Comparison of the Peripheral Reactive Hyperemia Index with Myocardial Perfusion Reserve by 82Rb PET/CT in HIV-Infected Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics
2.3. Data Collection
2.3.1. EndoPAT
2.3.2. PET Imaging
2.3.3. Blood Markers
2.3.4. Risk Score
2.4. Statistical Analyses
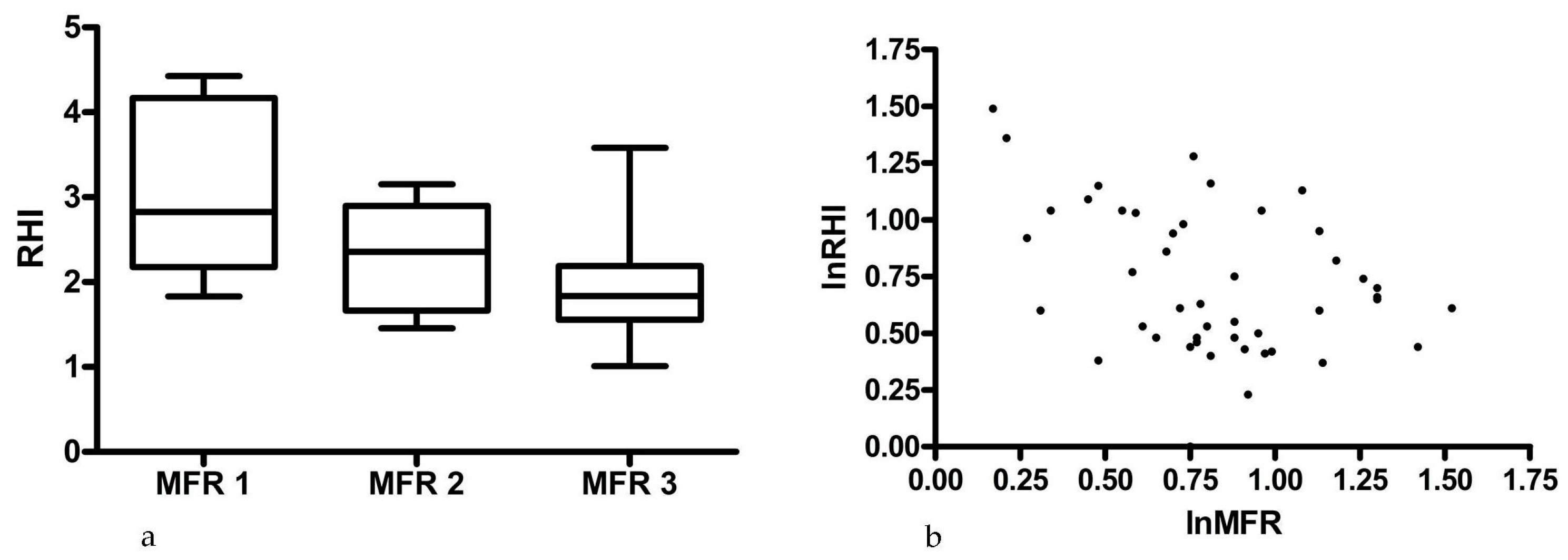
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Obel, N.; Omland, L.H.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Pedersen, G.; Sørensen, H.T.; Gerstoft, J. Impact of Non-HIV and HIV Risk Factors on Survival in HIV-Infected Patients on HAART: A Population-Based Nationwide Cohort Study. PLoS ONE 2011, 6, e22698. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Hasse, B.; Ledergerber, B.; Furrer, H.; Battegay, M.; Hirschel, B.; Cavassini, M.; Bertisch, B.; Bernasconi, E.; Weber, R. Swiss HIV Cohort Study. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin. Infect. Dis. 2011, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, T.A.T.C. Causes of Death in HIV-1—Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies. Clin. Infect. Dis. 2010, 50, 1387–1396. [Google Scholar]
- Post, W.S.; Budoff, M.; Kingsley, L.; Palella, J.; Frank, J.; Witt, M.D.; Li, X.; George, R.T.; Brown, T.T.; Jacobson, L.P. Associations Between HIV Infection and Subclinical Coronary AtherosclerosisAssociations Between HIV Infection and Subclinical Coronary Atherosclerosis. Ann. Intern. Med. 2014, 160, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.-C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Bidwell Goetz, M.; Leaf, D.; Oursler, K.A.; et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Plutzky, J. The Biology of Atherosclerosis: General Paradigms and Distinct Pathogenic Mechanisms Among HIV-Infected Patients. J. Infect. Dis. 2012, 205, S368–S374. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Deeks, S.G.; Hunt, P.W. Immunologic Basis of Cardiovascular Disease in HIV-Infected Adults. J. Infect. Dis. 2012, 205, S375–S382. [Google Scholar] [CrossRef] [PubMed]
- Seaberg, E.C.; Benning, L.; Sharrett, A.R.; Lazar, J.M.; Hodis, H.N.; Mack, W.J.; Siedner, M.J.; Phair, J.P.; Kingsley, L.A.; Kaplan, R.C. Association Between Human Immunodeficiency Virus Infection and Stiffness of the Common Carotid Artery. Stroke 2010, 41, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Ordovas, K.; Lee, T.; Reddy, G.; Gotway, M.; Schnell, A.; Ho, J.E.; Selby, V.; Madden, E.; Martin, J.N.; et al. Carotid Intima-Media Thickness Among Human Immunodeficiency Virus–Infected Patients Without Coronary Calcium. Am. J. Cardiol. 2012, 109, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Tawakol, A.; Burdo, T.H.; Abbara, S.; Wei, J.; Vijayakumar, J.; Corsini, E.; Abdelbaky, A.; Zanni, M.V.; Hoffmann, U.; et al. Arterial inflammation in patients with HIV. J. Am. Med. Assoc. 