An Assessment of Early Response to Targeted Therapy via Molecular Imaging: A Pilot Study of 3′-deoxy-3′[(18)F]-Fluorothymidine Positron Emission Tomography 18F-FLT PET/CT in Prostate Adenocarcinoma
Abstract
:1. Introduction
2. Methods
2.1. Radiosynthesis of 18F-FLT
2.2. Patient Selection, Preparation, and Clinical Trial Description
2.3. The Positron Emission Tomography/Computerized Tomography (PET/CT) Study
2.4. Image Analysis
2.5. 18F-FLT PET/CT Scan Interpretation
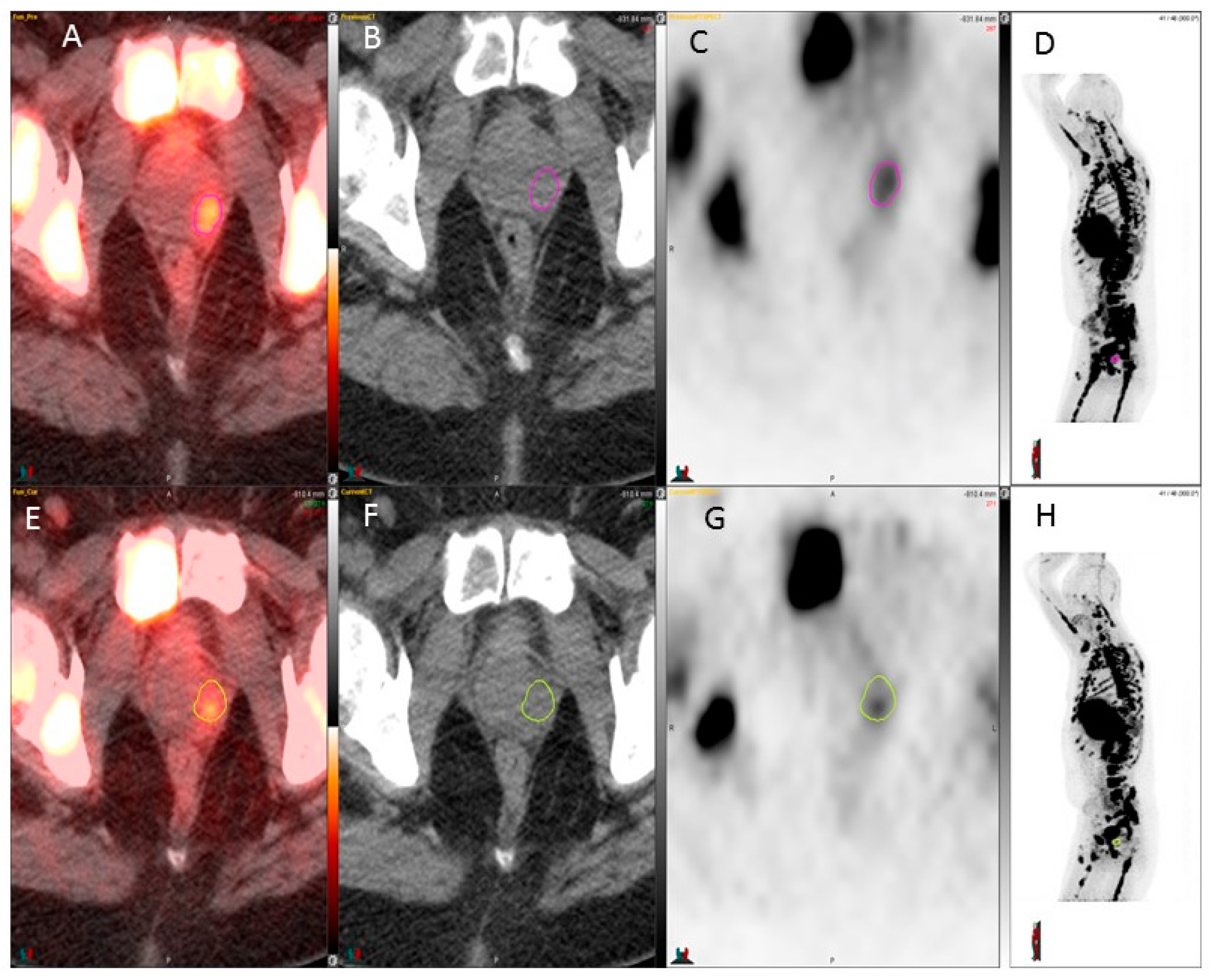
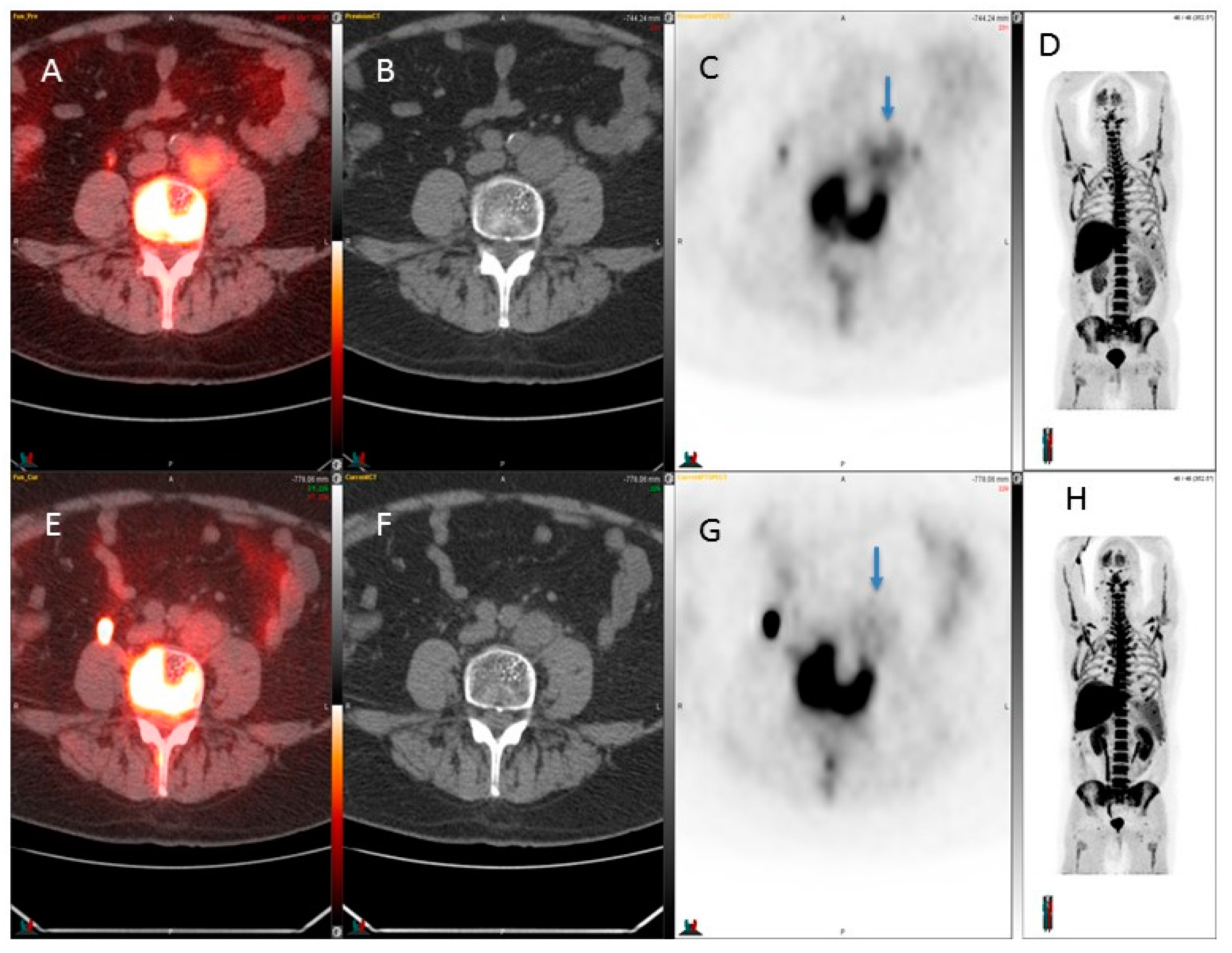
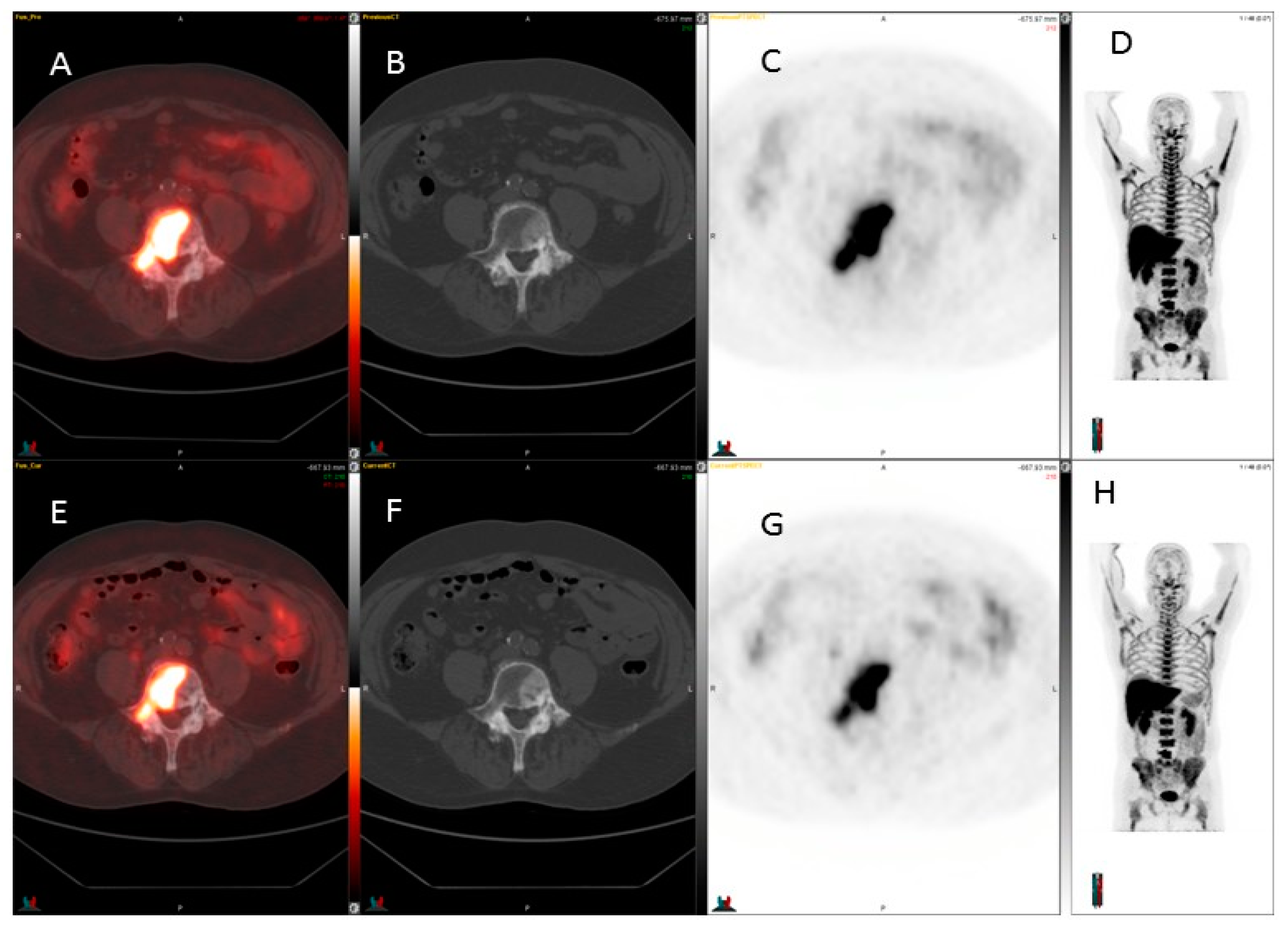
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.; Laubenbacher, C.; Block, T.; Breul, J.; Hartung, R.; Schwaiger, M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur. Urol. 1999, 36, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.J.; Zafar, M.B.; Lai, Y.H.; Segall, G.M.; Terris, M.K. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 2001, 57, 108–111. [Google Scholar] [CrossRef]
- Salminen, E.; Hogg, A.; Binns, D.; Frydenberg, M.; Hicks, R. Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002, 41, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, H.; Karapolat, I. F-fluorodeoxyglucose PET/CT for detection of disease in patients with prostate-specific antigen relapse following radical treatment of a local-stage prostate cancer. Oncol. Lett. 2016, 11, 316–322. [Google Scholar] [PubMed]
- Meirelles, G.S.; Schoder, H.; Ravizzini, G.C.; Gonen, M.; Fox, J.J.; Humm, J.; Morris, M.J.; Scher, H.I.; Larson, S.M. Prognostic value of baseline [18F] fluorodeoxyglucose positron emission tomography and 99mTC-MDP bone scan in progressing metastatic prostate cancer. Clin. Cancer Res. 2010, 16, 6093–6099. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Bommer, M.; Stilgenbauer, S.; Juweid, M.; Glatting, G.; Schirrmeister, H.; Mattfeldt, T.; Tepsic, D.; Bunjes, D.; Mottaghy, F.M.; et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006, 66, 11055–11061. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, S.; Shields, A.F. The role of DNA synthesis imaging in cancer in the era of targeted therapeutics. Cancer Metastasis Rev. 2008, 27, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Kukuk, D.; Reischl, G.; Raguin, O.; Wiehr, S.; Judenhofer, M.S.; Calaminus, C.; Honndorf, V.S.; Quintanilla-Martinez, L.; Schonberger, T.; Duchamp, O.; et al. Assessment of PET tracer uptake in hormone-independent and hormone-dependent xenograft prostate cancer mouse models. J. Nucl. Med. 2011, 52, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Ponde, D.E.; Dence, C.; Kim, J.; Tai, Y.C.; Welch, M.J. Monitoring of therapy in androgen-dependent prostate tumor model by measuring tumor proliferation. J. Nucl. Med. 2004, 45, 519–525. [Google Scholar] [PubMed]
- Oyama, N.; Hasegawa, Y.; Kiyono, Y.; Kobayashi, M.; Fujibayashi, Y.; Ponde, D.E.; Dence, C.; Welch, M.J.; Yokoyama, O. Early response assessment in prostate carcinoma by (18)F-fluorothymidine following anticancer therapy with docetaxel using preclinical tumour models. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Briganti, A.; Fanti, S.; Joniau, S.; Reske, S.; Schiavina, R.; Stief, C.; Thalmann, G.N.; Picchio, M. New clinical indications for 18F/11C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: A systematic review of the literature. Eur. Urol. 