Weight Loss at First Month and Development of Tolerance as Possible Predictors of 30 mg Phentermine Efficacy at 6 Months
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Analysis
3. Results
3.1. Demographic Data
3.2. Efficacy of Phentermine Treatment
3.3. Effect of 30 mg Phentermine on Cardiometabbolic Parameters
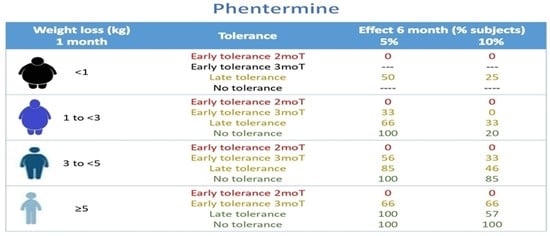
3.4. Impact of 1 mo-BWLkg, Development of Tolerance, and Obesity Class on the Efficacy of Phentermine
3.5. Three- and Six-Month Phentermine Efficacy Projections According to 1 mo-BWLkg, Development of Tolerance, and Obesity Class
3.6. Phentermine Efficacy in Class 3 Obese Subjects
3.7. Safety of 30 mg Phentermine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharm. Econ. 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Butryn, M.L.; Webb, V.; Wadden, T.A. Behavioral treatment of obesity. Psychiatr Clin. North Am. 2011, 34, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Sternberg, J.A.; Letizia, K.A.; Stunkard, A.J.; Foster, G.D. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: A five-year perspective. Int. J. Obes. 1989, 13 (Suppl. S2), 39–46. [Google Scholar]
- Dent, R.; McPherson, R.; Harper, M.E. Factors affecting weight loss variability in obesity. Metabolism 2020, 113, 154388. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E. Sibutramine—A review of clinical efficacy. Int. J. Obes. Relat. Metab. Disord. 1997, 21 (Suppl. S1), S30–S39. [Google Scholar]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pr. 2017, 29 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, A.; Apovian, C.M. Updates on obesity pharmacotherapy. Ann. N. Y. Acad. Sci. 2018, 1411, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Arbaizar, B.; Gómez-Acebo, I.; Llorca, J. Efficacy of topiramate in bulimia nervosa and binge-eating disorder: A systematic review. Gen. Hosp. Psychiatry 2008, 30, 471–475. [Google Scholar] [CrossRef]
- Yip, S.W.; Potenza, M.N. Treatment of Gambling Disorders. Curr. Treat. Options Psychiatry 2014, 1, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Apolzan, J.W.; Berthoud, H.R. Pharmacotherapy for Patients with Obesity. Clin. Chem. 2018, 64, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhurandhar, N.V.; Blank, R.C.; Schumacher, D.; Atkinson, R.L. Initial weight loss as a predictor of response to obesity drugs. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 1333–1336. [Google Scholar] [CrossRef] [Green Version]
- Maccora, C.; Ciuoli, C.; Goracci, A.; Benenati, N.; Formichi, C.; Pilli, T.; Verdino, V.; Mnutr, O.N.; Bufano, A.; Tirone, A.; et al. One month weight loss predicts the efficacy of liraglutide in obese patients: Data from a single center. Endocr. Pract. 2020, 26, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Baumann, M.H.; Dersch, C.M.; Romero, D.V.; Rice, K.C.; Carroll, F.I.; Partilla, J.S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001, 39, 32–41. [Google Scholar] [CrossRef]
- Apovian, C.M.; Istfan, N.W. Obesity: Guidelines, Best Practices, New Research. Endocrinol. Metab. Clin. North Am. 2016, 45, 17–18. [Google Scholar] [CrossRef]
- Saxon, D.R.; Iwamoto, S.J.; Mettenbrink, C.J.; McCormick, E.; Arterburn, D.; Daley, M.F.; Oshiro, C.E.; Koebnick, C.; Horberg, M.; Young, D.R.; et al. Antiobesity Medication Use in 2.2 Million Adults Across Eight Large Health Care Organizations: 2009–2015. Obesity 2019, 27, 1975–1981. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y.; Kang, J.H.; Park, Y.W.; Park, S.W. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes. Metab. 2010, 12, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tak, Y.J.; Lee, S.Y. Anti-Obesity Drugs: Long-Term Efficacy and Safety: An Updated Review. World J. Mens. Heal. 2021, 39, 208–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K.H.; Fischer, H.; Ard, J.; Barton, L.; Bessesen, D.H.; Daley, M.F.; Desai, J.; Fitzpatrick, S.L.; Horberg, M.; Koebnick, C.; et al. Safety and Effectiveness of Longer-Term Phentermine Use: Clinical Outcomes from an Electronic Health Record Cohort. Obesity 2019, 27, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, E.J.; Greenway, F.L.; Westman, E.C.; Gupta, A.K. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity 2011, 19, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Cruz, M.; Kammar-García, A.; Huerta-Cruz, J.C.; Carrasco-Portugal, M.; Barranco-Garduño, L.M.; Rodríguez-Silverio, J.; Rocha González, H.I.; Reyes-García, J.G. Three- and six-month efficacy and safety of phentermine in a Mexican obese population. Int. J. Clin. Pharm. 2021, 59, 539–548. [Google Scholar] [CrossRef]
- SuprenzaTM (Phentermine Hydrochloride) Orally Disintegrating Tablet. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202088s005lbl.pdf (accessed on 9 August 2021).
