Effect of Anterioposterior Weight-Shift Training with Visual Biofeedback in Patients with Step Length Asymmetry after Subacute Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
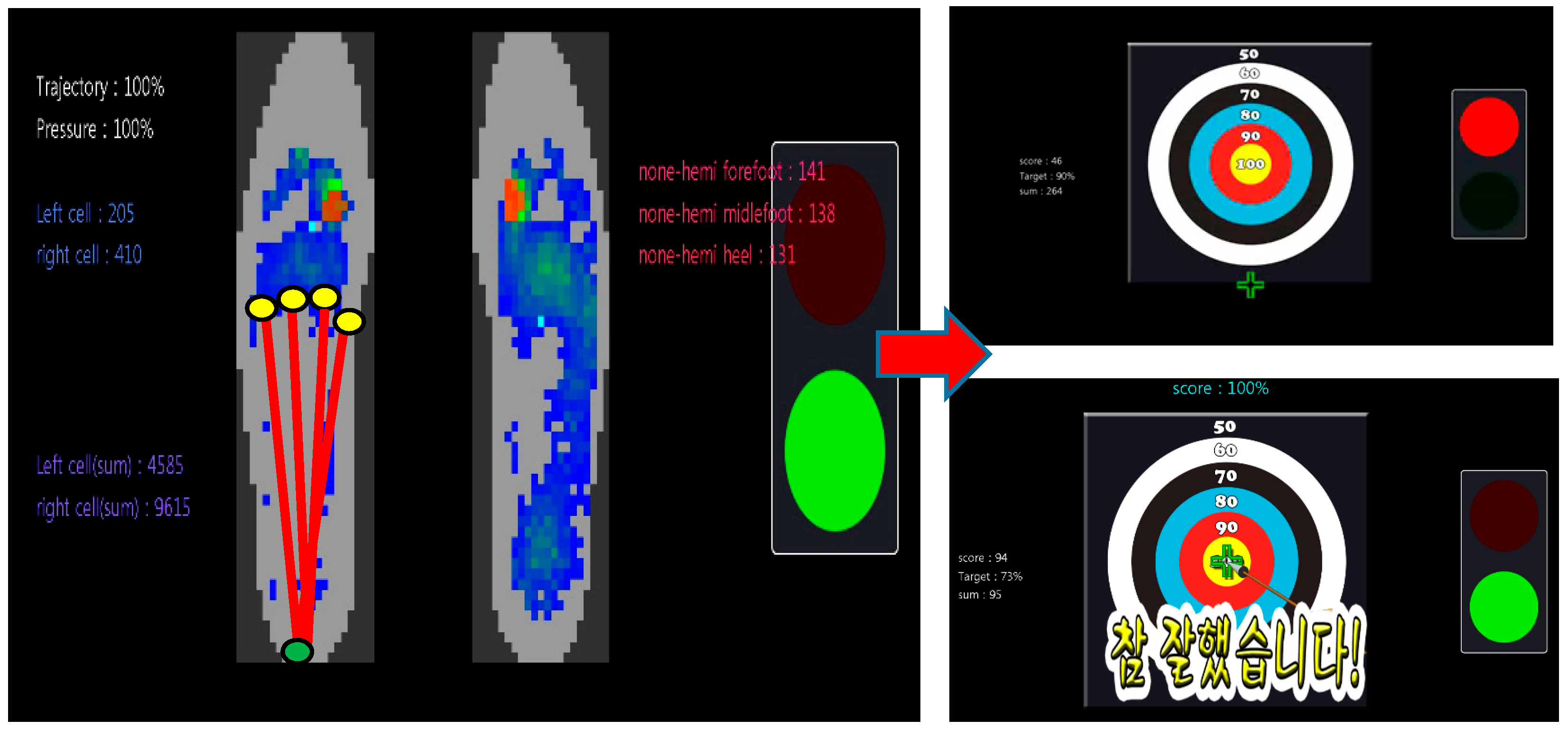
2.3. Anterioposterior Weight-Shift Training Using Visual Feedback
2.4. Outcome Measurements
2.4.1. Primary Outcomes
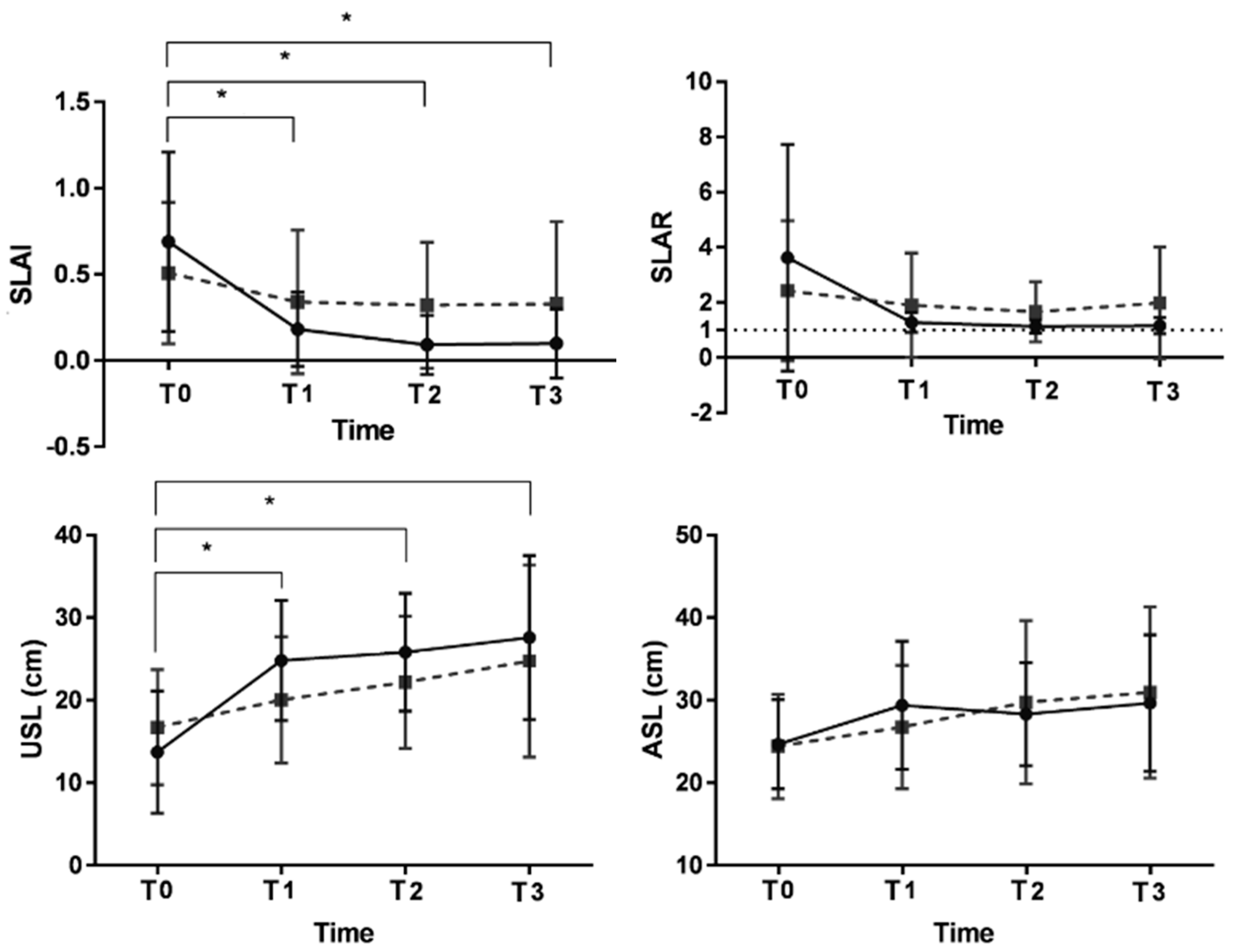
Gait Asymmetry
2.4.2. Secondary Outcomes
Plantar Pressure Analysis
Behavioral Parameters
Energy Consumption and 3D Motion Analysis
2.5. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
3.2.1. Plantar Pressure Analysis
3.2.2. Behavioral Parameters
3.2.3. Energy Consumption and 3D Motion Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP training | Anterioposterior weight-shift training with visual biofeedback |
| ASL | Affected side-step length |
| BBS | Berg balance scale |
| CoP | Center of pressure |
| FAC | Functional Ambulation Category |
| FIM-mobility | Functional Independent Measure-mobility |
| FMA | Fugl-Meyer assessment |
| K-MMSE | Korean Mini Mental State Examination |
| ML | Mediolateral |
| MRC | Medical Research Council scale |
| MSWS | Maximum safe walking speed |
| NOB | Number of back movement |
| RMANOVA | Repeated-measure analysis of variance |
| SLAI | Step length asymmetric index |
| SLAR | Step length asymmetric ratio |
| SSWS | Self-selected walking speed |
| TUG | Timed up and go test |
| USL | Unaffected side-step length |
References
- Balasubramanian, C.K.; Bowden, M.G.; Neptune, R.R.; Kautz, S.A. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 2007, 88, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Bradley, C.E.; Wutzke, C.J.; Zinder, S.M. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J. Appl. Biomech. 2014, 30, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Joo, M.C. Change in bone mineral density in stroke patients with osteoporosis or osteopenia. Int. J. Environ. Res. Public Health 2022, 19, 8954. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lauziere, S.; Betschart, M.; Aissaoui, R.; Nadeau, S. Understanding spatial and temporal gait asymmetries in individuals post stroke. Int. J. Phys. Med. Rehabil. 2014, 2, 201. [Google Scholar]
- Padmanabhan, P.; Rao, K.S.; Gulhar, S.; Cherry-Allen, K.M.; Leech, K.A.; Roemmich, R.T. Persons post-stroke improve step length symmetry by walking asymmetrically. J. Neuroeng. Rehabil. 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture 2011, 33, 538–543. [Google Scholar] [CrossRef]
- Roerdink, M.; Beek, P.J. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: Impairments and compensatory gait. Neurorehabilit. Neural Repair 2011, 25, 253–258. [Google Scholar] [CrossRef]
