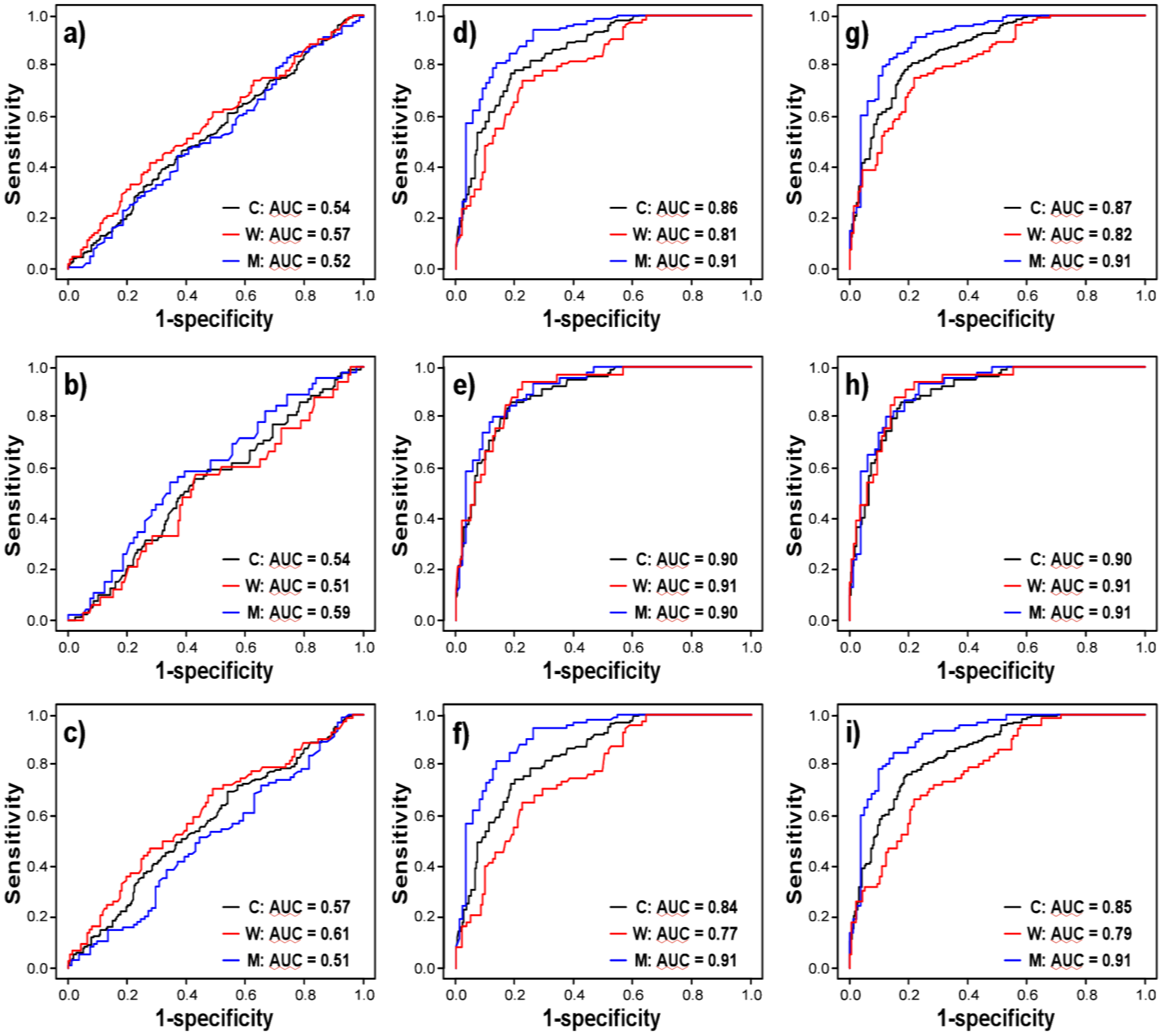
3.3. sHER2 is a Weak, Independent Discriminatory Biomarker of Adenocarcinoma
| Univariate Logistic Regression Models | ||||||
| Patient GroupsParameters | Maximum Likelihood Estimate | Standard Error | Wald Χ2 | Χ2/FDRPvalue | AUC | |
| All Lung Cancer Cases vs. Controls | ||||||
| Age | 0.106 | 0.010 | 125.216 | <0.0001/<0.0005 | 0.858 | |
| Gender | −0.766 | 0.187 | 16.728 | <0.0001/<0.0005 | 0.594 | |
| Log sHER2 | 0.429 | 0.328 | 1.711 | 0.191/0.298 | 0.537 | |
| Squamous Cell Carcinomas vs. Controls | ||||||
| Age | 0.129 | 0.016 | 66.308 | <0.0001/<0.0005 | 0.895 | |
| Gender | −0.818 | 0.265 | 9.499 | 0.002/0.007 | 0.601 | |
| Log sHER2 | −0.565 | 0.534 | 1.119 | 0.290/0.415 | 0.539 | |
| Adenocarcinomas vs. Controls | ||||||
| Age | 0.098 | 0.010 | 95.433 | <0.0001/<0.0005 | 0.840 | |
| Gender | −0.741 | 0.207 | 12.788 | 0.0003/0.0012 | 0.591 | |
| Log sHER2 | 0.777 | 0.350 | 4.937 | 0.026/0.057 | 0.573 | |
| Multivariate Logistic Regression Models | ||||||
| Patient GroupsParameters | Maximum Likelihood Estimate | Standard Error | Wald Χ2 | Χ2/FDRPvalue | AUC | |
| All Lung Cancer Cases vs. Controls | ||||||
| Age | 0.112 | 0.010 | 121.132 | <0.0001/<0.0005 | ||
