Validation of the Performance of International Ovarian Tumor Analysis (IOTA) Methods in the Diagnosis of Early Stage Ovarian Cancer in a Non-Screening Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
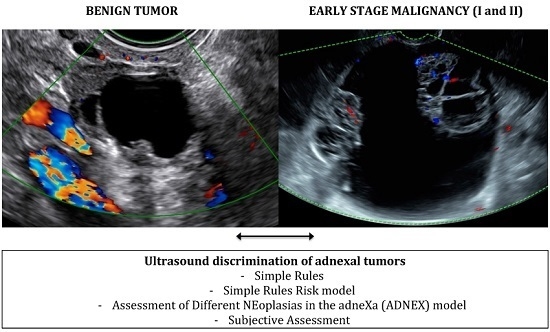
2.2. Diagnostic Models
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pavlik, E.J.; Ueland, F.R.; Miller, R.W.; Ubellacker, J.M.; DeSimone, C.P.; Elder, J.; Hoff, J.; Baldwin, L.; Kryscio, R.J.; van Nagell, J.R., Jr. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet. Gynecol. 2013, 122, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Alcazar, J.L.; Jurado, M. Natural history of sonographically detected simple unilocular adnexal cysts in asymptomatic postmenopausal women. Gynecol. Oncol. 2004, 92, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Borgfeldt, C.; Andolf, E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet. Gynecol. 1999, 13, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.P. Management of the Adnexal Mass. Gynecol. Oncol. 1994, 55, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Available online: www.cancerresearchuk.org (accessed on 20 November 2016).
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Heintz, A.P.M.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Benedet, J.L.; Creasman, W.T.; Ngan, H.Y.S.; Pecorelli, S.; Beller, U. Carcinoma of the Ovary. Int. J. Gynecol. Obstet. 2006, 95, S161–S192. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S. Effect of Screening on Ovarian Cancer Mortality. JAMA 2011, 305, 2295. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Paulsen, T.; Kjaerheim, K.; Kaern, J.; Tretli, S.; Tropé, C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int. J. Gynecol. Cancer 2006, 16, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.J.; Kos, H.E.; Willemse, P.H.; Aalders, J.G.; de Vries, E.G.; Schaapveld, M.; Otter, R.; van der Zee, A.G. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer 2006, 106, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Earle, C.C.; Schrag, D.; Neville, B.A.; Yabroff, K.R.; Topor, M.; Fahey, A.; Trimble, E.L.; Bodurka, D.C.; Bristow, R.E.; Carney, M.; et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J. Natl. Cancer Inst. 2006, 98, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.L.; Kyrgiou, M.; Bryant, A.; Everett, T.; Dickinson, H.O. Centralisation of services for gynaecological cancer—A Cochrane Systematic Review. Gynecol. Oncol. 2012, 126, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Valentin, L.; Hagen, B.; Tingulstad, S.; Eik-Nes, S. Comparison of “pattern recognition” and logistic regression models for discrimination between benign and malignant pelvic masses: A prospective cross validation. Ultrasound Obstet. Gynecol. 2001, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D. The use of mathematical models to evaluate pelvic masses; can they beat an expert operator? Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Meys, E.M.; Kaijser, J.; Kruitwagen, R.F.; Slangen, B.F.; van Calster, B.; Aertgeerts, B.; Verbakel, J.Y.; Timmerman, D.; van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; van Holsbeke, C.; Paladini, D.; van Calster, B.; Vergote, I.; van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. Management of Suspected Ovarian Masses in Premenopausal Women; Green-top Guideline No. 62; Royal College of Obstetricians and Gynaecologists: London, UK, 2011. [Google Scholar]
- The American College of Obstetricians and Gynecologists. Practice bulletin—Evaluation and Management of Adnexal Masses. Obstet. Gynecol. 2016, 128, e210–e226. [Google Scholar]
- Kaijser, J.; Sayasneh, A.; van Hoorde, K.; Ghaem-Maghami, S.; Bourne, T.; Timmerman, D.; van Calster, B. Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. International Ovarian Tumour Analysis, G. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef] [PubMed]
