An Innovative Scoring System to Select the Optimal Surgery in Breast Cancer after Neoadjuvant Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Operative Protocol, Surgical Technique, and Pathological Evaluation
2.2. Adjuvant Treatment
2.3. Statistical Analysis and Score Processing
2.4. Evaluation of Oncological, Aesthetic Outcomes, and Patient Quality of Life (QoL)
2.5. Assessment of the Adherence of Score with Type of Surgery
3. Results
3.1. Demographic, Clinical, and Biological Features
3.2. Pre- and Post-NACT Radiological Assessment
3.3. Pathological Response
3.4. Adjuvant Treatments
3.5. Outcomes According to the Type of Surgery
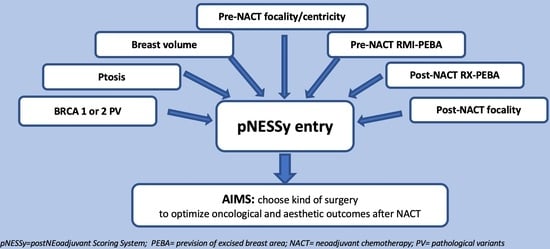
3.6. Definition of “pNESSy”
3.6.1. Univariate and Multivariable Analysis for BCS (Table S3)
3.6.2. Univariate and Multivariable Analysis for OPS (Table S4)
3.6.3. Univariate and Multivariable Analysis for CMR (Table S5)
3.7. Assessment of pNESSy Adherence with Surgery and Evaluation of Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ofri, A.; Elstner, K.; Mann, G.; Kumar, S.; Warrier, S. Neoadjuvant chemotherapy in non-metastatic breast cancer: The surgeon’s perspective. Surgeon 2023, S1479-666X(23)00042-2. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Tong, T.; Xu, D.; Cheng, F.; Fang, C.; He, C.; Wang, J.; Wang, B.; Yang, X.; Wang, K.; et al. Deep learning radiomics of ultrasonography for comprehensively predicting tumor and axillary lymph node status after neoadjuvant chemotherapy in breast cancer patients: A multicenter study. Cancer 2023, 129, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.; Krishnamurthy, A.; Shukla, H.S. Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg. Oncol. 2008, 17, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Garufi, G.; Carbognin, L.; Schettini, F.; Seguí, E.; Di Leone, A.; Franco, A.; Paris, I.; Scambia, G.; Tortora, G.; Fabi, A. Updated Neoadjuvant Treatment Landscape for Early Triple Negative Breast Cancer: Immunotherapy, Potential Predictive Biomarkers, and Novel Agents. Cancers 2022, 14, 4064. [Google Scholar] [CrossRef]
- Veronesi, U.; Stafyla, V.; Petit, J.-Y.; Veronesi, P. Conservative mastectomy: Extending the idea of breast conservation. Lancet Oncol. 2012, 13, e311–e317. [Google Scholar] [CrossRef]
- Franceschini, G.; Di Leone, A.; Natale, M.; Sanchez, M.A.; Masett, R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Ann. Ital. di Chir. 2018, 89, 290. [Google Scholar]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Parmar, V.; Krishnamurthy, A.; Hawaldar, R.; Nadkarni, M.; Sarin, R.; Chinoy, R.; Nair, R.; Dinshaw, K.; Badwe, R. Breast conservation treatment in women with locally advanced breast cancer—Experience from a single centre. Int. J. Surg. 2006, 4, 106–114. [Google Scholar] [CrossRef]
- von Minckwitz, G. Preoperative therapy: What, when and for whom? Ann. Oncol. 2008, 19 (Suppl. S5), v113–v116. [Google Scholar] [CrossRef]