- Szopa, A.; Domagalska-Szopa, M.; Lasek-Bal, A.; Żak, A. The link between weight shift asymmetry and gait disturbances in chronic hemiparetic stroke patients. Clin. Interv. Aging 2017, 12, 2055. [Google Scholar] [CrossRef]
- Bishop, L.; Khan, M.; Martelli, D.; Quinn, L.; Stein, J.; Agrawal, S. Exploration of two training paradigms using forced induced weight shifting with the tethered pelvic assist device to reduce asymmetry in individuals after stroke: Case reports. Am. J. Phys. Med. Rehabil. 2017, 96, S135–S140. [Google Scholar] [CrossRef]
- Tsaklis, P.V.; Grooten, W.J.; Franzen, E. Effects of weight-shift training on balance control and weight distribution in chronic stroke: A pilot study. Top. Stroke Rehabil. 2012, 19, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.Y.; Kim, J.H.; Choi, J.H.; Joo, S.Y.; Kang, N.K.; Baek, Y.S. Development of weight shifting training system using biofeedback for post-stroke hemiplegic patients with step length asymmetry. J. Korean Soc. Precis. Eng. 2013, 30, 450–458. [Google Scholar] [CrossRef]
- Branco, J.P.; Oliveira, S.; Sargento-Freitas, J.; Laíns, J.; Pinheiro, J. Assessing functional recovery in the first six months after acute ischemic stroke: A prospective, observational study. Eur. J. Phys. Rehabil. Med. 2019, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abdul Razak, A.H.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot plantar pressure measurement system: A review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Lim, S.H. Assessment of lower limb motor function, ambulation, and balance after stroke. Brain Neurorehabil. 2022, 15, e17. [Google Scholar] [CrossRef] [PubMed]
- Beaman, C.B.; Peterson, C.L.; Neptune, R.R.; Kautz, S.A. Differences in self-selected and fastest-comfortable walking in post-stroke hemiparetic persons. Gait Posture 2010, 31, 311–316. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.-G.; Sheng, J.; Ma, S.-J. Virtual reality for improving balance in patients after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2015, 30, 432–440. [Google Scholar] [CrossRef]
- Danielsson, A.; Willén, C.; Sunnerhagen, K.S. Measurement of energy cost by the physiological cost index in walking after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1298–1303. [Google Scholar] [CrossRef]
- Hendrickson, J.; Patterson, K.K.; Inness, E.L.; McIlroy, W.E.; Mansfield, A. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Posture 2014, 39, 177–181. [Google Scholar] [CrossRef]
- Chow, J.W.; Stokic, D.S. Longitudinal changes in temporospatial gait characteristics during the first year post-stroke. Brain Sci. 2021, 11, 1648. [Google Scholar] [CrossRef]
