Functional Imaging with 18F-FDG PET/CT and Diffusion Weighted Imaging (DWI) in Early Response Evaluation of Combination Therapy of Elotuzumab, Lenalidomide, and Dexamethasone in a Relapsed Multiple Myeloma Patient
Abstract
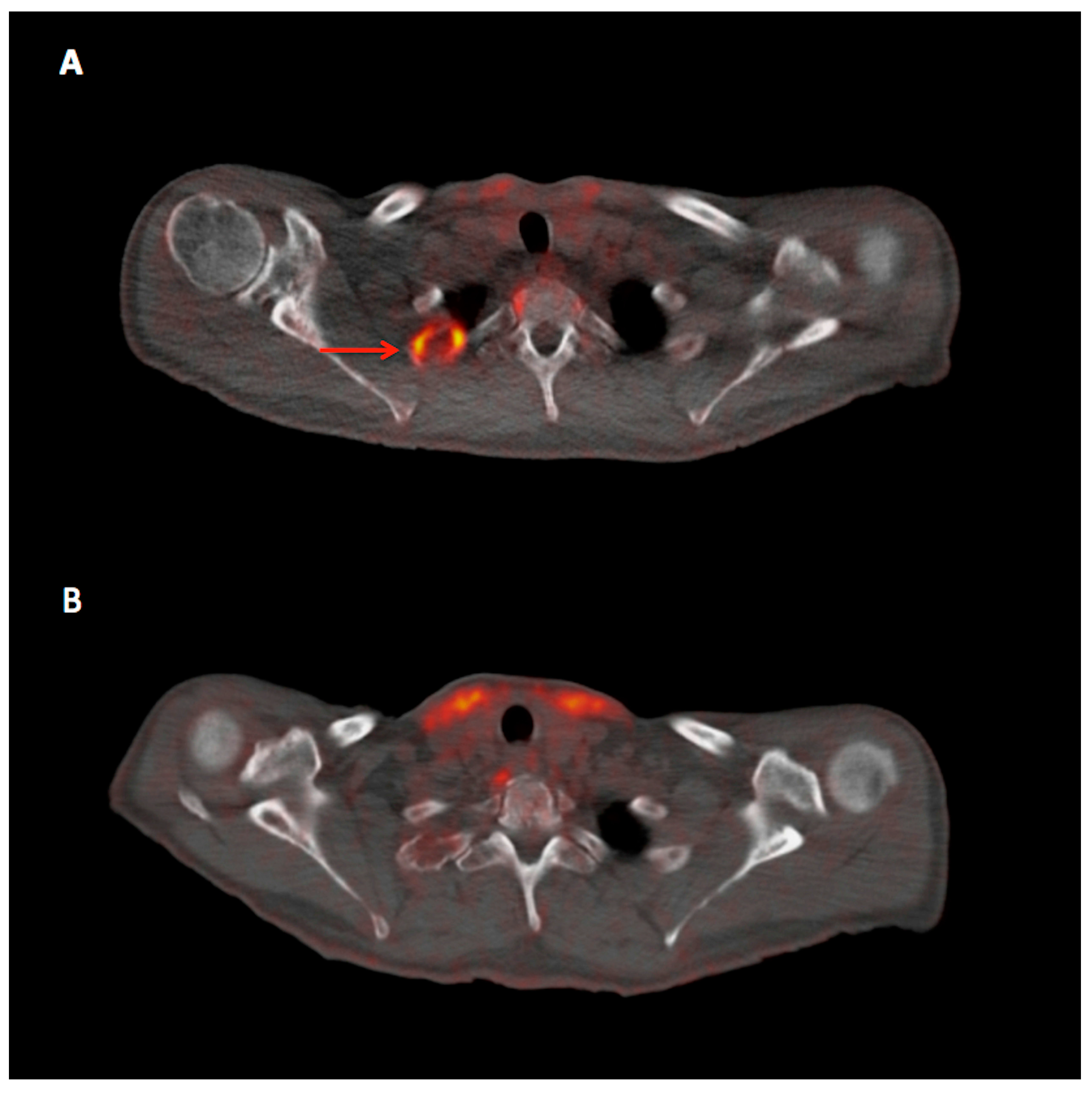
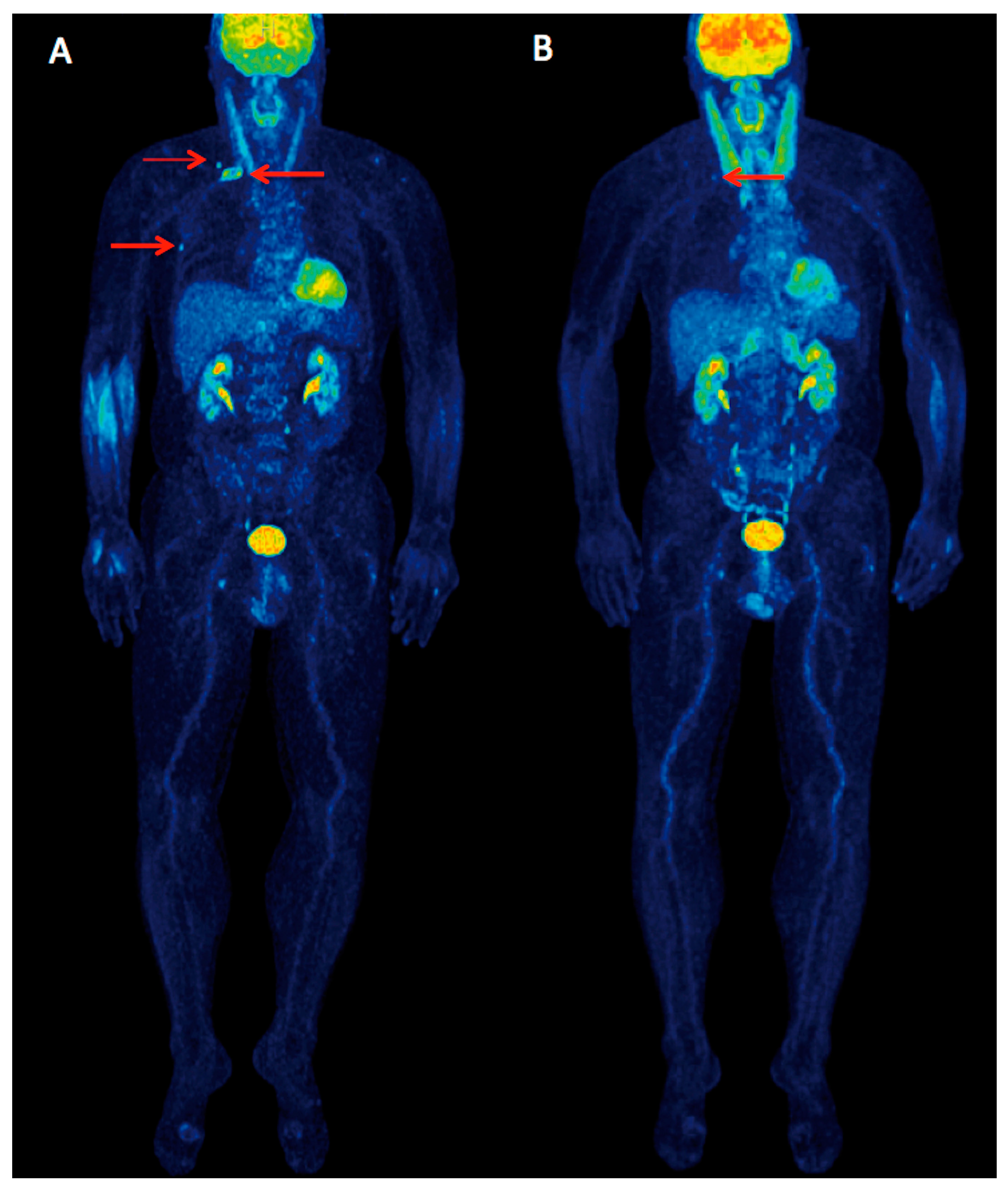
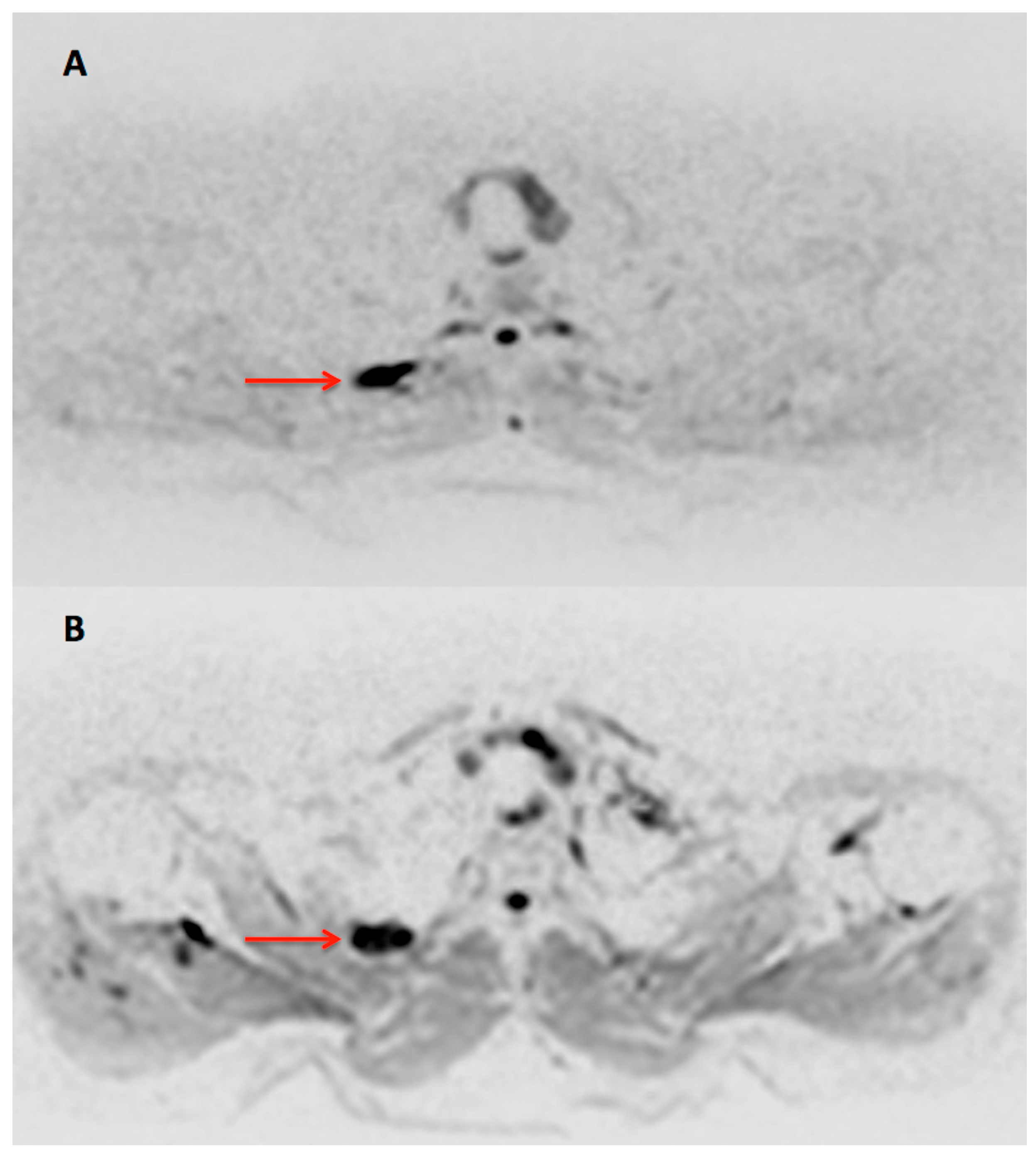
:
References
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Palumbo, A.; San-Miguel, J.; Shpilberg, O.; Anderson, K.; Grosicki, S.; Spicka, I.; et al. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br. J. Haematol. 2017, 178, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Mai, E.K.; Goldschmidt, H.; Hillengass, J.; Hose, D.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. (18)F-FDG dynamic PET/CT in patients with multiple myeloma: Patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin. Nucl. Med. 2015, 40, e300–e307. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou-Strauss, A.; Hoffmann, M.; Bergner, R.; Uppenkamp, M.; Haberkorn, U.; Strauss, L.G. Prediction of progression-free survival in patients with multiple myeloma following anthracycline-based chemotherapy based on dynamic FDG-PET. Clin. Nucl. Med. 2009, 34, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bartel, T.B.; Haessler, J.; Brown, T.L.; Shaughnessy, J.D., Jr.; van Rhee, F.; Anaissie, E.; Alpe, T.; Angtuaco, E.; Walker, R.; Epstein, J.; et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 2009, 114, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Tacchetti, P.; Terragna, C.; Cavo, M. Multiple myeloma: Disease response assessment. Expert Rev. Hematol. 2016, 9, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Giles, S.; Collins, D.J.; West, S.; Davies, F.E.; Morgan, G.J.; Desouza, N.M. Assessing response of myeloma bone disease with diffusion-weighted MRI. Br. J. Radiol. 2012, 85, e1198–e1203. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.L.; Messiou, C.; Collins, D.J.; Morgan, V.A.; Simpkin, C.J.; West, S.; Davies, F.E.; Morgan, G.J.; de Souza, N.M. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 2014, 271, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Lacognata, C.; Crimì, F.; Guolo, A.; Varin, C.; De March, E.; Vio, S.; Ponzoni, A.; Barilà, G.; Lico, A.; Branca, A.; et al. Diffusion-weighted whole-body MRI for evaluation of early response in multiple myeloma. Clin Radiol. 2017, 72, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Mosebach, J.; Freitag, M.T.; Wilhelm, T.; Mai, E.K.; Goldschmidt, H.; Haberkorn, U.; Schlemmer, H.P.; Delorme, S.; Dimitrakopoulou-Strauss, A. Application of (18)F-FDG PET and diffusion weighted imaging (DWI) in multiple myeloma: Comparison of functional imaging modalities. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 479–492. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachpekidis, C.; Dimitrakopoulou-Strauss, A.; Delorme, S.; Goldschmidt, H. Functional Imaging with 18F-FDG PET/CT and Diffusion Weighted Imaging (DWI) in Early Response Evaluation of Combination Therapy of Elotuzumab, Lenalidomide, and Dexamethasone in a Relapsed Multiple Myeloma Patient. Diagnostics 2017, 7, 61. https://doi.org/10.3390/diagnostics7040061
Sachpekidis C, Dimitrakopoulou-Strauss A, Delorme S, Goldschmidt H. Functional Imaging with 18F-FDG PET/CT and Diffusion Weighted Imaging (DWI) in Early Response Evaluation of Combination Therapy of Elotuzumab, Lenalidomide, and Dexamethasone in a Relapsed Multiple Myeloma Patient. Diagnostics. 2017; 7(4):61. https://doi.org/10.3390/diagnostics7040061
Chicago/Turabian StyleSachpekidis, Christos, Antonia Dimitrakopoulou-Strauss, Stefan Delorme, and Hartmut Goldschmidt. 2017. "Functional Imaging with 18F-FDG PET/CT and Diffusion Weighted Imaging (DWI) in Early Response Evaluation of Combination Therapy of Elotuzumab, Lenalidomide, and Dexamethasone in a Relapsed Multiple Myeloma Patient" Diagnostics 7, no. 4: 61. https://doi.org/10.3390/diagnostics7040061