- Hunt, K.K.; Yi, M.; Mittendorf, E.A.; Guerrero, C.; Babiera, G.V.; Bedrosian, I.; Hwang, R.F.; Kuerer, H.M.; Ross, M.I.; Meric-Bernstam, F. Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy is Accurate and Reduces the Need for Axillary Dissection in Breast Cancer Patients. Ann. Surg. 2009, 250, 558–566. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Volders, J.H.; Negenborn, V.L.; Spronk, P.E.; Krekel, N.M.A.; Schoonmade, L.J.; Meijer, S.; Rubio, I.T.; Tol, M.P.v.D. Breast-conserving surgery following neoadjuvant therapy-a systematic review on surgical outcomes. Breast Cancer Res. Treat. 2018, 168, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Morrow, M.; Khan, A.J. Locoregional Management After Neoadjuvant Chemotherapy. J. Clin. Oncol. 2020, 38, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.; Croitoru, A.; Cristian, D.; Jitea, N.; Scaunasu, R.; Aldea, C.; Popa, I.; Burcos, T. Surgical Features after Neoadjuvant Treatment for Breast Cancer. Chirurgia 2021, 116, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Cen, C.; Chun, J.; Kaplowitz, E.; Axelrod, D.; Shapiro, R.; Guth, A.; Schnabel, F. Margin Assessment and Re-excision Rates for Patients Who Have Neoadjuvant Chemotherapy and Breast-Conserving Surgery. Ann. Surg. Oncol. 2021, 28, 5142–5148. [Google Scholar] [CrossRef] [PubMed]
- Di Leone, A.; Franco, A.; Terribile, D.A.; Magno, S.; Fabi, A.; Sanchez, A.M.; D’archi, S.; Scardina, L.; Natale, M.; Mason, E.J.; et al. Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience. Cancers 2022, 14, 1275. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Magno, S.; Fabbri, C.; Chiesa, F.; Di Leone, A.; Moschella, F.; Scafetta, I.; Scaldaferri, A.; Fragomeni, S.M.; Barone, L.A.; et al. Conservative and radical oncoplastic approches in the surgical treatment of breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 387–396. [Google Scholar]
- Sanchez, A.M.; Franceschini, G.; D’Archi, S.; De Lauretis, F.; Scardina, L.; Di Giorgio, D.; Accetta, C.; Masetti, R. Results obtained with level II oncoplastic surgery spanning 20 years of breast cancer treatment: Do we really need further demonstration of reliability? Breast J. 2020, 26, 125–132. [Google Scholar] [CrossRef]
- Franceschini, G.; Scardina, L.; Di Leone, A.; Terribile, D.A.; Sanchez, A.M.; Magno, S.; D’archi, S.; Franco, A.; Mason, E.J.; Carnassale, B.; et al. Immediate Prosthetic Breast Reconstruction after Nipple-Sparing Mastectomy: Traditional Subpectoral Technique versus Direct-to-Implant Prepectoral Reconstruction without Acellular Dermal Matrix. J. Pers. Med. 2021, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Hershko, D. Surgical Management of the Breast and Axilla after Neoadjuvant Therapy. Chirurgia 2021, 116, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Clinical Practice Guidalines in Oncology. Breast Cancer (NCCN Guidelines). Versione 01. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 31 January 2022).
- Innovative “Scoring System” in Breast Cancer Post Neoadiuvant Chemotherapy (BreastNESSy): NCT05213403. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05213403 (accessed on 1 August 2023).
- Di Leone, A.; Terribile, D.; Magno, S.; Sanchez, A.M.; Scardina, L.; Mason, E.J.; D’archi, S.; Maggiore, C.; Rossi, C.; Di Micco, A.; et al. Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes. J. Pers. Med. 2021, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Rancati, A.O.; Angrigiani, C.H.; Hammond, D.C.; Nava, M.B.; Gonzalez, E.G.; Dorr, J.C.; Gercovich, G.F.; Rocco, N.; Rostagno, R.L. Direct to Implant Reconstruction in Nipple Sparing Mastectomy: Patient Selection by Preoperative Digital Mammogram. Plast. Reconstr. Surg.—Glob. Open 2017, 5, e1369. [Google Scholar] [CrossRef]