- Selves, C.; Stoquart, G.; Lejeune, T. Gait rehabilitation after stroke: Review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol. Belg. 2020, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Son, S.M.; Kim, K. Changes of gait parameters following constrained-weight shift training in patients with stroke. J. Phys. Ther. Sci. 2017, 29, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.O.; Barela, J.A.; Prado-Medeiros, C.L.; Salvini, T.F.; Barela, A.M. Gait training with partial body weight support during overground walking for individuals with chronic stroke: A pilot study. J. Neuroeng. Rehabil. 2011, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Azarpazhooh, M.R.; Hosseini, H.A. Randomized comparison trial of gait training with and without compelled weight-shift therapy in individuals with chronic stroke. Clin. Rehabil. 2016, 30, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Rodà, A.; Avanzini, F.; Masiero, S. On the role of auditory feedback in robot-assisted movement training after stroke: Review of the literature. Comput. Intell. Neurosci. 2013, 2013, 586138. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Lee, W.H. Virtual walking training program using a real-world video recording for patients with chronic stroke: A pilot study. Am. J. Phys. Med. Rehabil. 2013, 92, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Thikey, H.; Grealy, M.; van Wijck, F.; Barber, M.; Rowe, P. Augmented visual feedback of movement performance to enhance walking recovery after stroke: Study protocol for a pilot randomised controlled trial. Trials 2012, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.S.; McLean, H.; Keller, J.; Danks, K.A.; Bastian, A.J. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabilit. Neural Repair 2013, 27, 460–468. [Google Scholar] [CrossRef]
- Wutzke, C.J.; Faldowski, R.A.; Lewek, M.D. Individuals poststroke do not perceive their spatiotemporal gait asymmetries as abnormal. Phys. Ther. 2015, 95, 1244–1253. [Google Scholar] [CrossRef]
- Sánchez, N.; Finley, J.M. Individual differences in locomotor function predict the capacity to reduce asymmetry and modify the energetic cost of walking poststroke. Neurorehabilit. Neural Repair 2018, 32, 701–713. [Google Scholar] [CrossRef]
- De Nunzio, A.; Zucchella, C.; Spicciato, F.; Tortola, P.; Vecchione, C.; Pierelli, F.; Bartolo, M. Biofeedback rehabilitation of posture and weightbearing distribution in stroke: A center of foot pressure analysis. Funct. Neurol. 2014, 29, 127–134. [Google Scholar] [PubMed]