- Fernstrom, J.D.; Choi, S. The development of tolerance to drugs that suppress food intake. Pharm. Ther. 2008, 117, 105–122. [Google Scholar] [CrossRef]
- Del Valle-Laisequilla, C.F.; Trejo-Jasso, C.; Huerta-Cruz, J.C.; Barranco-Garduño, L.M.; Rodríguez-Silverio, J.; Rocha-González, H.I.; Reyes-García, J.G. Efficacy and safety of a fixed-dose combination of D-norpseudoephedrine, triiodothyronine, atropine, aloin, and diazepam in obese patients. Int. J. Clin. Pharm. 2018, 56, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2013, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Available online: www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 9 August 2021).
- Hainer, V.; Toplak, H.; Mitrakou, A. Treatment modalities of obesity: What fits whom? Diabetes Care 2008, 31 (Suppl. 2), S269–S277. [Google Scholar] [CrossRef] [Green Version]
- Toplak, H.; Woodward, E.; Yumuk, V.; Oppert, J.M.; Halford, J.C.; Frühbeck, G. 2014 EASO Position Statement on the Use of Anti-Obesity Drugs. Obes. Facts 2015, 8, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; Finer, N. New medications for treatment of obesity: Metabolic and cardiovascular effects. Can. J. Cardiol. 2015, 31, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Lee, J.A.; Suh, H.W.; Kim, Y.S.; Kim, B.S.; Ahn, E.S.; Roh, Y.J.; Jung, S.G.; Kim, J.M.; Kang, M.K.; et al. Postmarketing surveillance study of the efficacy and safety of phentermine in patients with obesity. Korean J. Fam. Med. 2013, 34, 298–306. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Total Sample (n = 166) | Reference Value |
|---|---|---|
| Women, n (%) | 144 (86.7) | - |
| Age, years (SD) | 37.5 (9.7) | - |
| Weight, kg (SD) | 86.2 (13.9) | - |
| Body Mass Index, kg/m2 (SD) | 33.9 (3.7) | - |
| Class 1 obesity, n (%) | 78 (47.1) | 30–34.9 kg/m2 |
| Class 2 obesity, n (%) | 70 (42.) | 35–39.9 kg/m2 |
| Class 3 obesity, n (%) | 18 (10.8) | ≥ 40 kg/m2 |
| SBP, mmHg (SD) | 110.1 (11.0) | <120 mmHg |
| DBP, mmHg (SD) | 76.7 (7.6) | <80 mmHg |
| Fasting glycemia, mg/dL (SD) | 104.4 (25.4) | 75–<99 mg/dL |
| Triglycerides, mg/dL (SD) | 182.9 (113.0) | <150 mg/dL |
| Total Cholesterol, mg/dL (SD) | 187 (38.4) | <200 mg/dL |
| LDL-Cholesterol, mg/dL (SD) | 108.9 (34.8) | <130 mg/dL |
| HDL-Cholesterol, mg/dL (SD) | 42.3 (9.5) | ≥40 mg/dL |
| Parameter | Baseline | 3 Months | 6 Months |
|---|---|---|---|
| Waist circumference, cm (SD) | 110.6 ± 10.2 | 101.1 ± 10.0 * | 97.8 ± 10.5 * |