- Regnault, P. Breast ptosis. Definition and treatment. Clin. Plast. Surg. 1976, 3, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Feig, B.A.B.; Hsiang, D.J.-B.; Butler, J.A.; Mehta, R.S.; Bahri, S.; Nalcioglu, O.; Su, M.-Y. Impact of MRI-Evaluated Neoadjuvant Chemotherapy Response on Change of Surgical Recommendation in Breast Cancer. Ann. Surg. 2009, 249, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Povoski, S.P.; E Jimenez, R.; Wang, W.P.; Xu, R.X. Standardized and reproducible methodology for the comprehensive and systematic assessment of surgical resection margins during breast-conserving surgery for invasive breast cancer. BMC Cancer 2009, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, I.L.; Wartenberg, D.E.; Greco, R.S. Three dimensional staging of breast cancer. Breast Cancer Res. Treat. 1996, 41, 15–19. [Google Scholar] [CrossRef]
- Woeste, M.R.; Bhutiani, N.; Donaldson, M.; McMasters, K.M.; Ajkay, N. Evaluating the effect of neoadjuvant chemotherapy on surgical outcomes after breast conserving surgery. J. Surg. Oncol. 2021, 123, 439–445. [Google Scholar] [CrossRef]
- Choi, J.; Laws, A.; Hu, J.; Barry, W.; Golshan, M.; King, T. Margins in Breast-Conserving Surgery After Neoadjuvant Therapy. Ann. Surg. Oncol. 2018, 25, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Untch, M.; Fasching, P.A.; Konecny, G.E.; Hasmüller, S.; Lebeau, A.; Kreienberg, R.; Camara, O.; Müller, V.; du Bois, A.; Kühn, T.; et al. Pathologic Complete Response After Neoadjuvant Chemotherapy Plus Trastuzumab Predicts Favorable Survival in Human Epidermal Growth Factor Receptor 2–Overexpressing Breast Cancer: Results from the TECHNO Trial of the AGO and GBG Study Groups. J. Clin. Oncol. 2011, 29, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Lambertini, M.; Pondé, N.; Fumagalli, D.; de Azambuja, E.; Piccart, M. Post-neoadjuvant treatment and the management of residual disease in breast cancer: State of the art and perspectives. Ther. Adv. Med. Oncol. 2019, 11, 1758835919827714. [Google Scholar] [CrossRef]
- Huo, X.; Li, J.; Zhao, F.; Ren, D.; Ahmad, R.; Yuan, X.; Du, F.; Zhao, J. The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Boyraz, B.; Sendur, M.A.; Aksoy, S.; Babacan, T.; Roach, E.C.; Kizilarslanoglu, M.C.; Petekkaya, I.; Altundag, K. Trastuzumab emtansine (T-DM1) for HER2-positive breast cancer. Curr. Med. Res. Opin. 2013, 29, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Recht, A.; Comen, E.A.; Fine, R.E.; Fleming, G.F.; Hardenbergh, P.H.; Ho, A.Y.; Hudis, C.A.; Hwang, E.S.; Kirshner, J.J.; Morrow, M.; et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract. Radiat. Oncol. 2016, 6, e219–e234. [Google Scholar] [CrossRef]
- Makris, A.; Powles, T.J.; Ashley, S.E.; Chang, J.; Hickish, T.; Tidy, V.A.; Nash, A.G.; Ford, H.T. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann. Oncol. 1998, 9, 1179–1184. [Google Scholar] [CrossRef]