- Pignolo, L.; Basta, G.; Carozzo, S.; Bilotta, M.; Todaro, M.R.; Serra, S.; Ciancarelli, I.; Tonin, P.; Cerasa, A. A body-weight-supported visual feedback system for gait recovering in stroke patients: A randomized controlled study. Gait Posture 2020, 82, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Van Peppen, R.P.; Kortsmit, M.; Lindeman, E.; Kwakkel, G. Effects of visual feedback therapy on postural control in bilateral standing after stroke: A systematic review. J. Rehabil. Med. 2006, 38, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sungkarat, S.; Fisher, B.E.; Kovindha, A. Efficacy of an insole shoe wedge and augmented pressure sensor for gait training in individuals with stroke: A randomized controlled trial. Clin. Rehabil. 2011, 25, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Pak, N.W.; Lee, J.H. Effects of visual feedback training and visual targets on muscle activation, balancing, and walking ability in adults after hemiplegic stroke: A preliminary, randomized, controlled study. Int. J. Rehabil. Res. 2020, 43, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.E.; Reisman, D.S. The split-belt walking paradigm: Exploring motor learning and spatiotemporal asymmetry poststroke. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.S.; Wityk, R.; Silver, K.; Bastian, A.J. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 2007, 130, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Bovonsunthonchai, S.; Hiengkaew, V.; Vachalathiti, R.; Vongsirinavarat, M. Gait symmetrical indexes and their relationships to muscle tone, lower extremity function, and postural balance in mild to moderate stroke. J. Med. Assoc. Thai 2011, 94, 476–484. [Google Scholar]
- Meidian, A.C.; Yige, S.; Irfan, M.; Rahayu, U.B.; Amimoto, K. Immediate effect of adding mirror visual feedback to lateral weight-shifting training on the standing balance control of the unilateral spatial neglect model. J. Phys. Ther. Sci. 2021, 33, 809–817. [Google Scholar] [CrossRef]
- Yoshioka, K.; Watanabe, T.; Maruyama, N.; Yoshioka, M.; Iino, K.; Honda, K.; Hayashida, K. Two-month individually supervised exercise therapy improves walking speed, step length, and temporal gait symmetry in chronic stroke patients: A before–after trial. Healthcare 2022, 10, 527. [Google Scholar]