- Meys, E.M.; Jeelof, L.S.; Achten, N.M.; Slangen, B.F.; Lambrechts, S.; Kruitwagen, R.F.; van Gorp, T. Estimating the risk of malignancy in adnexal masses: An external validation of the ADNEX model and comparison with other frequently used ultrasound methods. Ultrasound Obstet. Gynecol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sayasneh, A.; Ferrara, L.; de Cock, B.; Saso, S.; Al-Memar, M.; Johnson, S.; Kaijser, J.; Carvalho, J.; Husicka, R.; Smith, A.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model: A multicentre external validation study. Br. J. Cancer 2016, 115, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Szubert, S.; Wojtowicz, A.; Moszynski, R.; Zywica, P.; Dyczkowski, K.; Stachowiak, A.; Sajdak, S.; Szpurek, D.; Alcazar, J.L. External validation of the IOTA ADNEX model performed by two independent gynecologic centers. Gynecol. Oncol. 2016, 142, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Araujo, K.G.; Jales, R.M.; Pereira, P.N.; Yoshida, A.; de Angelo Andrade, L.; Sarian, L.O.; Derchain, S. Performance of the IOTA ADNEX model in the preoperative discrimination of adnexal masses in a gynecologic oncology center. Ultrasound Obstet. Gynecol. 2016. [Google Scholar] [CrossRef]
- Joyeux, E.; Miras, T.; Masquin, I.; Duglet, P.E.; Astruc, K.; Douvier, S. Before surgery predictability of malignant ovarian tumors based on ADNEX model and its use in clinical practice. Gynecol. Obstet. Fertil. 2016, 44, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Kaijser, J.; Wynants, L.; Fischerova, D.; van Holsbeke, C.; Franchi, D.; Savelli, L.; Epstein, E.; Czekierdowski, A.; Guerriero, S.; et al. Strategies to diagnose ovarian cancer: New evidence from phase 3 of the multicentre international IOTA study. Br. J. Cancer 2014, 111, 680–688. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.A.; Juliato, C.R.; Sarian, L.O.; Toledo, M.C.; Jales, R.M.; Morais, S.S.; Pitta, D.D.; Marussi, E.F.; Derchain, S. Ultrasound criteria and CA 125 as predictive variables of ovarian cancer in women with adnexal tumors. Ultrasound Obstet. Gynecol. 2012, 40, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, J.L.; Pascual, M.A.; Olartecoechea, B.; Graupera, B.; Auba, M.; Ajossa, S.; Hereter, L.; Julve, R.; Gaston, B.; Peddes, C.; et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: Prospective external validation. Ultrasound Obstet. Gynecol. 2013, 42, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Sayasneh, A.; Wynants, L.; Preisler, J.; Kaijser, J.; Johnson, S.; Stalder, C.; Husicka, R.; Abdallah, Y.; Raslan, F.; Drought, A.; et al. Multicentre external validation of IOTA prediction models and RMI by operators with varied training. Br. J. Cancer 2013, 108, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Ambler, G.; Foo, X.; Naftalin, J.; Widschwendter, M.; Jurkovic, D. Use of IOTA simple rules for diagnosis of ovarian cancer: Meta-analysis. Ultrasound Obstet. Gynecol. 2014, 44, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Tinnangwattana, D.; Vichak-ururote, L.; Tontivuthikul, P.; Charoenratana, C.; Lerthiranwong, T.; Tongsong, T. IOTA Simple Rules in Differentiating between Benign and Malignant Adnexal Masses by Non-expert Examiners. Asian Pac. J. Cancer Prev. 2015, 16, 3835–3838. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Gauna, B.; Rodriguez, D.; Olartecoechea, B.; Auba, M.; Jurado, M.; Gomez Roig, M.D.; Alcazar, J.L. Diagnostic performance of IOTA simple rules for adnexal masses classification: A comparison between two centers with different ovarian cancer prevalence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Knafel, A.; Banas, T.; Nocun, A.; Wiechec, M.; Jach, R.; Ludwin, A.; Kabzinska-Turek, M.; Pietrus, M.; Pitynski, K. The Prospective External Validation of International Ovarian Tumor Analysis (IOTA) Simple Rules in the Hands of Level I and II Examiners. Ultraschall Med. 2015, 37, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-M.; Kurman, R. Ovarian Tumorigenesis—A Proposed Model Based on Morphological and Molecular Genetic Analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Valentin, L.; Ameye, L.; Jurkovic, D.; Metzger, U.; Lecuru, F.; van Huffel, S.; Timmerman, D. Which extrauterine pelvic masses are difficult to correctly classify as benign or malignant on the basis of ultrasound findings and is there a way of making a correct diagnosis? Ultrasound Obstet. Gynecol. 2006, 27, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Valentin, L.; Ameye, L.; Savelli, L.; Fruscio, R.; Leone, F.P.; Czekierdowski, A.; Lissoni, A.A.; Fischerova, D.; Guerriero, S.; van Holsbeke, C.; et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: Logistic regression models do not help. Ultrasound Obstet. Gynecol. 2011, 38, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Amant, F.; Ameye, L.; Timmerman, D. Screening for ovarian carcinoma: Not quite there yet. Lancet Oncol. 2009, 10, 308–309. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Dundr, P.; Cibula, D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 2012, 17, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; de Brabander, J.; Fyles, A.; Bertelsen, K.; Einhorn, N.; Sevelda, P.; Gore, M.; Kaern, J.; Verrelst, H.; Sjövall, K.; et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001, 357, 176–182. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C.; Group, E.G.W. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi24–vi32. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Verheecke, M.; Wlodarska, I.; Dehaspe, L.; Brady, P.; Brison, N.; van Den Bogaert, K.; Dierickx, D.; Vandecaveye, V.; Tousseyn, T.; et al. Presymptomatic Identification of Cancers in Pregnant Women During Noninvasive Prenatal Testing. JAMA Oncol. 2015, 1, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichele, A.; Busschaert, P.; Smeets, D.; Landolfo, C.; van Nieuwenhuysen, E.; Leunen, K.; Neven, P.; Amant, F.; Mahner, S.; Braicu, E.I.; et al. Chromosomal Instability in Cell-Free DNA as a Highly Specific Biomarker for Detection of Ovarian Cancer in Women with Adnexal Masses. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
| Variable | Result | |
|---|---|---|
| Benign Tumor | Early Stage Malignancy (I and II) | |
| N | 1423 (86.1%) | 230 (13.9%) |
| Age (years) | 44 (33 to 56) | 55 (42 to 66) |
| Postmenopausal | 447 (31.4%) | 125 (54.3%) |
| CA125 (IU/L), if available a | 21 (12 to 46) | 55 (20 to 207) |
| Maximum tumor diameter (mm) | 64 (47 to 90) | 103 (68 to 143) |
| Presence of solid components | 474 (33.3%) | 214 (93.0%) |
| Maximum diameter of the solid component (if any, mm) | 28 (13 to 54) | 62 (37 to 93) |
| Locularity | ||
| Unilocular | 595 (41.8%) | 2 (0.9%) |
| Unilocular-solid | 141 (9.9%) | 34 (14.8%) |
| Multilocular | 354 (24.9%) | 14 (6.1%) |
| Multilocular-solid | 179 (12.6%) | 93 (40.4%) |
| Solid | 154 (10.8%) | 87 (37.8%) |
| Number of locules (if any) | 1 (1 to 3) | 5 (1.5 to 6) |
| Acoustic shadows | 265 (18.6%) | 17 (7.4%) |
| Intratumoral blood flow | ||
| No blood flow | 574 (40.3%) | 5 (2.2%) |
| Minimal blood flow | 563 (39.6%) | 54 (23.5%) |
| Moderate blood flow | 239 (16.8%) | 95 (41.3%) |
| Very strong blood flow | 47 (3.3%) | 76 (33.0%) |
| Irregular internal cyst wall | 385 (27.1%) | 151 (65.7%) |
| Presence of ascites | 18 (1.3%) | 33 (14.3%) |
| Presence of papillary structures | 180 (12.6%) | 54 (23.5%) |