- Cance, W.G.; A Carey, L.; Calvo, B.F.; Sartor, C.; Sawyer, L.; Moore, D.T.; Rosenman, J.; Ollila, D.W.; Graham, M. Long-Term Outcome of Neoadjuvant Therapy for Locally Advanced Breast Carcinoma: Effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann. Surg. 2002, 236, 295. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef]
- Hadar, T.; Koretz, M.; Nawass, M.; Allweis, T.M. Innovative Standards in Surgery of the Breast after Neoadjuvant Systemic Therapy. Breast Care 2021, 16, 590–597. [Google Scholar] [CrossRef] [PubMed]
- A McIntosh, S.; Ogston, K.N.; Payne, S.; Miller, I.D.; Sarkar, T.K.; Hutcheon, A.W.; Heys, S.D. Local recurrence in patients with large and locally advanced breast cancer treated with primary chemotherapy. Am. J. Surg. 2003, 185, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Meric-Bernstam, F.; Hunt, K.K.; Thames, H.D.; Oswald, M.J.; Outlaw, E.D.; Strom, E.A.; McNeese, M.D.; Kuerer, H.M.; Ross, M.I.; et al. Breast Conservation After Neoadjuvant Chemotherapy: The M.D. Anderson Cancer Center Experience. J. Clin. Oncol. 2004, 22, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Nardone, L.; Valentini, V.; Marino, L.; De Santis, M.C.; Terribile, D.; Franceschini, G.; Balducci, M.; Mantini, G.; Mattiucci, G.; Mulè, A.; et al. A Feasibility Study of Neo-Adjuvant Low-Dose Fractionated Radiotherapy with Two Different Concurrent Anthracycline-Docetaxel Schedules in Stage IIA/B-IIIA Breast Cancer. Tumori J. 2012, 98, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.H.; Strom, E.A.; Perkins, G.H.; Oh, J.L.; Chen, A.M.; Meric-Bernstam, F.; Hunt, K.K.; Sahin, A.A.; Hortobagyi, G.N.; Buchholz, T.A. Comparison of risk of local-regional recurrence after mastectomy or breast conservation therapy for patients treated with neoadjuvant chemotherapy and radiation stratified according to a prognostic index score. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 352–357. [Google Scholar] [CrossRef]
- Simons, J.M.; Jacobs, J.G.; Roijers, J.P.; Beek, M.A.; Winter, L.J.M.B.-D.; Rijken, A.M.; Gobardhan, P.D.; Wijsman, J.H.; Tetteroo, E.; Heijns, J.B.; et al. Disease-free and overall survival after neoadjuvant chemotherapy in breast cancer: Breast-conserving surgery compared to mastectomy in a large single-centre cohort study. Breast Cancer Res. Treat. 2021, 185, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kuerer, H.M.; Singletary, S.; Buzdar, A.U.; Ames, F.C.; Valero, V.; A Buchholz, T.; I Ross, M.; Pusztai, L.; Hortobagyi, G.N.; Hunt, K.K. Surgical conservation planning after neoadjuvant chemotherapy for stage II and operable stage III breast carcinoma. Am. J. Surg. 2001, 182, 601–608. [Google Scholar] [CrossRef]
- van la Parra, R.F.D.; Clough, K.B.; Thygesen, H.H.; Levy, E.; Poulet, B.; Sarfati, I.; Nos, C. Oncological Safety of Oncoplastic Level II Mammoplasties After Neoadjuvant Chemotherapy for Large Breast Cancers: A Matched-Cohort Analysis. Ann. Surg. Oncol. 2021, 28, 5920–5928. [Google Scholar] [CrossRef] [PubMed]
- Panico, C.; Ferrara, F.; Woitek, R.; D’angelo, A.; Di Paola, V.; Bufi, E.; Conti, M.; Palma, S.; Cicero, S.L.; Cimino, G.; et al. Staging Breast Cancer with MRI, the T. A Key Role in the Neoadjuvant Setting. Cancers 2022, 14, 5786. [Google Scholar] [CrossRef] [PubMed]