- Hsu, A.-L.; Tang, P.-F.; Jan, M.-H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Lee, S.; Packel, A.T.; Braitman, L. A randomized comparative study of manually assisted versus robotic-assisted body weight supported treadmill training in persons with a traumatic brain injury. PM R 2013, 5, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Alingh, J.F.; Fleerkotte, B.M.; Groen, B.E.; Rietman, J.S.; Weerdesteyn, V.; van Asseldonk, E.H.F.; Geurts, A.C.H.; Buurke, J.H. Effect of assist-as-needed robotic gait training on the gait pattern post stroke: A randomized controlled trial. J. NeuroEng. Rehabil. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Yang, H.S.; Jung, S.; Kang, C.S.; Jang, S.; Kim, D.H. Effect of reducing assistance during robot-assisted gait training on step length asymmetry in patients with hemiplegic stroke: A randomized controlled pilot trial. Medicine 2018, 97, e11792. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Lungholt, B.K.; Nyborg, K.; Nielsen, J.B. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp. Brain Res. 2004, 159, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.J.; Liu, H.; Komiyama, T. Learning in the rodent motor cortex. Annu. Rev. Neurosci. 2017, 40, 77–97. [Google Scholar] [CrossRef]
- Nikamp, C.D.M.; Hobbelink, M.S.H.; van der Palen, J.; Hermens, H.J.; Rietman, J.S.; Buurke, J.H. A randomized controlled trial on providing ankle-foot orthoses in patients with (sub-)acute stroke: Short-term kinematic and spatiotemporal effects and effects of timing. Gait Posture 2017, 55, 15–22. [Google Scholar] [CrossRef]
- Stanton, R.; Ada, L.; Dean, C.M.; Preston, E. Biofeedback improves activities of the lower limb after stroke: A systematic review. J. Physiother. 2011, 57, 145–155. [Google Scholar] [CrossRef]
- Spencer, J.; Wolf, S.L.; Kesar, T.M. Biofeedback for post-stroke gait retraining: A review of current evidence and future research directions in the context of emerging technologies. Front. Neurol. 2021, 12, 637199. [Google Scholar] [CrossRef]
- Ma, C.Z.-H.; Zheng, Y.-P.; Lee, W.C.-C. Changes in gait and plantar foot loading upon using vibrotactile wearable biofeedback system in patients with stroke. Top. Stroke Rehabil. 2018, 25, 20–27. [Google Scholar] [CrossRef]


| Characteristic | AP Training Group (n = 19) | Control Group (n = 21) | p Value |
|---|---|---|---|
| Age (years) | 57.7 ± 17.7 | 52.0 ± 14.0 | 0.267 |
| Sex (male) | 10 (52.6) | 14 (66.7) | 0.366 |
| Lesion side of stroke (left) | 9 (47.4) | 11 (52.4) | 0.752 |
| Type of stroke | 0.141 | ||
| Ischemic | 15 (78.9) | 12 (57.1) | |
| Hemorrhagic | 4 (21.1) | 9 (42.9) | |
| FAC | 0.587 | ||
| 3 | 13 (68.4) | 14 (66.7) | |
| 4 | 6 (31.5) | 7 (33.3) | |
| Duration from onset | 97.0 ± 59.8 | 81.1 ± 54.2 | 0.383 |
| FMA | 39.9 ± 17.0 | 43.6 ± 22.1 | 0.558 |
| FIM_mobility | 18.4 ± 2.7 | 17.1 ± 4.1 | 0.255 |
| BBS | 25.4 ± 7.3 | 24.6 ± 7.9 | 0.756 |
| MSWS (m/s) | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.408 |