| Gender | −0.831 | 0.252 | 10.831 | 0.001/0.004 | ||
| Log sHER2 | 0.801 | 0.461 | 3.024 | 0.082/0.144 | 0.877 | |
| Squamous Cell Carcinomas vs. Controls | ||||||
| Age | 0.140 | 0.018 | 63.664 | <0.0001/<0.0005 | ||
| Gender | −1.416 | 0.386 | 13.462 | 0.0002/0.0009 | ||
| Log sHER2 | 0.112 | 0.817 | 0.019 | 0.891/0.945 | 0.917 | |
| Adenocarcinomas vs. Controls | ||||||
| Age | 0.102 | 0.011 | 93.161 | <0.0001/<0.0005 | ||
| Gender | −0.697 | 0.267 | 6.807 | 0.009/0.024 | ||
| Log sHER2 | 1.080 | 0.472 | 5.235 | 0.022/0.051 | 0.859 | |
| Quintiles of Serum sHER2 | OR (95% CI) for Women | OR (95% CI) for Men |
|---|---|---|
| ≤1.85 ng/mL | referent | 2.01 (1.19, 3.39) |
| 2.40 ng/mL | 1.32 (1.04, 1.68) | 2.66 (1.52, 4.66) |
| 3.00 ng/mL | 1.69 (1.08, 2.64) | 3.38 (1.74, 6.57) |
| 3.65 ng/mL | 2.08 (1.11, 3.90) | 4.18 (1.90, 9.21) |
| ≥6.60 ng/mL | 3.95 (1.22, 12.81) | 7.93 (2.26, 27.82) |
4. Discussion
5. Conclusions
Acknowledgments
References
- Altekruse, S.F.; Kosary, C.L.; Krapcho, M.; Neyman, N.; Aminou, R.; Waldron, W.; Ruhl, J.; Howlader, N.; Tatalovich, Z.; Cho, H.; et al. SEER Cancer Statistics Review, 1975–2007. Available online: http://seer.cancer.gov/csr/1975_2007 (accessed on 17 November 2012).