2012, 308, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial Function and Dysfunction. Circulation 2007, 115, 1285–1295. [Google Scholar] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Shechter, M.; Shechter, A.; Koren-Morag, N.; Feinberg, M.S.; Hiersch, L. Usefulness of Brachial Artery Flow-Mediated Dilation to Predict Long-Term Cardiovascular Events in Subjects Without Heart Disease. Am. J. Cardiol. 2014, 113, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H. Carotid Artery Imaging: Insights Into Inflammation and Cardiovascular Disease Risk in Patients With HIV Infection. J. Am. Heart Assoc. 2012, 1, e001396. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, C.T.; Hoit, B.D. Imaging atherosclerosis in HIV: carotid intima-media thickness and beyond. Transl. Res. 2012, 159, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Benjamin, E.J. Assessment of Endothelial Function Using Digital Pulse Amplitude Tonometry. Trends Cardiovasc. Med. 2009, 19, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Zeiher, A.M.; Drexler, H.; Wollschläger, H.; Just, H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation 1991, 84, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Gaber, M.; Carli, G.D.; Blankstein, R.; Dorbala, S.; Sitek, A.; Pencina, M.J.; et al. Improved Cardiac Risk Assessment With Noninvasive Measures of Coronary Flow Reserve. Circulation 2011, 124, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Javadi, M.S.; Higuchi, T.; Lautamäki, R.; Merrill, J.; Nekolla, S.G.; Bengel, F.M. Prediction of Short-Term Cardiovascular Events Using Quantification of Global Myocardial Flow Reserve in Patients Referred for Clinical 82Rb PET Perfusion Imaging. J. Nucl. Med. 2011, 52, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Gaber, M.; Hainer, J.; Klein, J.; Dorbala, S.; Blankstein, R.; Carli, M.F.D. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients With and Without Diabetes Mellitus. Circulation 2012, 126, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Gaber, M.; Dorbala, S.; Charytan, D.M.; Blankstein, R.; Di Carli, M.F. Coronary Vascular Dysfunction and Prognosis in Patients with Chronic Kidney Disease. JACC Cardiovasc. Imaging 2012, 5, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Schelbert, H.R.; Quercioli, A.; Dilsizian, V. Cardiac PET Imaging for the Detection and Monitoring of Coronary Artery Disease and Microvascular Health. JACC Cardiovasc. Imaging 2010, 3, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.; Christensen, T.E.; Ghotbi, A.A.; Hasbak, P.; Lebech, A.-M.; Kjær, A.; Ripa, R.S. Normal Myocardial Flow Reserve in HIV-Infected Patients on Stable Antiretroviral Therapy: A Cross-Sectional Study Using Rubidium-82 PET/CT. Medicine (Baltimore) 2015, 94, e1886. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsson, K.; Ladelund, S.; Storgaard, M.; Rønsholt, F.F.; Johansen, I.S.; Pedersen, G.; Nielsen, L.N.; Bonde, J.; Westh, H.; Obel, N.; et al. Sexually transmitted infections and use of contraceptives in women living with HIV in Denmark—The SHADE cohort. BMC Infect. Dis. 2016, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Müller, P.; Chan, S.; Brunken, R.C.; Porenta, G.; Krivokapich, J.; Chen, K.; Chan, A.; Phelps, M.E.; Schelbert, H.R. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993, 88, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Anderson, K.M.; Odell, P.M.; Wilson, P.W.; Kannel, W.B. Cardiovascular disease risk profiles. Am. Heart J. 1991, 121, 293–298. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Flammer, A.J.; Lerman, L.O.; Elízaga, J.; Lerman, A.; Fernández-Avilés, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Uehata, A.; Gerhard, M.D.; Meredith, I.T.; Knab, S.; Delagrange, D.; Lieberman, E.H.; Ganz, P.; Creager, M.A.; Yeung, A.C.; et al. Close relation of endothelial function in the human coronary and peripheral circulations. J. Am. Coll. Cardiol. 1995, 26, 1235–1241. [Google Scholar] [CrossRef]
- Scholtens, A.M.; Tio, R.A.; Willemsen, A.; Dierckx, R.A.J.O.; Boersma, H.H.; Zeebregts, C.J.; Glaudemans, A.W.J.M.; Slart, R.H.J.A. Myocardial perfusion reserve compared with peripheral perfusion reserve: A [13N]ammonia PET study. J. Nucl. Cardiol. 2011, 18, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, M.M.; Mygind, N.D.; Pena, A.; Aziz, A.; Frestad, D.; Høst, N.; Prescott, E. Peripheral Reactive Hyperemia Index and Coronary Microvascular Function in Women With no Obstructive CAD: The iPOWER Study. JACC Cardiovasc. Imaging 2016, 9, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Bøttcher, M.; Madsen, M.M.; Refsgaard, J.; Buus, N.H.; Dørup, I.; Nielsen, T.T.; Sørensen, K. Peripheral Flow Response to Transient Arterial Forearm Occlusion Does Not Reflect Myocardial Perfusion Reserve. Circulation 2001, 103, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Lind, L. Relationships between three different tests to evaluate endothelium-dependent vasodilation and cardiovascular risk in a middle-aged sample. J. Hypertens. 2013, 31, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Bass, A.; Ellis, K.; Tran, B.; Steele, S.; Caughey, M.; Stouffer, G.A.; Hinderliter, A.L. Relation Between Digital Peripheral Arterial Tonometry and Brachial Artery Ultrasound Measures of Vascular Function in Patients With Coronary Artery Disease and in Healthy Volunteers. Am. J. Cardiol. 2012, 109, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.-G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef] [PubMed]
