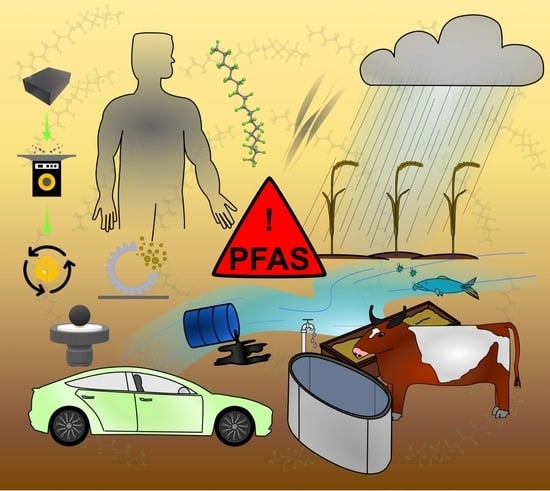
Forever Chemicals, Per-and Polyfluoroalkyl Substances (PFAS), in Lubrication
Abstract
:1. Introduction
2. PFAS in Lubrication
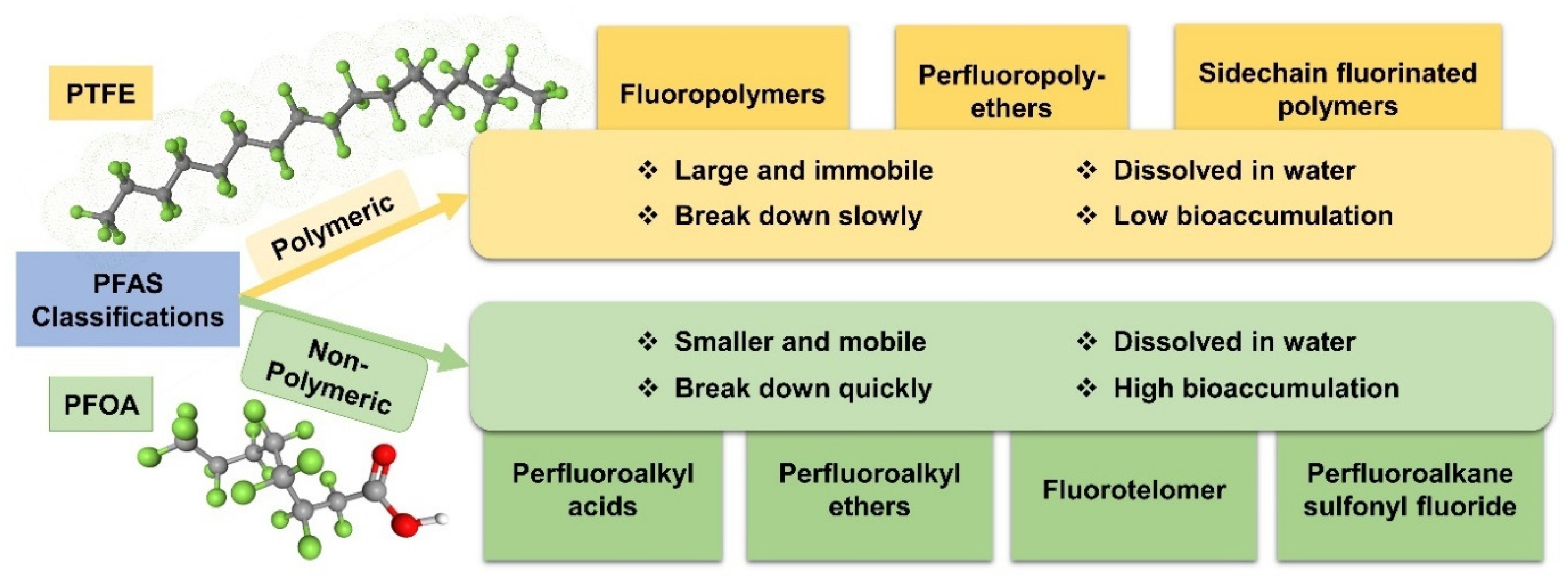