2016, 70, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Machulla, H.J.; Blocher, A.; Kuntzsch, M.; Piert, M.; Wei, R.; Grierson, J.R. Simplified labeling approach for synthesizing 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT). J. Radioanal. Nucl. Chem. 2000, 243, 843–846. [Google Scholar] [CrossRef]
- Jadvar, H. Molecular imaging of prostate cancer: PET radiotracers. Am. J. Roentgenol. 2012, 199, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Scher, B.; Seitz, M.; Albinger, W.; Tiling, R.; Scherr, M.; Becker, H.C.; Souvatzogluou, M.; Gildehaus, F.J.; Wester, H.J.; Dresel, S. Value of 11C-choline pet and PET/CT in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Brechbiel, M.W. Development of radioimmunotherapeutic and diagnostic antibodies: An inside-out view. Nucl. Med. Biol. 2007, 34, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Manyak, M.J. Indium-111 Capromab pendetide in the management of recurrent prostate cancer. Expert Rev. Anticancer Ther. 2008, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Manyak, M.J.; Hinkle, G.H.; Olsen, J.O.; Chiaccherini, R.P.; Partin, A.W.; Piantadosi, S.; Burgers, J.K.; Texter, J.H.; Neal, C.E.; Libertino, J.A.; et al. Immunoscintigraphy with indium-111-Capromab pendetide: Evaluation before definitive therapy in patients with prostate cancer. Urology 1999, 54, 1058–1063. [Google Scholar] [CrossRef]
- Liu, H.; Rajasekaran, A.K.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N.H. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998, 58, 4055–4060. [Google Scholar] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]gallium-labelled psma ligand as superior pet tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- van Waarde, A.; Cobben, D.C.; Suurmeijer, A.J.; Maas, B.; Vaalburg, W.; de Vries, E.F.; Jager, P.L.; Hoekstra, H.J.; Elsinga, P.H. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J. Nucl. Med. 2004, 45, 695–700. [Google Scholar] [PubMed]
- Li, Z.; Yu, Y.; Zhang, H.; Xu, G.; Chen, L. A meta-analysis comparing 18F-FLT PET with 18F-FDG PET for assessment of brain tumor recurrence. Nucl. Med. Commun. 2015, 36, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Kostakoglu, L.; Duan, F.; Idowu, M.O.; Jolles, P.R.; Bear, H.D.; Muzi, M.; Cormack, J.; Muzi, J.P.; Pryma, D.A.; Specht, J.M.; et al. A phase ii study of 3′-deoxy-3′-18F-fluorothymidine PET in the assessment of early response of breast cancer to neoadjuvant chemotherapy: Results from acrin 6688. J. Nucl. Med. 2015, 56, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, H.; Mori, T.; Yamamoto, Y.; Kishino, T.; Fukumura, T.; Samukawa, Y.; Mori, N.; Nishiyama, Y. Prognostic value comparison between (18)F-FLT PET/CT and (18)F-FDG PET/CT volume-based metabolic parameters in patients with head and neck cancer. Clin. Nucl. Med. 2015, 40, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; de Melo Gagliato, D.; Falchook, G.; Zinner, R.; Wheler, J.J.; Janku, F.; Subbiah, V.; Piha-Paul, S.A.; Fu, S.; Tannir, N.; et al. Met abnormalities in patients with genitourinary malignancies and outcomes with c-met inhibitors. Clin. Genitourin. Cancer 2015, 13, e19–e26. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Rosen, P.; Lockhart, A.C.; Fu, S.; Janku, F.; Kurzrock, R.; Khan, R.; Amore, B.; Caudillo, I.; Deng, H.; et al. A first-in-human study of amg 208, an oral met inhibitor, in adult patients with advanced solid tumors. Oncotarget 2015, 6, 18693–18706. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Corn, P.G.; Gaur, S.; Dayyani, F.; Logothetis, C.J.; Gallick, G.E. The role of HGF/C-MET signaling in prostate cancer progression and c-met inhibitors in clinical trials. Expert Opin. Investig. Drugs 2011, 20, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Feng, F.Y.; Wang, Y.; Cao, X.; Han, S.; Wilder-Romans, K.; Navone, N.M.; Logothetis, C.; Taichman, R.S.; Keller, E.T.; et al. Mechanistic support for combined MET and AR blockade in castration-resistant prostate cancer. Neoplasia 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Rosenthal, M.; Ng, S.; Alumkal, J.; Picus, J.; Gravis, G.; Fizazi, K.; Forget, F.; Machiels, J.P.; Srinivas, S.; et al. Targeted met inhibition in castration-resistant prostate cancer: A randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin. Cancer Res. 2013, 19, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, X.; Sun, S.; Gao, X.; Zhou, D. C-met inhibitor su11274 enhances the response of the prostate cancer cell line DU145 to ionizing radiation. Biochem. Biophys. Res. Commun. 2012, 427, 659–665. [Google Scholar] [CrossRef] [PubMed]
| Patient/Age | Prostate SUVmax BL-> 4 Weeks | Lymph Nodes SUVmax BL-> 4 Weeks Size (cm) BL-> 4 Weeks | Skeletal Disease SUVmax BL-> 4 Weeks | Soft Tissue | PSA Response |
|---|---|---|---|---|---|
| 1/67 | 2.5→2.8 3.6→2.3 | 1.6→1.5 1.1 × 1.0→1.0 × 0.9 (para-aortic) | 1.6→1.5 (cortex) 6.8→5.2 (marrow) | 9.8→12.8 (liver) | 0.2→0.5 |
| 2/74 | Ø | Ø | defects | Ø | 387→871 |
| 3/65 | Ø | 4.1→2.4 3.6 × 3.4→3.1 × 2.6 (iliaca comm L) | Ø | Ø | 95→62 |