| SSWS (m/s) | 0.2 ± 0.1 | 0.2± 0.1 | 0.869 |
| TUG (s) | 46.7 ± 24.6 | 56.1 ± 64.1 | 0.555 |
| SLAR | 3.6 ± 4.1 | 2.5 ± 2.5 | 0.301 |
| SLAI | 0.7 ± 0.5 | 0.5 ± 0.4 | 0.285 |
| Before Training | During Training | After Training | Follow Up | |
|---|---|---|---|---|
| Primary outcome | ||||
| Gait asymmetry | V | V | V | V |
| Secondary outcomes | ||||
| Plantar pressure analysis | V | V | V | V |
| Behavior parameters | V | V | V | V |
| 3D motion analysis | V | V | ||
| Energy consumption | V | V |
| AP Training Group | Control Group | ||||
|---|---|---|---|---|---|
| Asymmetric Index | Before Training | After Training | Before Training | After Training | p Value |
| Stance time (s) | −0.10 ± 0.08 | −0.14 ± 0.11 | −0.15 ± 0.09 | −0.16 ± 0.09 | 0.666 |
| Swing time (s) | 0.60 ± 0.34 | 0.50 ± 0.29 | 0.61 ± 0.30 | 0.49 ± 0.24 | 0.679 |
| Double support time (s) | 0.01 ± 0.04 | 0.01 ± 0.06 | 0.01 ± 0.06 | 0.00 ± 0.07 | 0.666 |
| SW/ST | 0.69 ± 0.37 | 0.61 ± 0.32 | 0.74 ± 0.30 | 0.63 ± 0.26 | 0.634 |
| AP Training Group | Control Group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Training | During Training | After Training | Follow Up | Before Training | During Training | After Training | Follow Up | ||
| Affected side | |||||||||
| Contact area (mm2) | |||||||||
| Forefoot | 27.1 ± 30.6 | 39.2 ± 24.5 | 57.5 ± 34.9 * | 56.0 ± 42.1 * | 26.3 ± 21.0 | 20.7 ± 21.8 | 32.7 ± 26.5 | 40.8 ± 28.7 | 0.029 |
| Midfoot | 92.7 ± 49.0 | 120.6 ± 4 * | 149.7 ± 54.2 * | 150.7 ± 66.0 * | 106.4 ± 64.0 | 99.2 ± 53.0 | 116.0 ± 52.5 | 117.0 ± 58.0 | 0.004 |
| Hindfoot | 119.6 ± 40.5 | 131.0 ± 50.7 | 125.2 ± 51.4 | 129.3 ± 43.4 | 103.0 ± 33.1 | 97.1 ± 38.1 | 108.8 ± 34.1 | 97.4 ± 40.5 | 0.189 |
| Total | 239.5 ± 93.5 | 290.8 ± 89.6 * | 331.9 ± 111.9 * | 337.4 ± 132.4 * | 235.5 ± 90.1 | 216.9 ± 89.4 | 257.5 ± 82.5 | 255.2 ± 91.1 | 0.012 |
| Contact pressure (kPa) | |||||||||
| Forefoot | 26.4 ± 34.6 | 51.7 ± 46.9 * | 80.0 ± 64.6 * | 80.7 ± 63.3 * | 25.3 ± 18.05 | 22.0 ± 19.1 | 32.9 ± 25.0 | 48.4 ± 47.3 | 0.006 |
| Midfoot | 96.4 ± 54.9 | 140.8 ± 73.4 | 173.4 ± 76.4 * | 202.9 ± 107.3 * | 135.9 ± 96.7 | 143.5 ± 84.3 | 159.4 ± 89.8 | 152.7 ± 94.6 | 0.006 |
| Hindfoot | 185.2 ± 80.2 | 213.2 ± 88.7 | 190.5 ± 67.0 | 211.6 ± 114.2 | 181.1 ± 97.6 | 174.5 ± 101.8 | 180.2 ± 88.6 | 173.4 ± 117.7 | 0.523 |
| Total | 308.1 ± 92.7 | 400.4 ± 84.8 * | 420.0 ± 121.3 | 487.4 ± 214.0 * | 342.4 ± 82.3 | 339.5 ± 86.3 | 372.1 ± 90.1 | 374.3 ± 159.9 | 0.033 |
| Peak | 418.7 ± 134.4 | 477.8 ± 118.6 | 489.5 ± 161.8 | 566.1 ± 256.3 | 430.7 ± 99.0 | 417.5 ± 94.7 | 473.7 ± 152.2 | 479.6 ± 163.4 | 0.306 |
| Trajectory (mm) | |||||||||