- Margolese, R.G. Surgical Considerations in Preoperative Chemotherapy of Breast Cancer. Recent Results Cancer Res. 1998, 152, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kuerer, H.M.; Newman, L.A.; Buzdar, A.U.; Hunt, K.K.; Dhingra, K.; Buchholz, T.A.; Binkley, S.M.; Ames, F.C.; Feig, B.W.; Ross, M.I.; et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am. J. Surg. 1998, 176, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; von Minckwitz, G.; Mamounas, E.P.; Cameron, D.; Carey, L.A.; Cristofanilli, M.; Denkert, C.; Eiermann, W.; Gnant, M.; Harris, J.R.; et al. Recommendations from an International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann. Surg. Oncol. 2012, 19, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Oncology, F.T.A.F.C.T.I.; Cirrincione, C.T.; Sikov, W.M.; Carey, L.A.; Berry, D.A.; Overmoyer, B.; Henry, N.L.; Somlo, G.; Port, E.; et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II–III HER2-positive breast cancer: Surgical results of CALGB 40601 (Alliance). Breast Cancer Res. Treat. 2016, 160, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Terribile, D.A.; Mason, E.J.; Murando, F.; DI Leone, A.; Sanchez, A.M.; Scardina, L.; Magno, S.; Franco, A.; D’archi, S.; Natale, M.; et al. Surgical management of BRCA pathogenic variant carriers with breast cancer: A recent literature review and current state of the art. Minerva Surg. 2021, 76, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Terribile, D.; Fabbri, C.; Magno, S.; D’Alba, P.; Chiesa, F.; Di Leone, A.; Masetti, R. Management of locally advanced breast cancer. Mini-review. Minerva Chir. 2007, 62, 249–255. [Google Scholar] [PubMed]
- Sun, Y.; Liao, M.; He, L.; Zhu, C. Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after good response to neoadjuvant chemotherapy: A PRISMA-compliant systematic review and meta-analysis. Medicine 2017, 96, e8367. [Google Scholar] [CrossRef] [PubMed]
- Arlow, R.L.; Paddock, L.E.; Niu, X.; Kirstein, L.; Haffty, B.G.; Goyal, S.; Kearney, T.; Toppmeyer, D.; Stroup, A.M.; Khan, A.J. Breast-conservation Therapy After Neoadjuvant Chemotherapy Does Not Compromise 10-Year Breast Cancer–specific Mortality. Am. J. Clin. Oncol. 2018, 41, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Sagona, A.; De Carlo, C.; Fernandes, B.; Barbieri, E.; Grimaldi, S.D.M.; Jacobs, F.; Vatteroni, G.; Scardina, L.; Biondi, E.; et al. Pathologic response and residual tumor cellularity after neo-adjuvant chemotherapy predict prognosis in breast cancer patients. Breast 2023, 69, 323–329. [Google Scholar] [CrossRef]
- Jankowski, C.; Michel, E.; Vincent, L.; Beltjens, F.; Arnould, L.; Ladoire, S.; Coutant, C. Axillary pathologic response after neoadjuvant chemotherapy and surgery according to breast cancers subtypes and survival impact. Bull. Cancer 2023, 110, 605–615. [Google Scholar] [CrossRef]
- de Oliveira-Junior, I.; Silva, I.d.A.d.; da Silva, F.C.B.; da Silva, J.J.; Sarri, A.J.; Paiva, C.E.; Vieira, R.A.d.C. Oncoplastic Surgery in Breast-Conserving Treatment: Patient Profile and Impact on Quality of Life. Breast Care 2021, 16, 243–253. [Google Scholar] [CrossRef]






| BCS 118 (46.3%) | OPS 49 (19.2%) | CMR 88 (34.5%) | p-Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (y) | 51.9 ± 11.5 (44.2–60.3) | 48.3 ± 9.1 (42.2–53) | 45. 8 ± 9.7 (39.6–52.4) | p < 0.0001 |