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Bach, P.B.; Kelley, M.J.; Tate, R.C.; McCrory, D.C. Screening for lung cancer: A review of the current literature. Chest 2003, 123, 72–82. [Google Scholar] [CrossRef]
- Bach, P.B.; Niewoehner, D.E.; Black, W.C. Screening for lung cancer: The guidelines. Chest 2003, 123, 83–88. [Google Scholar] [CrossRef]
- Gohagan, J.K.; Prorok, P.C.; Hayes, R.B.; Kramer, B.S. The prostate, lung, colorectal and ovarian (PLCO) cancer screening trial of the national cancer institute: History, organization, and status. Control. Clin. Trials 2000, 21, 251–272. [Google Scholar] [CrossRef]
- Prorok, P.C.; Andriole, G.L.; Bresalier, R.S.; Buys, S.S.; Chia, D.; Crawford, E.D.; Fogel, R.; Gelmann, E.P.; Gilbert, F.; Hasson, M.A.; et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control. Clin. Trials 2000, 21, 273–309. [Google Scholar] [CrossRef]
- Oken, M.M.; Marcus, P.M.; Hu, P.; Beck, T.M.; Hocking, W.; Kvale, P.A.; Cordes, J.; Riley, T.L.; Winslow, S.D.; Peace, S.; et al. Baseline chest radiograph for lung cancer detection in the randomized prostate, lung, colorectal and ovarian cancer screening trial. J. Natl. Cancer Inst. 2005, 97, 1832–1839. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Miller, A.; Kramer, B.S.; Church, T.; Reding, D.; Prorok, P.; Gelmann, E.; Schoen, R.E.; Buys, S.; Hayes, R.B.; Berg, C.D. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am. J. Epidemiol. 2007, 165, 874–881. [Google Scholar] [CrossRef]
- Marcus, P.M. Lung cancer screening: An update. J. Clin. Oncol. 2001, 19, 83–86. [Google Scholar]
- Nawa, T.; Nakagawa, T.; Kusano, S.; Kawasaki, Y.; Sugawara, Y.; Nakata, H. Lung cancer screening using low-dose spiral CT*: Results of baseline and 1-year follow-up studies. Chest 2002, 122, 15–20. [Google Scholar] [CrossRef]
- Ma, P.C.; Blaszkowsky, L.; Bharti, A.; Ladanyi, A.; Kraeft, S.K.; Bruno, A.; Skarin, A.T.; Chen, L.B.; Salgia, R. Circulating tumor cells and serum tumor biomarkers in small cell lung cancer. Anticancer Res. 2003, 23, 49–62. [Google Scholar] [PubMed]
- Brambilla, C.; Fievet, F.; Jeanmart, M.; de Fraipont, F.; Lantuejoul, S.; Frappat, V.; Ferretti, G.; Brichon, P.Y.; Moro-Sibilot, D. Early detection of lung cancer: Role of biomarkers. Eur. Respir. J. Suppl. 2003, 39, 36–44. [Google Scholar]
- Chanin, T.D.; Merrick, D.T.; Franklin, W.A.; Hirsch, F.R. Recent developments in biomarkers for the early detection of lung cancer: perspectives based on publications 2003 to present. Curr. Opin. Pulm. Med. 2004, 10, 242–247. [Google Scholar] [CrossRef]
- Hilbe, W.; Dirnhofer, S.; Greil, R.; Woll, E. Biomarkers in non-small cell lung cancer prevention. Eur. J. Cancer Prev. 2004, 13, 425–436. [Google Scholar] [CrossRef]
- Yang, S.Y.; Xiao, X.Y.; Zhang, W.G.; Zhang, L.J.; Zhang, W.; Zhou, B.; Chen, G.; He, D.C. Application of serum SELDI proteomic patterns in diagnosis of lung cancer. BMC Cancer 2005, 5. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, F.R.; Varella-Garcia, M.; Cappuzzo, F. Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene 2009, 28 Suppl 1, S32–S37. [Google Scholar] [PubMed]
- Hynes, N.E.; Stern, D.F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta 2009, 1198, 165–184. [Google Scholar]
- Shi, D.; He, G.; Cao, S.; Pan, W.; Zhang, H.Z.; Yu, D.; Hung, M.C. Overexpression of the c-erbB-2/neu-encoded p185 protein in primary lung cancer. Mol. Carcinog. 1992, 5, 213–218. [Google Scholar] [CrossRef]