| SBP, mmHg (SD) | 109.9 ± 10.8 | 108.1 ± 8.9 | 107.3 ± 9.1 |
| DPB, mmHg (SD) | 77.0 ± 6.9 | 75.0 ± 7.7 | 74.6 ± 8.5 * |
| Fasting glycemia, mg/dL (SD) | 104.7 ± 25.5 | 95.9 ± 16.7 * | 91.5 ± 14.3 * |
| Triglycerides, mg/dL (SD) | 184.9 ± 114.2 | 136.7 ± 123.5 * | 137.8 ± 74.7 * |
| Total Cholesterol, mg/dL (SD) | 188.0 ± 37.7 | 176.2 ± 38.7 * | 173.3 ± 35.5 * |
| LDL-Cholesterol, mg/dL (SD) | 108.7 ± 34.7 | 105.3 ± 30.3 | 99.9 ± 28.7 |
| HDL-Cholesterol, mg/dL (SD) | 42.4 ± 9.4 | 43.7 ± 9.7 | 45.8 ± 8.9 * |
| Adverse Event | Total (%) | Mild (%) | Moderate (%) |
|---|---|---|---|
| Dry mouth | 128 (15.9) | 128 (15.9) | - |
| Headache | 89 (11.1) | 56 (7.0) | 33 (4.1) |
| Hyperhidrosis | 58 (7.2) | 58 (7.2) | - |
| Dysgeusia | 56 (7.0) | 56 (7.0) | - |
| Constipation | 47 (5.9) | 41 (5.1) | 6 (0.7) |
| Nervousness | 41 (5.1) | 41 (5.1) | - |
| Insomnia | 36 (4.5) | 36 (4.5) | - |
| Drowsiness | 36 (4.5) | 36 (4.5) | - |
| Thirst | 35 (4.4) | 35 (4.4) | - |
| Nausea | 34 (4.2) | 33 (4.1) | 1 (0.1) |
| Anxiety | 26 (3.2) | 26 (3.2) | - |
| Fatigue | 24 (3.0) | 24 (3.0) | - |
| Irregular bowel sounds | 15 (1.9) | 14 (1.7) | 1 (0.1) |
| Diarrhea | 14 (1.7) | 11 (1.4) | 3 (0.4) |
| Abdominal pain | 13 (1.6) | 9 (1.1) | 4 (0.5) |
| Others | 151 (18.8) | 142 (17.7) | 9 (1.1) |
| Total | 803 (100%) | 746 (92.9%) | 57 (7.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-González, H.I.; De la Cruz-Álvarez, L.E.; Kammar-García, A.; Canizales-Quinteros, S.; Huerta-Cruz, J.C.; Barranco-Garduño, L.M.; Reyes-García, J.G. Weight Loss at First Month and Development of Tolerance as Possible Predictors of 30 mg Phentermine Efficacy at 6 Months. J. Pers. Med. 2021, 11, 1354. https://doi.org/10.3390/jpm11121354
Rocha-González HI, De la Cruz-Álvarez LE, Kammar-García A, Canizales-Quinteros S, Huerta-Cruz JC, Barranco-Garduño LM, Reyes-García JG. Weight Loss at First Month and Development of Tolerance as Possible Predictors of 30 mg Phentermine Efficacy at 6 Months. Journal of Personalized Medicine. 2021; 11(12):1354. https://doi.org/10.3390/jpm11121354
Chicago/Turabian StyleRocha-González, Héctor Isaac, Lidia Elizabeth De la Cruz-Álvarez, Ashuin Kammar-García, Samuel Canizales-Quinteros, Juan Carlos Huerta-Cruz, Lina Marcela Barranco-Garduño, and Juan Gerardo Reyes-García. 2021. "Weight Loss at First Month and Development of Tolerance as Possible Predictors of 30 mg Phentermine Efficacy at 6 Months" Journal of Personalized Medicine 11, no. 12: 1354. https://doi.org/10.3390/jpm11121354