2.1. Classifications
2.2. Manufacturing Techniques
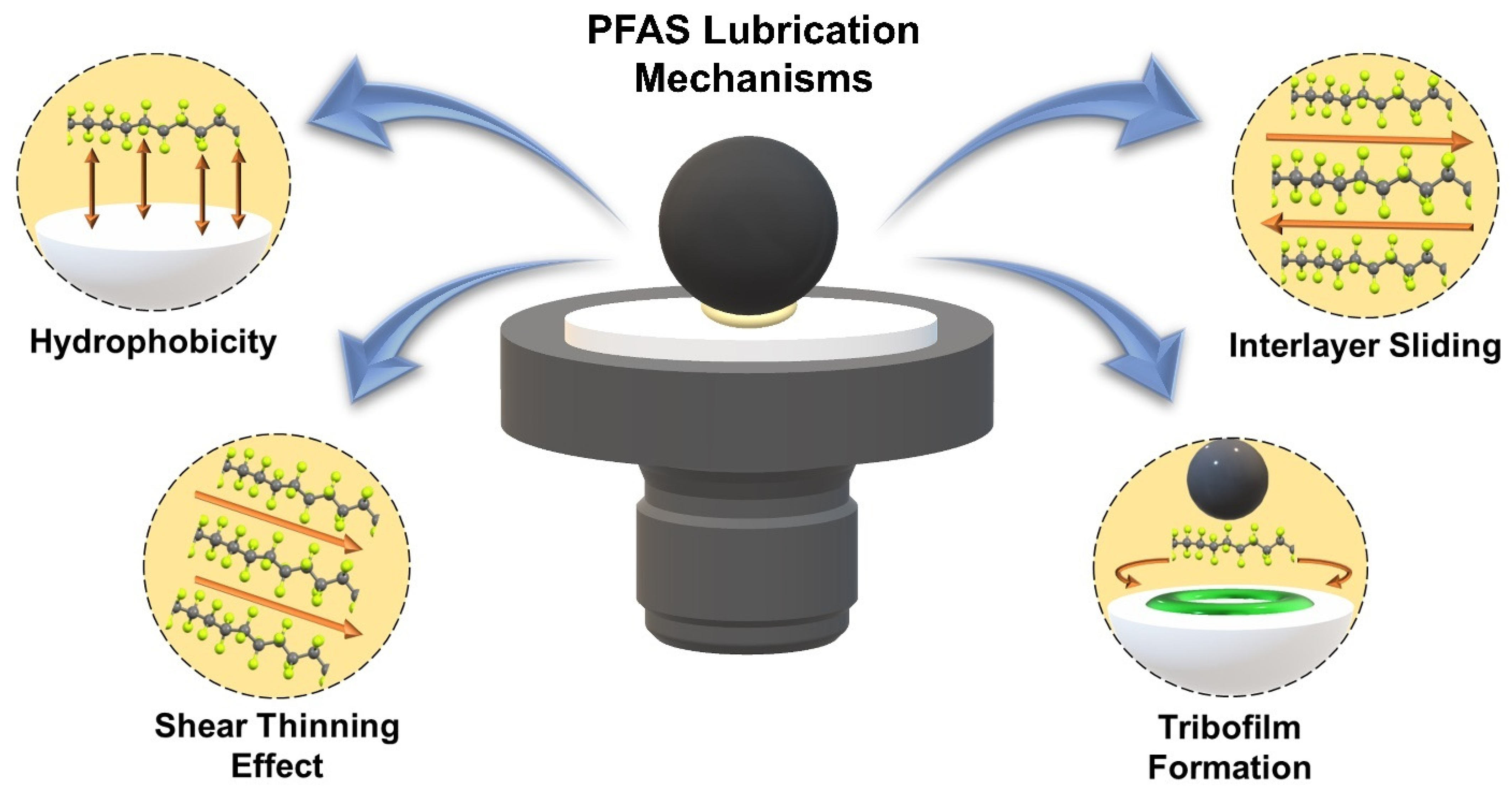
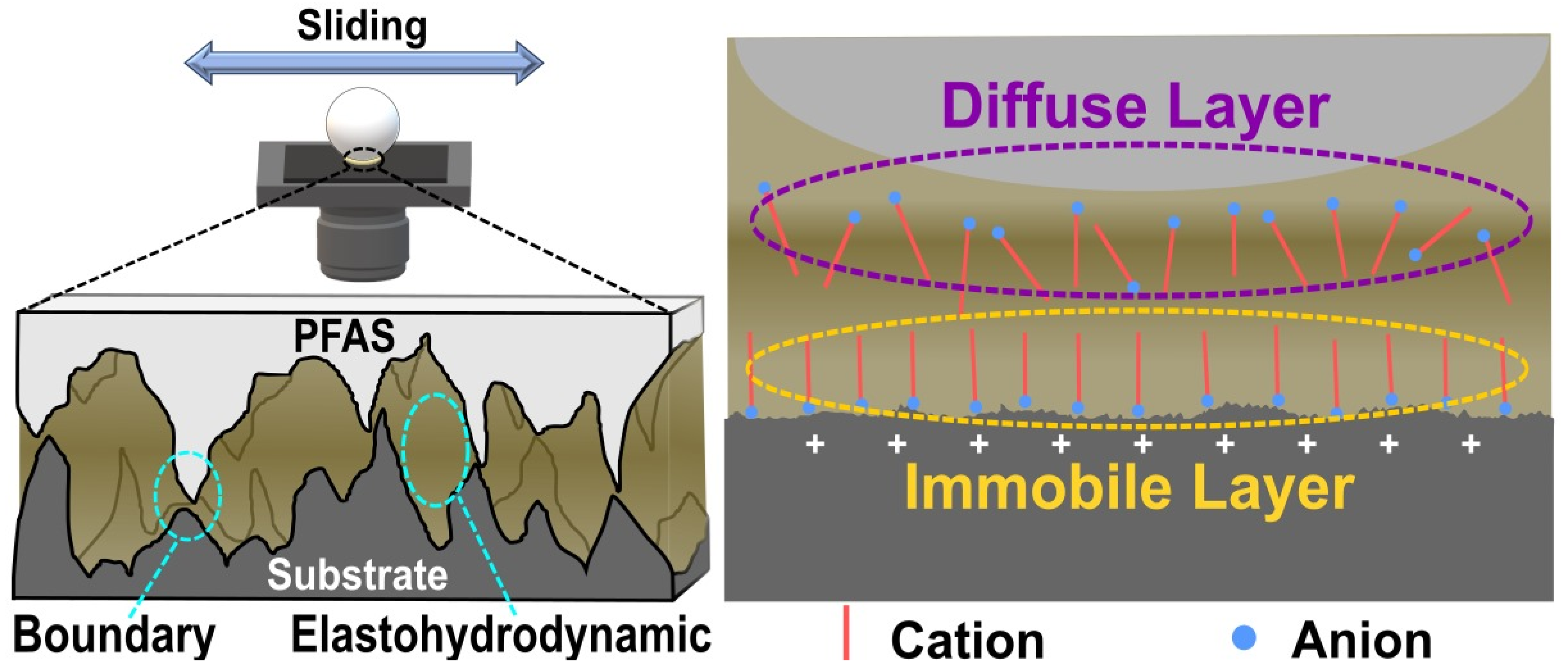
2.3. Lubrication Mechanisms of PFAS