- Harpole, D.H., Jr.; Marks, J.R.; Richards, W.G.; Herndon, J.E., II; Sugarbaker, D.J. Localized adenocarcinoma of the lung: Oncogene expression of erbB-2 and p53 in 150 patients. Clin. Cancer Res. 1995, 1, 659–664. [Google Scholar] [PubMed]
- Scheurle, D.; Jahanzeb, M.; Aronsohn, R.S.; Watzek, L.; Narayanan, R. HER-2/neu expression in archival non-small cell lung carcinomas using FDA-approved Hercep test. Anticancer Res. 2000, 20, 2091–2096. [Google Scholar] [PubMed]
- Pellegrini, C.; Falleni, M.; Marchetti, A.; Cassani, B.; Miozzo, M.; Buttitta, F.; Roncalli, M.; Coggi, G.; Bosari, S. HER-2/Neu alterations in non-small cell lung cancer: A comprehensive evaluation by real time reverse transcription-PCR, fluorescence in situ hybridization, and immunohistochemistry. Clin. Cancer Res. 2003, 9, 3645–3652. [Google Scholar] [PubMed]
- Heinmoller, P.; Gross, C.; Beyser, K.; Schmidtgen, C.; Maass, G.; Pedrocchi, M.; Ruschoff, J. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin. Cancer Res. 2003, 9, 5238–5243. [Google Scholar] [PubMed]
- Tateishi, M.; Ishida, T.; Mitsudomi, T.; Kaneko, S.; Sugimachi, K. Prognostic value of c-erbB-2 protein expression in human lung adenocarcinoma and squamous cell carcinoma. Eur. J. Cancer 1991, 27, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.A.; Slebos, R.J.; Top, B.; Rodenhuis, S.; Lager, D.; Robinson, R.A.; Weiner, D.; Schwartz, D.A. C-erbB-2 expression and codon 12 K-ras mutations both predict shortened survival for patients with pulmonary adenocarcinomas. J. Clin. Invest. 1994, 93, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Zabrecky, J.R.; Lam, T.; McKenzie, S.J.; Carney, W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J. Biol. Chem. 1991, 266, 1716–1720. [Google Scholar] [PubMed]
- Pupa, S.M.; Menard, S.; Morelli, D.; Pozzi, B.; De Palo, G.; Colnaghi, M.I. The extracellular domain of the c-erbB-2 oncoprotein is released from tumor cells by proteolytic cleavage. Oncogene 1993, 8, 2917–2923. [Google Scholar] [PubMed]
- Scott, G.K.; Robles, R.; Park, J.W.; Montgomery, P.A.; Daniel, J.; Holmes, W.E.; Lee, J.; Keller, G.A.; Li, W.L.; Fendly, B.M. A truncated intracellular HER2/neu receptor produced by alternative RNA processing affects growth of human carcinoma cells. Mol. Cell. Biol. 1993, 13, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Codony-Servat, J.; Albanell, J.; Lopez-Talavera, J.C.; Arribas, J.; Baselga, J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999, 59, 1196–1201. [Google Scholar] [PubMed]
- Doherty, J.K.; Bond, C.; Jardim, A.; Adelman, J.P.; Clinton, G.M. The HER-2/neu receptor tyrosine kinase gene encodes a secreted autoinhibitor. Proc. Natl. Acad. Sci. USA 1999, 96, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Brandt-Rauf, P.W. The c-erbB transmembrane growth factor receptors as serum biomarkers in human cancer studies. Mutat. Res. 1995, 333, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Jo, J.; Filella, X.; Bruix, J.; Castells, A.; Hague, M.; Ballesta, A.M. Serum levels of C-erbB-2 (HER-2/neu) in patients with malignant and non-malignant diseases. Tumour Biol. 1997, 18, 188–196. [Google Scholar] [CrossRef]
- Brandt-Rauf, P.W.; Luo, J.C.; Carney, W.P.; Smith, S.; De Vivo, I.; Milling, C.; Hemminki, K.; Koskinen, H.; Vainio, H.; Neugut, A.I. Detection of increased amounts of the extracellular domain of the c-erbB-2 oncoprotein in serum during pulmonary carcinogenesis in humans. Int. J. Cancer 1994, 56, 383–386. [Google Scholar] [CrossRef]
- Osaki, T.; Mitsudomi, T.; Oyama, T.; Nakanishi, R.; Yasumoto, K. Serum level and tissue expression of c-erbB-2 protein in lung adenocarcinoma. Chest 1995, 108, 157–162. [Google Scholar] [CrossRef]