| BMI (kg/m2) | 25.4 ± 4.7 (22.7–27.3) | 27.4 ± 5.3 (23.3–30.1) | 23.2 ± 3.5 (20.8–24.5) | p < 0.0001 |
Menopausal status
| 58 (49.2%) 60 (50.8%9 | 19 (38.8%) 30 (61.2%) | 24 (27.3%) 64 (72.7%) | p = 0.006 |
| Pathological mutations of BRCA 1 or 2 | 5 (4.2%) | 1 (2%) | 18 (20.5%) | p < 0.0001 |
| Anatomic-morphological mammary characteristics of breasts | ||||
Bra size
| 26 (22.0%) 50 (42.4%) 42 (35.6%) | 8 (16.3%) 13 (26.5%) 28 (57.2%) | 8 (44.4%) 30 (34.1%) 19 (21.5%) | p < 0.0001 |
Ptosis
| 20 (16.9%) 53 (44.9%) 45 (38.2%) | 5 (10.2%) 13 (26.5%) 31 (63.3%) | 44 (50%) 30 (34.1%) 14 (15.9%) | p < 0.0001 |
| Location of cancer | ||||
| 78 (66.1%) 8 (6.8%) 4 (3.4%) 20 (16.9%) 8 (6.8%) | 36 (73.5%) 4 (8.2%) 1 (2%) 6 (12.2%) 2 (4.1%) | 65 (73.9%) 7 (8%) 2 (2.3%) 11 (12.5%) 3 (3.4%) | p = 0.948 |
| Biological characteristics of cancer | ||||
Histotype
| 86 (72.9%) 8 (6.8%) 24 (20.3%) | 35 (71.5%) 3 (6.1%) 11 (22.4%) | 63 (71.6%) 6 (6.8%) 19 (21.6%) | p = 0.997 |
Grading
| 0 (0%) 33 (28%) 85 (72%) | 0 (0%) 15 (30.6%) 34 (69.4%) | 1 (1.1%) 24 (27.3%) 63 (71.6%) | p = 0.830 |
Immunophenotype
| 59 (50%) 39 (33.1%) 20 (16.9%) | 22 (44.9%) 16 (32.7%) 11 (22.4%) | 38 (43.2%) 31 (35.2%) 19 (21.6%) | p = 0.825 |
| Initial stage of cancer ** | ||||
| 6 (5.1%) 32 (27.1%) 40 (33.9%) 29 (24.6%) 7 (5.9%) 4 (3.4%) | 1 (8.3%) 9 (18.4%) 18 (36.7%) 18 (36.7%) 2 (4.1%) 1 (20%) | 5 (5.7%) 24 (27.3%) 26 (29.5%) 24 (27.3%) 6 (6.8%) 3 (3.4%) | p = 0.904 |
| BCS 118 (46.3%) | OPS 49 (19.2%) | CMR 88 (34.5%) | p-Value | |
|---|---|---|---|---|
| Assessment Pre-NACT | ||||
Rancati score
| 31 (26.3%) 64 (54.2%) 23 (19.5%) | 8 (16.3%) 30 (61.3%) 11 (22.4%) | 39 (44.3%) 43 (48.9%) 6 (6.8%) | p = 0.001 |
Breast volume (cm3) *
| 33 (28%) 53 (44.9%) 32 (27.1%) | 5 (10.2%) 16 (32.7%) 28 (57.1%) | 53 (60.2%) 24 (27.3%) 11 (12.5%) | p < 0.0001 |
Microcalcification extension
| 90 (76.3%) 27 (22.9%) 1 (0.8%) | 20 (40.8%) 26 (53.1%) 3 (6.1%) | 42 (47.7%) 36 (40.9%) 10 (10.4%) | p < 0.0001 |
No. involved quadrants
| 77 (65.3%) 31 (26.3%) 10 (8.5%) | 17 (34.7%) 16 (32.7%) 16 (32.7%) | 29 (33.0%) 18 (20.5%) 41 (46.6%) | p < 0.0001 |
Pre-NACT RX-PEBA
| 71 (60.2%) 33 (28.1%) 14 (11.7%) | 14 (28.6%) 30 (61.2%) 5 (10.2%) | 20 (22.7%) 12 (13.6%) 56 (63.7%) | p < 0.0001 |
Pre-NACT focality
| 84 (71.2%) 27 (22.9%) 7 (5.9%) | 12 (24.5%) 34 (69.4%) 3 (6.1%) | 28 (31.8%) 17 (19.3%) 43 (48.9%) | p < 0.0001 |
Pre-NACT MRI-PEBA
| 74 (62.7%) 38 (32.2%) 6 (5.1%) | 10 (20.4%) 33 (67.4%) 6 (12.2%) | 19 (21.6%) 30 (34.1%) 39 (44.3%) | p < 0.0001 |
| Assessment post-NACT | ||||
Radiological response
| 66 (55.9%) 38 (32.3%) 12 (10.2%) 2 (1.7%) | 16 (32.7%) 29 (59.2%) 4 (8.2%) 0 (0%) | 38 (43.2%) 33 (37.5%) 16 (18.2%) 1 (1.1%) | p = 0.016 |
Post-NACT RX-PEBA
| 87 (73.7%) 27 (22.9%) 4 (3.4%) | 14 (28.6%) 35 (71.4%) 0 (0%) | 27 (30.7%) 40 (45.5%) 21 (9.8%) | p < 0.0001 |
Post-NACT focality
| 65 (55.1%) 42 (35.6%) 9 (7.6%) 2 (1.7%) | 15 (30.6%) 12 (23.9%) 20 (40.8%) 2 (4.1%) | 36 (40.9%) 21 (23.9%) 6 (6.8%) 25 (28.4%) | p < 0.0001 |
Post-NACT MRI-PEBA
| 75 (63.6%) 36 (30.5%) 7 (5.9%) | 14 (28.6%) 24 (49%) 11 (22.4%) | 39 (44.3%) 16 (18.2%) 33 (37.5%) | p < 0.0001 |
| BRCA 1 or 2 Genes | No Pathogenetic Variant | Pathological Mutation | |