| Number of papillary structures (if present) | 1 (1 to 3) | 3 (2 to 4) |
| Histology | N (%) |
|---|---|
| Endometrioma | 344 (20.8%) |
| Teratoma | 231 (14.0%) |
| Simple cyst + parasalpingeal cyst | 106 (6.4%) |
| Functional cyst | 40 (2.4%) |
| Hydrosalpinx + salpingitis | 47 (2.8%) |
| Peritoneal pseudocyst | 18 (1.1%) |
| Abscess | 17 (1.0%) |
| Fibroma | 130 (7.9%) |
| Serous cystadenoma | 259 (15.7%) |
| Mucinous cystadenoma | 183 (11.1%) |
| Rare benign | 48 (2.9%) |
| Primary invasive (epithelial) cancer stage I | 128 (7.7%) |
| Primary invasive (epithelial) cancer stage II | 47 (2.8%) |
| Rare primary invasive malignancy stage I or II * | 55 (3.3%) |
| Risk Threshold | Statistic | ADNEX | SRR |
|---|---|---|---|
| 1% | Sensitivity | 100.0% | 100.0% |
| (98.4% to 100.0%) | (98.4% to 100.0%) | ||
| Specificity | 19.4% | 38.0% | |
| (17.4% to 21.5%) | (35.5% to 40.6%) | ||
| 10% | Sensitivity | 97.4% | 97.0% |
| (94.4% to 98.8%) | (93.9% to 98.5%) | ||
| Specificity | 69.5% | 65.1% | |
| (67.1% to 71.8%) | (62.6% to 67.6%) | ||
| 20% | Sensitivity | 91.3% | 94.3% |
| (87.0% to 94.3%) | (90.6% to 96.7%) | ||
| Specificity | 79.7% | 74.3% | |
| (77.5% to 81.7%) | (71.9% to 76.5%) | ||
| 30% | Sensitivity | 84.5% | 88.3% |
| (80.5% to 89.6%) | (83.5% to 91.8%) | ||
| Specificity | 84.5% | 81.1% | |
| (82.6% to 86.3%) | (79.0% to 83.0%) |
| Risk Threshold | Statistic | ADNEX | SRR | ||
|---|---|---|---|---|---|
| Premenopausal | Postmenopausal | Premenopausal | Postmenopausal | ||
| 1% | Sens | 100.0% | 100.0% | 100.0% | 100% |
| (96.5% to 100.0%) | (97.0% to 100%) | (96.5% to 100.0%) | (97.0% to 100.0%) | ||
| Spec | 25.8% | 5.4% | 41.4% | 30.6% | |
| (23.2% to 28.7%) | (3.6% to 7.9%) | (38.3% to 44.5%) | (26.6% to 35.1%) | ||
| 10% | Sens | 94.3% | 100% | 98.1% | 96.0% |
| (88.1% to 97.4%) | (97.0% to 100%) | (93.3% to 99.5%) | (91.0% to 98.3%) | ||
| Spec | 77.8% | 51.5% | 70.6% | 53.2% | |
| (75.1% to 80.3%) | (46.8% to 56.1%) | (67.7% to 73.4%) | (48.6% to 57.8%) | ||
| 20% | Sens | 86.7% | 95.2% | 94.3% | 94.4% |
| (78.9% to 91.9%) | (89.9% to 97.8%) | (88.1% to 97.4%) | (88.9% to 97.3%) | ||
| Spec | 85.6% | 66.9% | 78.5% | 65.1% | |
| (83.2% to 87.6%) | (62.4% to 71.1%) | (75.8% to 80.9%) | (60.6% to 69.4%) | ||
| 30% | Sens | 78.1% | 92.0% | 84.8% | 91.2% |
| (69.3% to 84.9%) | (85.9% to 95.6%) | (76.7% to 90.4%) | (84.9% to 95.0%) | ||
| Spec | 89.5% | 73.6% | 84.1% | 74.5% | |
| (87.5% to 81.3%) | (96.3% to 77.5%) | (81.7% to 86.3%) | (70.3% to 78.3%) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froyman, W.; Wynants, L.; Landolfo, C.; Bourne, T.; Valentin, L.; Testa, A.; Sladkevicius, P.; Franchi, D.; Fischerova, D.; Savelli, L.; et al. Validation of the Performance of International Ovarian Tumor Analysis (IOTA) Methods in the Diagnosis of Early Stage Ovarian Cancer in a Non-Screening Population. Diagnostics 2017, 7, 32. https://doi.org/10.3390/diagnostics7020032
Froyman W, Wynants L, Landolfo C, Bourne T, Valentin L, Testa A, Sladkevicius P, Franchi D, Fischerova D, Savelli L, et al. Validation of the Performance of International Ovarian Tumor Analysis (IOTA) Methods in the Diagnosis of Early Stage Ovarian Cancer in a Non-Screening Population. Diagnostics. 2017; 7(2):32. https://doi.org/10.3390/diagnostics7020032
Chicago/Turabian StyleFroyman, Wouter, Laure Wynants, Chiara Landolfo, Tom Bourne, Lil Valentin, Antonia Testa, Povilas Sladkevicius, Dorella Franchi, Daniela Fischerova, Luca Savelli, and et al. 2017. "Validation of the Performance of International Ovarian Tumor Analysis (IOTA) Methods in the Diagnosis of Early Stage Ovarian Cancer in a Non-Screening Population" Diagnostics 7, no. 2: 32. https://doi.org/10.3390/diagnostics7020032