- Yoshimura, C.; Nomura, S.; Yamaoka, M.; Ohtani, T.; Matsuzakiz, T.; Yamaguchi, K.; Fukuharal, S. Analysis of serum ErbB-2 protein and HLA-DRB1 in Japanese patients with lung cancer. Cancer Lett. 2000, 152, 87–95. [Google Scholar] [CrossRef]
- Filiberti, R.; Marroni, P.; Paganuzzi, M.; Izzo, V.; Padovani, P.; Cafferata, M.; Ardizzoni, A.; Neri, M.; Raimondi, L.; Puntoni, R. c-erbB-2 protein in serum of primary lung cancer patients. Cancer Detect. Prev. 2002, 26, 64–68. [Google Scholar] [CrossRef]
- Baron, A.T.; Lafky, J.M.; Connolly, D.C.; Peoples, J.; O'Kane, D.J.; Suman, V.J.; Boardman, C.H.; Podratz, K.C.; Maihle, N.J. A sandwich type acridinium-linked immunosorbent assay (ALISA) detects soluble ErbB1 (sErbB1) in normal human sera. J. Immunol. Method. 1998, 219, 23–43. [Google Scholar] [CrossRef]
- Yang, P.; Allen, M.S.; Aubry, M.C.; Wampfler, J.A.; Marks, R.S.; Edell, E.S.; Thibodeau, S.; Adjei, A.A.; Jett, J.; Deschamps, C. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest 2005, 128, 452–462. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Storey, J.D.; Tibshirani, R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Meth. Mol. Biol. 2003, 224, 149–157. [Google Scholar]
- Dati, C.; Antoniotti, S.; Taverna, D.; Perroteau, I.; De Bortoli, M. Inhibition of c-erbB-2 oncogene expression by estrogens in human breast cancer cells. Oncogene 1990, 5, 1001–1006. [Google Scholar] [PubMed]
- Read, L.D.; Keith, D., Jr.; Slamon, D.J.; Katzenellenbogen, B.S. Hormonal modulation of HER-2/neu protooncogene messenger ribonucleic acid and p185 protein expression in human breast cancer cell lines. Cancer Res. 1990, 50, 3947–3951. [Google Scholar] [PubMed]
- Warri, A.M.; Laine, A.M.; Majasuo, K.E.; Alitalo, K.K.; Harkonen, P.L. Estrogen suppression of erbB2 expression is associated with increased growth rate of ZR-75-1 human breast cancer cells in vitro and in nude mice. Int. J. Cancer 1991, 49, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Warri, A.M.; Isola, J.J.; Harkonen, P.L. Anti-oestrogen stimulation of ERBB2 ectodomain shedding from BT-474 human breast cancer cells with ERBB2 gene amplification. Eur. J. Cancer 1996, 32A, 134–140. [Google Scholar] [PubMed]
- Taverna, D.; Antoniotti, S.; Maggiora, P.; Dati, C.; De Bortoli, M.; Hynes, N.E. erbB-2 expression in estrogen-receptor-positive breast-tumor cells is regulated by growth-modulatory reagents. Int. J. Cancer. 1994, 56, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Lagasse, L.D.; Karlan, B.Y. Steroid hormonal independence of HER-2/neu mRNA expression in four human ovarian carcinoma cell lines. Gynecol. Oncol. 1994, 55, 421–426. [Google Scholar] [CrossRef]
- Shi, Y.; Brands, F.H.; Chatterjee, S.; Feng, A.C.; Groshen, S.; Schewe, J.; Lieskovsky, G.; Cote, R.J. Her-2/neu expression in prostate cancer: High level of expression associated with exposure to hormone therapy and androgen independent disease. J. Urol. 2001, 166, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Meden, H.; Mielke, S.; Marx, D.; Wuttke, W.; Kuhn, W. Hormonal treatment with sex steroids in women is associated with lower p105 serum concentrations. Anticancer Res. 1997, 17, 3075–3077. [Google Scholar] [PubMed]
- Meden, H.; Mielke, S.; Schauer, A.; Kuhn, W. Serum levels of the c-erbB-2 (HER2/neu) encoded oncoprotein fragment p105 in normal pregnancies. In Vivo 1997, 11, 51–54. [Google Scholar] [PubMed]