|---|---|---|---|
| 0 | 2.867 | ||
| Ptosis | Grade 0 | Grade 1 | Grade 2 or 3 |
| 1.389 | 0.511 | 1.352 | |
| Breast volume evaluated with MRI | <645.99 cm3 | 646.00–1009.39 cm3 | >1009.4 cm3 |
| 1.375 | 0.900 | 1.526 | |
| Pre-NACT Focality | Unifocality | Multifocality | Multicentricity |
| 1.368 | 1.193 | 2.309 | |
| Pre-NACT MRI-PEBA | <3.52 | 3.53–9.99 | >10.00 |
| 1.251 | 1.391 | 1.860 | |
| Post-NACT Focality | Clinical complete response or unifocal | Multifocality | Multicentricity |
| 1.536 | 2.274 | 2.007 | |
| Post-NACT RX-PEBA | <0.041 | 0.042–4.61 | >4.62 |
| 1.505 | 2.020 | 1.589 | |
| SCORE | 6.896–8.724 | 8.725–9.375 | 9.376–14.245 |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| TYPE OF SURGERY | BCS | OPS | CMR |
| G1 164 (64.3%) | G2 91 (35.7%) | p-Value | |
|---|---|---|---|
| Oncological radicality | |||
| Reached Failed | 152 (95%) 8 (5%) | 81 (85.3%) 14 (14.7%) | p = 0.010 |
| Aesthetic Outcomes evaluate on patients who responded to “Breast Q” questionnaire * | |||
| G1 123 (75%) | G2 69 (75.8%) | ||
| Q1. Satisfaction with breasts <40 41–70 >71 | 29 (23.6%) 45 (36.6%) 49 (39.8%) | 30 (43.5%) 20 (29.0%) 19 (27.5%) | p = 0.017 |
| Q2. Psychological wellbeing <40 41–70 >71 | 21 (17.1%) 37 (30.1%) 65 (52.8%) | 18 (26.5%) 21 (30.9%) 29 (49.2%) | p = 0.245 |
| Q3. Sexual wellbeing <40 41–70 >71 | 37 (30.1%) 46 (37.4%) 40 (32.5%) | 31 (45.6%) 19 (27.9%) 18 (26.5%) | p = 0.108 |
| Q4. Physical wellbeing: chest <40 41–70 >71 | 77 (62.6%) 38 (30.9%) 8 (6.5%) | 35 (51.5%) 15 (22.1%) 18 (26.5%) | p = 0.001 |
| Breast sensitivity Preserved Lost | 49 (39.8%) 74 (60.2%) | 22 (32.4%) 46 (67.6%) | p = 0.350 |
| Evaluation in patients with loss of breast sensitivity ** | |||
| G1 76 (61.7%) | G2 44 (63.7%) | ||
| Percentage of breast sensitivity lost 10–30% 40–70% 70–100% | 35 (46.1%) 25 (32.9%) 16 (21.1%) | 23 (52.3%) 13 (29.5%) 8 (18.2%) | p = 0.825 |
| Influence on daily life 0–30% 40–70% 70–100% | 35 (46.1%) 25 (32.9%) 16 (21.1%) | 23 (52.3%) 13 (29.5%) 8 (18.2%) | p = 0.825 |
| Influence on sexual life 0–30% 40–70% 70–100% | 23 (30.3%) 23 (30.3%) 30 (39.5%) | 17 (38.6%) 13 (29.5%) 14 (31.8%) | p = 0.607 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Di Leone, A.; Conti, M.; Fabi, A.; Carbognin, L.; Terribile, A.D.; Belli, P.; Orlandi, A.; Sanchez, M.A.; Moschella, F.; et al. An Innovative Scoring System to Select the Optimal Surgery in Breast Cancer after Neoadjuvant Chemotherapy. J. Pers. Med. 2023, 13, 1280. https://doi.org/10.3390/jpm13081280
Franco A, Di Leone A, Conti M, Fabi A, Carbognin L, Terribile AD, Belli P, Orlandi A, Sanchez MA, Moschella F, et al. An Innovative Scoring System to Select the Optimal Surgery in Breast Cancer after Neoadjuvant Chemotherapy. Journal of Personalized Medicine. 2023; 13(8):1280. https://doi.org/10.3390/jpm13081280
Chicago/Turabian StyleFranco, Antonio, Alba Di Leone, Marco Conti, Alessandra Fabi, Luisa Carbognin, Andreina Daniela Terribile, Paolo Belli, Armando Orlandi, Martin Alejandro Sanchez, Francesca Moschella, and et al. 2023. "An Innovative Scoring System to Select the Optimal Surgery in Breast Cancer after Neoadjuvant Chemotherapy" Journal of Personalized Medicine 13, no. 8: 1280. https://doi.org/10.3390/jpm13081280