| Parameters | N = 48 (%) | ||
|---|---|---|---|
| Sex | |||
| Male | 30 (63) | ||
| Female | 18 (37) | ||
| Age, years | 55 ± 1 | ||
| Interscan duration (years) # | 3.3 ± 0.1 | ||
| Smoking | |||
| Active | 9 (19) | ||
| Former | 21 (44) | ||
| Never | 18 (37) | ||
| Medication | |||
| Antihypertensive | 13 (27) | ||
| Statin | 9 (19) | ||
| Anticoagulant | 8 (17) | ||
| Perfusion defects on PET/CT | 3 (6) | ||
| FRS (CHD 10 years, %) | 11.2 ± 1.5 | ||
| ΔFRS (CHD 10 years, %) ¤ | 3.4 ± 0.9 | ||
| Lipids | |||
| Total cholesterol, mmol/L | 5.6 ± 0.2 | ||
| HDL, mmol/L | 1.4 ± 0.1 | ||
| LDL, mmol/L | 3.4 ± 0.1 | ||
| Triglycerids, mmol/L | 1.9 ± 0.3 | ||
| Systolic Blood Pressure, mmHg | 132 ± 3 | ||
| Diastolic Blood Pressure, mmHg | 78 ± 2 | ||
| BMI | 24.8 ± 0.7 | ||
| Diabetes mellitus | 3 (6) | ||
| Blood glucose, mmol/L | 6.0 ± 0.3 | ||
| Parameters | N = 48 |
|---|---|
| CD 4 cell count (106/L), median (IQR) | 672 (528–844) |
| HIV RNA (copies/mL), median (IQR) | 19 (19–19) |
| HIV duration, years, median (IQR) | 18.0 (12–24) |
| ART duration, years, median (IQR) | 16 (11–19) |
| ART regimens | |
| 2 NRTI + 1 NNRTI | 25 (52) |
| 2 NRTI + PI | 8 (17) |
| 2 NRTI + PI + IH | 5 (10) |
| Other | 10 (21) |
| Parameters | Value |
|---|---|
| Myocardial flow reserve, mean ± SEM | 2.4 ± 1.1 |
| Myocardial flow reserve tertiles | |
| <1.5 | 11% |
| ≥1.5 < 2.0 | 19% |
| ≥2.0 | 70% |
| CACS, median (range) | 0 (0–1884) |
| RHI, median (range) | 1.9 (1.0–4.4) |
| Low RHI (<1.67) | 33% |
| Normal RHI (≥1.67) | 67% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ørbæk, M.; Hasbak, P.; Sejersten Ripa, R.; Kjær, A.; Lebech, A.-M.; Knudsen, A. Comparison of the Peripheral Reactive Hyperemia Index with Myocardial Perfusion Reserve by 82Rb PET/CT in HIV-Infected Patients. Diagnostics 2017, 7, 31. https://doi.org/10.3390/diagnostics7020031
Ørbæk M, Hasbak P, Sejersten Ripa R, Kjær A, Lebech A-M, Knudsen A. Comparison of the Peripheral Reactive Hyperemia Index with Myocardial Perfusion Reserve by 82Rb PET/CT in HIV-Infected Patients. Diagnostics. 2017; 7(2):31. https://doi.org/10.3390/diagnostics7020031
Chicago/Turabian StyleØrbæk, Mathilde, Philip Hasbak, Rasmus Sejersten Ripa, Andreas Kjær, Anne-Mette Lebech, and Andreas Knudsen. 2017. "Comparison of the Peripheral Reactive Hyperemia Index with Myocardial Perfusion Reserve by 82Rb PET/CT in HIV-Infected Patients" Diagnostics 7, no. 2: 31. https://doi.org/10.3390/diagnostics7020031
APA StyleØrbæk, M., Hasbak, P., Sejersten Ripa, R., Kjær, A., Lebech, A.-M., & Knudsen, A. (2017). Comparison of the Peripheral Reactive Hyperemia Index with Myocardial Perfusion Reserve by 82Rb PET/CT in HIV-Infected Patients. Diagnostics, 7(2), 31. https://doi.org/10.3390/diagnostics7020031