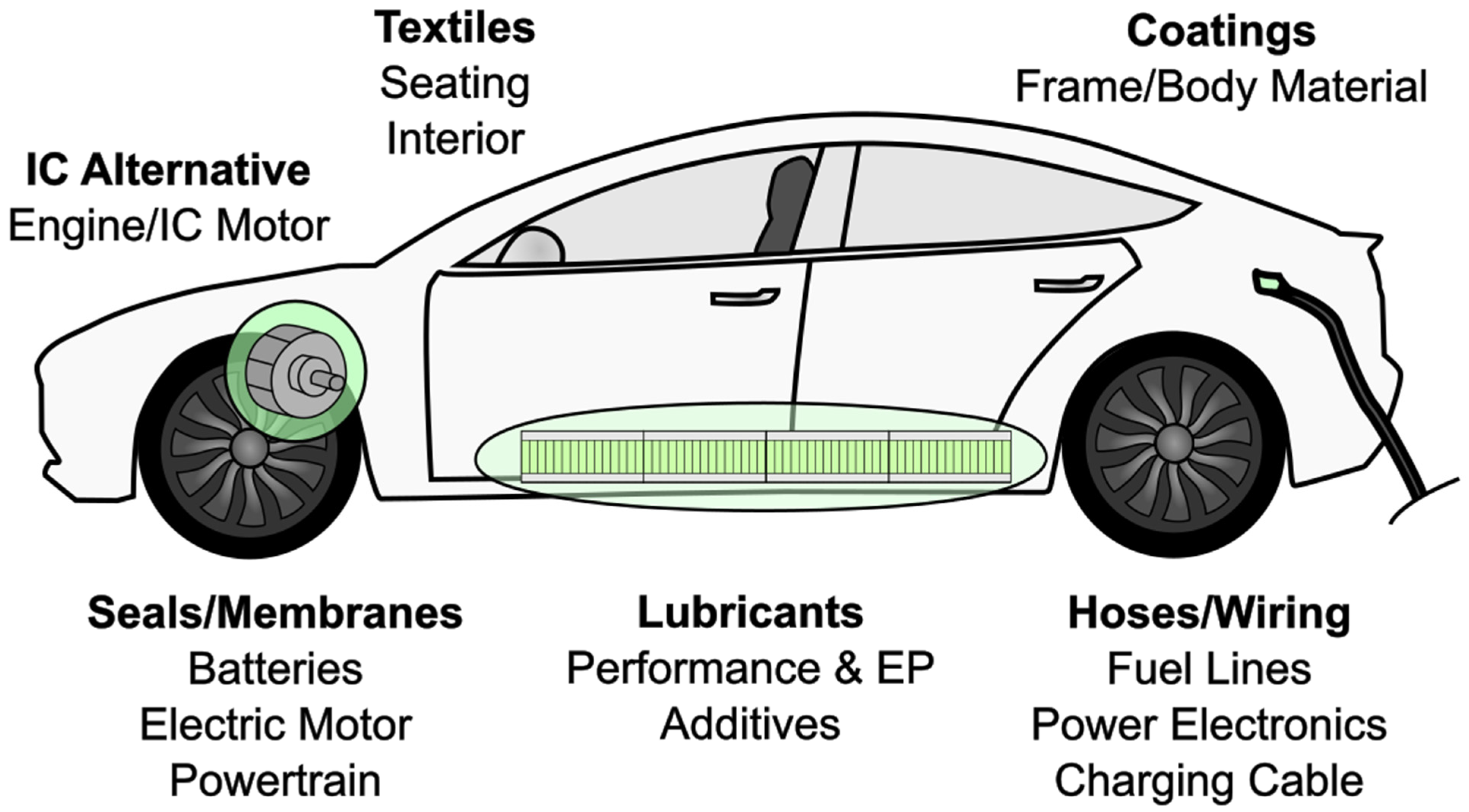
2.4. Applications of PFAS in Modern Automobiles
2.5. PFAS in Coatings and Composite Materials
2.5.1. PTFE Coatings
2.5.2. PVDF Coatings
2.5.3. Composite Materials