- Meden, H.; Mielke, S.; Wuttke, W.; Kuhn, W. Elevated serum levels of the c-erbB-2 encoded oncoprotein fragment in cases of pure preeclampsia and HELLP syndrome. J. Obstet Gynaecol. Res. 1997, 23, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.; Meden, H.; Kuhn, W. Expression of the c-erbB-2-encoded oncoprotein p185 (HER-2/neu) in pregnancy as a model for oncogene-induced carcinogenesis. Med. Hypotheses 1998, 50, 359–362. [Google Scholar] [CrossRef]
- Meden, H.; Mielke, S.; Wuttke, W.; Kuhn, W. Increased serum level of c-erbB-2-coded protein p105 in patients with pre-eclampsia. Gynakologisch-geburtshilfliche Rundschau 1995, 35 Suppl 1, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.C.; Mitchell, P.; Anderson, M.; Lasley, B.L. Cigarette smoking and effects on hormone function in premenopausal women. Environ. Health Perspect. 2005, 113, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Rohrmann, S.; Menke, A.; Selvin, E.; Crespo, C.J.; Rifai, N.; Dobs, A.; Feinleib, M.; Guallar, E.; Platz, E.A. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Cause. Control 2009, 20, 877–886. [Google Scholar] [CrossRef]
- Breuer, B.; Smith, S.; Osborne, M.P.; Simmons, R.M.; Carney, W.P.; Brandt-Rauf, P.W. ErbB-2 protein levels in healthy, asymptomatic women. Biomarkers 1996, 1, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K. Lung cancer risk models come of age. Cancer Prev. Res. 2008, 1, 226–228. [Google Scholar] [CrossRef]
- Freedman, A.N.; Seminara, D.; Gail, M.H.; Hartge, P.; Colditz, G.A.; Ballard-Barbash, R.; Pfeiffer, R.M. Cancer risk prediction models: A workshop on development, evaluation, and application. J. Nat. Cancer Inst. 2005, 97, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.B.; Kattan, M.W.; Thornquist, M.D.; Kris, M.G.; Tate, R.C.; Barnett, M.J.; Hsieh, L.J.; Begg, C.B. Variations in lung cancer risk among smokers. J. Nat. Cancer Inst. 2003, 95, 470–478. [Google Scholar] [CrossRef]
- Cassidy, A.; Duffy, S.W.; Myles, J.P.; Liloglou, T.; Field, J.K. Lung cancer risk prediction: A tool for early detection. Int. J. Cancer 2007, 120, 1–6. [Google Scholar] [PubMed]
- Cassidy, A.; Myles, J.P.; van Tongeren, M.; Page, R.D.; Liloglou, T.; Duffy, S.W.; Field, J.K. The LLP risk model: An individual risk prediction model for lung cancer. Brit. J. Cancer 2008, 98, 270–276. [Google Scholar] [CrossRef]
- Cronin, K.A.; Gail, M.H.; Zhaohui, Z.; Bach, P.B.; Virtamo, J.; Albanes, D. Validation of a model of lung cancer risk prediction among smokers. J. Nat. Cancer Inst. 2006, 98, 637–640. [Google Scholar] [CrossRef]
- Etzel, C.J.; Kachroo, S.; Liu, M.; D’Amelio, A.; Dong, Q.; Cote, M.L.; Wenzlaff, A.S.; Hong, W.K.; Greisinger, A.J.; Schwartz, A.G.; Spitz, M.R. Development and validation of a lung cancer risk prediction model for African-Americans. Cancer Prev. Res. 2008, 1, 255–265. [Google Scholar] [CrossRef]
- Peto, R.; Darby, S.; Doll, R.; Deo, H.; Silcocks, P.; Whitley, E. Smoking, smoking cessation, and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. Brit. Med. J. 2000, 321, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Spitz, M.A.; Waun Ki, H.; Amos, C.I.; Xifeng, W.; Schabath, M.B.; Qiong, D.; Sanjay, S.; Etzel, C.J. A risk model for prediction of lung cancer. J. Nat. Cancer Inst. 2007, 99, 715–726. [Google Scholar] [CrossRef]
- Spitz, M.R.; Etzel, C.J.; Dong, Q.; Amos, C.I.; Wei, Q.; Wu, X.; Hong, W.K. An expanded risk prediction model for lung cancer. Cancer Prev. Res. 2008, 1, 250–254. [Google Scholar] [CrossRef]