| AP | 43.3 ± 65.9 | 72.6 ± 56.0 * | 78.6 ± 56.9 * | 91.1 ± 57.3 * | 70.6 ± 50.1 | 64.4 ± 51.5 | 68.8 ± 63.1 | 69.0 ± 61.1 | 0.001 |
| ML | 6.3 ± 5.0 | 9.2 ± 7.8 | 10.2 ± 8.0 * | 10.8 ± 8.1 * | 10.9 ± 6.5 | 9.7 ± 7.3 | 8.3 ± 4.9 | 8.7 ± 7.1 | 0.018 |
| NOB | 1.4 ± 0.6 | 1.6 ± 0.8 | 1.5 ± 0.7 | 1.6 ± 0.9 | 1.3 ± 0.7 | 1.7 ± 0.7 | 1.3 ± 0.5 | 1.3 ± 0.5 | 0.553 |
| Unaffected side | |||||||||
| Contact area (mm2) | |||||||||
| Forefoot | 69.7 ± 36.9 | 89.9 ± 43.3 | 84.9 ± 35.3 | 84.0 ± 34.31 | 82.4 ± 46.0 | 76.7 ± 38.3 | 73.9 ± 38.1 | 78.5 ± 32.1 | 0.036 |
| Midfoot | 146.4 ± 56.3 | 164.9 ± 55.2 | 162.1 ± 57.7 | 158.7 ± 68.4 | 177.0 ± 49.9 | 174.0 ± 51.3 | 161.0 ± 50.0 | 168.3 ± 55.0 | 0.177 |
| Hindfoot | 126.5 ± 31.1 | 138.5 ± 33.7 | 135.1 ± 18.6 | 132.7 ± 29.2 | 139.5 ± 26.7 | 139.2 ± 26.7 | 137.0 ± 20.8 | 127.1 ± 29.2 | 0.269 |
| Total | 342.4 ± 106.5 | 393.3 ± 119.9 | 382.0 ± 88.4 | 375.3 ± 115.5 | 398.9 ± 104.0 | 389.9 ± 99.7 | 372.0 ± 83.1 | 374.1 ± 103.1 | 0.071 |
| Contact pressure (kPa) | |||||||||
| Forefoot | 113.5 ± 70.9 | 124.8 ± 56.8 | 155.4 ± 97.6 | 148.3 ± 86.7 | 120.6 ± 73.7 | 104.6 ± 66.8 | 122.7 ± 91.2 | 127.0 ± 64.2 | 0.267 |
| Midfoot | 180.0 ± 93.2 | 194.2 ± 93.4 | 225.1 ± 103.1 | 217.1 ± 95.7 | 194.6 ± 58.3 | 205.3 ± 101.7 | 200.4 ± 94.3 | 207.6 ± 84.3 | 0.411 |
| Hindfoot | 239.2 ± 105.3 | 229.9 ± 63.5 | 237.0 ± 49.9 | 254.7 ± 146.7 | 247.4 ± 69.4 | 231.3 ± 78.6 | 272.2 ± 72.7 | 223.4 ± 95.4 | 0.272 |
| Total | 532.8 ± 213.3 | 549.0 ± 178.2 | 608.7 ± 176.8 | 620.2 ± 222.7 | 562.6 ± 143.5 | 540.9 ± 185.7 | 595.3 ± 174.0 | 558.2 ± 179.4 | 0.528 |
| Peak | 701.4 ± 275.8 | 667.5 ± 228.5 | 758.7 ± 270.1 | 753.2 ± 268.2 | 716.0 ± 210.9 | 678.5 ± 243.2 | 734.9 ± 216.8 | 710.4 ± 233.9 | 0.866 |
| Trajectory (mm) | |||||||||
| AP | 140.0 ± 29.9 | 145.2 ± 42.7 | 155.0 ± 26.4 | 154.5 ± 23.1 | 159.7 ± 24.0 | 157.5 ± 26.8 | 141.4 ± 39.3 | 153.0 ± 33.2 | 0.053 |
| ML | 7.3 ± 5.8 | 7.3 ± 5.4 | 7.8 ± 6.0 | 6.7 ± 5.7 | 8.4 ± 6.7 | 6.6 ± 4.3 | 9.9 ± 7.7 | 9.1 ± 4.6 | 0.621 |
| NOB | 2.6 ± 2.6 | 2.4 ± 1.6 | 2.5 ± 1.5 | 2.4 ± 1.7 | 2.5 ± 1.3 | 2.5 ± 1.1 | 2.1 ± 1.0 | 2.4 ± 1.2 | 0.739 |
| AP Training Group | Control Group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Training | During Training | After Training | Follow Up | Before Training | During Training | After Training | Follow Up | ||
| MRC on | |||||||||
| Hip flexor | 3.1 ± 0.6 | 3.2 ± 0.6 | 3.5 ± 0.8 | 3.7 ± 0.7 | 3.1 ± 0.7 | 3.3 ± 0.7 | 3.3 ± 0.7 | 3.5 ± 0.8 | 0.288 |
| Hip extensor | 3.3 ± 0.7 | 3.5 ± 0.8 | 3.7 ± 0.7 | 3.9 ± 0.7 | 3.1 ± 0.8 | 3.3 ± 0.8 | 3.3 ± 0.8 | 3.5 ± 0.8 | 0.340 |
| Knee flexor | 3.0 ± 0.7 | 3.2 ± 0.7 | 3.5 ± 0.8 | 3.7 ± 0.7 | 2.9 ± 0.8 | 3.1 ± 0.8 | 3.1 ± 0.9 | 3.3 ± 0.9 | 0.081 |