| 4/51 | 4.2→3.9 | 4.3→3.6 3.7 × 4.4→3.3 × 4.2 (iliaca comm R) | Ø | Ø | 820→2138 |
| 5/72 | Ø | Ø | defects | Ø | 47.8→98.0 |
| 6/74 | Ø | Ø | defects | Ø | 3.1→6.3 |
| 7/61 | Ø | Ø | Ø | liver defects | 16.6→18.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairemo, K.; Ravizzini, G.C.; Macapinlac, H.A.; Subbiah, V. An Assessment of Early Response to Targeted Therapy via Molecular Imaging: A Pilot Study of 3′-deoxy-3′[(18)F]-Fluorothymidine Positron Emission Tomography 18F-FLT PET/CT in Prostate Adenocarcinoma. Diagnostics 2017, 7, 20. https://doi.org/10.3390/diagnostics7020020
Kairemo K, Ravizzini GC, Macapinlac HA, Subbiah V. An Assessment of Early Response to Targeted Therapy via Molecular Imaging: A Pilot Study of 3′-deoxy-3′[(18)F]-Fluorothymidine Positron Emission Tomography 18F-FLT PET/CT in Prostate Adenocarcinoma. Diagnostics. 2017; 7(2):20. https://doi.org/10.3390/diagnostics7020020
Chicago/Turabian StyleKairemo, Kalevi, Gregory C. Ravizzini, Homer A. Macapinlac, and Vivek Subbiah. 2017. "An Assessment of Early Response to Targeted Therapy via Molecular Imaging: A Pilot Study of 3′-deoxy-3′[(18)F]-Fluorothymidine Positron Emission Tomography 18F-FLT PET/CT in Prostate Adenocarcinoma" Diagnostics 7, no. 2: 20. https://doi.org/10.3390/diagnostics7020020
APA StyleKairemo, K., Ravizzini, G. C., Macapinlac, H. A., & Subbiah, V. (2017). An Assessment of Early Response to Targeted Therapy via Molecular Imaging: A Pilot Study of 3′-deoxy-3′[(18)F]-Fluorothymidine Positron Emission Tomography 18F-FLT PET/CT in Prostate Adenocarcinoma. Diagnostics, 7(2), 20. https://doi.org/10.3390/diagnostics7020020