- Prindiville, S.A.; Byers, T.; Hirsch, F.R.; Franklin, W.A.; Miller, Y.E.; Vu, K.O.; Wolf, H.J.; Barón, A.E.; Shroyer, K.R.; Zeng, C.; et al. Sputum cytological atypia as a predictor of incident lung cancer in a cohort of heavy smokers with airflow obstruction. Canc. Epidemiol. Biomarkers Prev. 2003, 12, 987–993. [Google Scholar]
- Blay, J.-Y.; Le Cesne, A.; Alberti, L.; Ray-Coquart, I. Targeted cancer therapies. Bull. Cancer 2005, 92, E13–E18. [Google Scholar] [CrossRef]
- Bacus, S.; Spector, N.L.; Yarden, Y. The era of ErbB-receptor-targeted therapies: Advances toward personalized medicine. Personalized Med. 2005, 2, 301–315. [Google Scholar] [CrossRef]
- Baselga, J.; Albanell, J. Epithelial growth factor receptor interacting agents. Hematol. Oncol. Clin. N. Amer. 2002, 16, 1041–1063. [Google Scholar] [CrossRef]
- Metro, G.; Finocchiaro, G.; Toschi, L.; Bartolini, S.; Magrini, E.; Cancellieri, A.; Trisolini, R.; Castaldini, L.; Tallini, G.; Crino, L.; Cappuzzo, F. Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC). Rev. Recent Clin. Trials 2006, 1, 1–13. [Google Scholar] [CrossRef]
- Friedrich, M.J. Using EGFR status to personalize treatment: Lung cancer researchers reach a milestone. J. Nat. Cancer Inst. 2009, 101, 1039–1041. [Google Scholar] [CrossRef]
- Perez-Soler, R. Individualized therapy in non-small-cell lung cancer: Future versus current clinical practice. Oncogene 2009, 28 Suppl 1, S38–S45. [Google Scholar] [CrossRef]
- Johnson, B.E.; Jänne, P.A. Rationale for a phase II trial of pertuzumab, a HER-2 dimerization inhibitor, in patients with non-small cell lung cancer. Clin. Cancer Res. 2006, 12, 4436–4440. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nambu, Y.; Fujimoto, T.; Koshiji, M. A landmark point analysis with cytotoxic agents for advanced NSCLC. J. Thoracic Oncol. 2009, 4, 697–701. [Google Scholar] [CrossRef]
- Zinner, R.G.; Glisson, B.S.; Fossella, F.V.; Pisters, K.M.; Kies, M.S.; Lee, P.M.; Massarelli, E.; Sabloff, B.; Fritsche, H.A., Jr.; Ro, J.Y.; et al. Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer 2004, 44, 99–110. [Google Scholar] [CrossRef]
- Swanton, C.; Futreal, A.; Eisen, T. Her2-targeted therapies in non-small cell lung cancer. Clin. Cancer Res. 2006, 12, 4377–4383. [Google Scholar] [CrossRef]
- Yatabe, Y. Molecular diagnosis of solid tumors. Nippon Rinsho. Jpn. J. Clin. Med. 2005, 63, 434–440. [Google Scholar]
- Allison, M. The HER2 testing conundrum. Nat. Biotechnol. 2010, 28, 117–119. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cosentino-Boehm, A.L.; Lafky, J.M.; Greenwood, T.M.; Kimbler, K.D.; Buenafe, M.C.; Wang, Y.; Branscum, A.J.; Yang, P.; Maihle, N.J.; Baron, A.T. Soluble Human Epidermal Growth Factor Receptor 2 (sHER2) as a Potential Risk Assessment, Screening, and Diagnostic Biomarker of Lung Adenocarcinoma. Diagnostics 2013, 3, 13-32. https://doi.org/10.3390/diagnostics3010013
Cosentino-Boehm AL, Lafky JM, Greenwood TM, Kimbler KD, Buenafe MC, Wang Y, Branscum AJ, Yang P, Maihle NJ, Baron AT. Soluble Human Epidermal Growth Factor Receptor 2 (sHER2) as a Potential Risk Assessment, Screening, and Diagnostic Biomarker of Lung Adenocarcinoma. Diagnostics. 2013; 3(1):13-32. https://doi.org/10.3390/diagnostics3010013
Chicago/Turabian StyleCosentino-Boehm, Abby L., Jacqueline M. Lafky, Tammy M. Greenwood, Kimberly D. Kimbler, Marites C. Buenafe, Yuxia Wang, Adam J. Branscum, Ping Yang, Nita J. Maihle, and Andre T. Baron. 2013. "Soluble Human Epidermal Growth Factor Receptor 2 (sHER2) as a Potential Risk Assessment, Screening, and Diagnostic Biomarker of Lung Adenocarcinoma" Diagnostics 3, no. 1: 13-32. https://doi.org/10.3390/diagnostics3010013