| Knee extensor | 3.2 ± 0.9 | 3.4 ± 0.8 | 3.7 ± 0.7 * | 3.8 ± 0.8 * | 3.2 ± 0.9 | 3.3 ± 0.7 | 3.2 ± 0.8 | 3.3 ± 1.0 | 0.007 |
| Ankle dorsiflexor | 1.8 ± 1.1 | 2.4 ± 1.1 * | 2.6 ± 1.1 * | 3.0 ± 1.4 * | 2.0 ± 1.2 | 2.0 ± 1.2 | 2.2 ± 1.1 | 2.5 ± 1.2 | 0.036 |
| Ankle plantarflexor | 2.4 ± 1.2 | 2.8 ± 1.3 | 2.9 ± 1.3 | 3.2 ± 1.3 | 2.1 ± 1.4 | 2.2 ± 1.3 | 2.3 ± 1.3 | 2.6 ± 1.2 | 0.270 |
| MSWS (m/s) | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.5 ± 0.3 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.944 |
| SSWS (m/s) | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.914 |
| TUG (s) | 46.8 ± 24.6 | 38.9 ± 24.2 | 34.3 ± 19.9 | 28.7 ± 10.7 | 56.1 ± 64.2 | 51.8 ± 55.6 | 43.1 ± 48.2 | 38.6 ± 31.1 | 0.836 |
| FMA | 39.9 ± 17.0 | 49.5 ± 20.9 * | 54.7 ± 22.1 * | 58.5 ± 20.6 * | 43.7 ± 22.1 | 48.2 ± 22.3 | 51.4 ± 21.1 | 53.7 ± 21.9 | 0.033 |
| FAC | 3.3 ± 0.5 | 3.8 ± 0.6 | 4.2 ± 0.6 | 4.4 ± 0.6 | 3.3 ± 0.5 | 3.6 ± 0.6 | 3.9 ± 0.7 | 4.0 ± 0.7 | 0.210 |
| FIM mobility | 18.4 ± 2.7 | 21.3 ± 3.8 | 24.6 ± 4.6 | 26.6 ± 5.6 | 17.1 ± 4.1 | 19.1 ± 4.2 | 22.3 ± 5.5 | 24.4 ± 6.2 | 0.857 |
| BBS | 25.4 ± 7.3 | 36.8 ± 6.6 * | 43.8 ± 6.9 * | 45.0 ± 8.6 * | 24.7 ± 7.9 | 29.3 ± 8.9 | 34.5 ± 9.6 | 38.3 ± 9.7 | 0.005 |
| Asymmetric Index | AP Training Group | Control Group | p Value | ||
|---|---|---|---|---|---|
| Before Training | After Training | Before Training | After Training | ||
| O2 cost (mL/kg/m) | 0.7 ± 0.4 | 0.6 ± 0.4 | 0.8 ± 0.4 | 0.5 ± 0.3 * | 0.042 |
| O2 rate (mL/min/kg) | 8.0 ± 2.3 | 7.9 ± 1.8 | 7.9 ± 1.7 | 7.8 ± 2.1 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.J.; Kim, D.H.; Kim, S.; Kim, J.H.; Choi, J.H.; Park, J.B.; Baek, Y.S.; Park, Y.G.; Kim, D.Y. Effect of Anterioposterior Weight-Shift Training with Visual Biofeedback in Patients with Step Length Asymmetry after Subacute Stroke. J. Pers. Med. 2023, 13, 1726. https://doi.org/10.3390/jpm13121726
Jo YJ, Kim DH, Kim S, Kim JH, Choi JH, Park JB, Baek YS, Park YG, Kim DY. Effect of Anterioposterior Weight-Shift Training with Visual Biofeedback in Patients with Step Length Asymmetry after Subacute Stroke. Journal of Personalized Medicine. 2023; 13(12):1726. https://doi.org/10.3390/jpm13121726
Chicago/Turabian StyleJo, Yea Jin, Dae Hyun Kim, Seeun Kim, Jung Hoon Kim, Jong Hyun Choi, Jong Bum Park, Yoon Su Baek, Yoon Ghil Park, and Deog Young Kim. 2023. "Effect of Anterioposterior Weight-Shift Training with Visual Biofeedback in Patients with Step Length Asymmetry after Subacute Stroke" Journal of Personalized Medicine 13, no. 12: 1726. https://doi.org/10.3390/jpm13121726