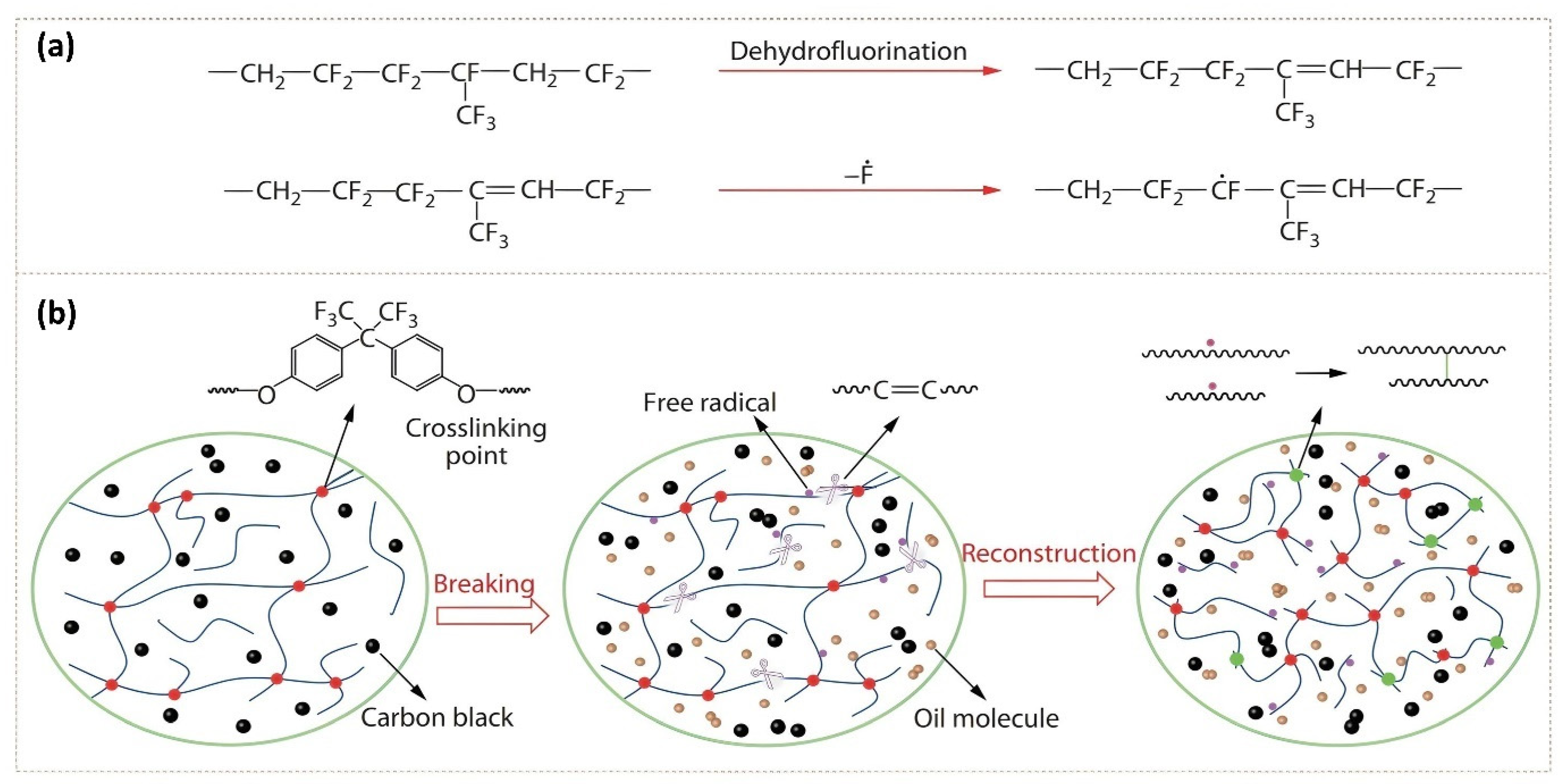
2.6. PFAS as Additives
2.6.1. Particulate Additives
2.6.2. Extreme Pressure Additives
2.6.3. Synthetic Lubricant Additives
2.7. PFAS in Ionic Liquids
2.8. PFAS in Seals and O-Rings
3. Impacts of PFAS
3.1. Environmental Impacts
3.2. Human Health and Toxicology Impacts
4. PFAS Remediation and Alternatives
4.1. Regulatory Considerations and Industry Practices
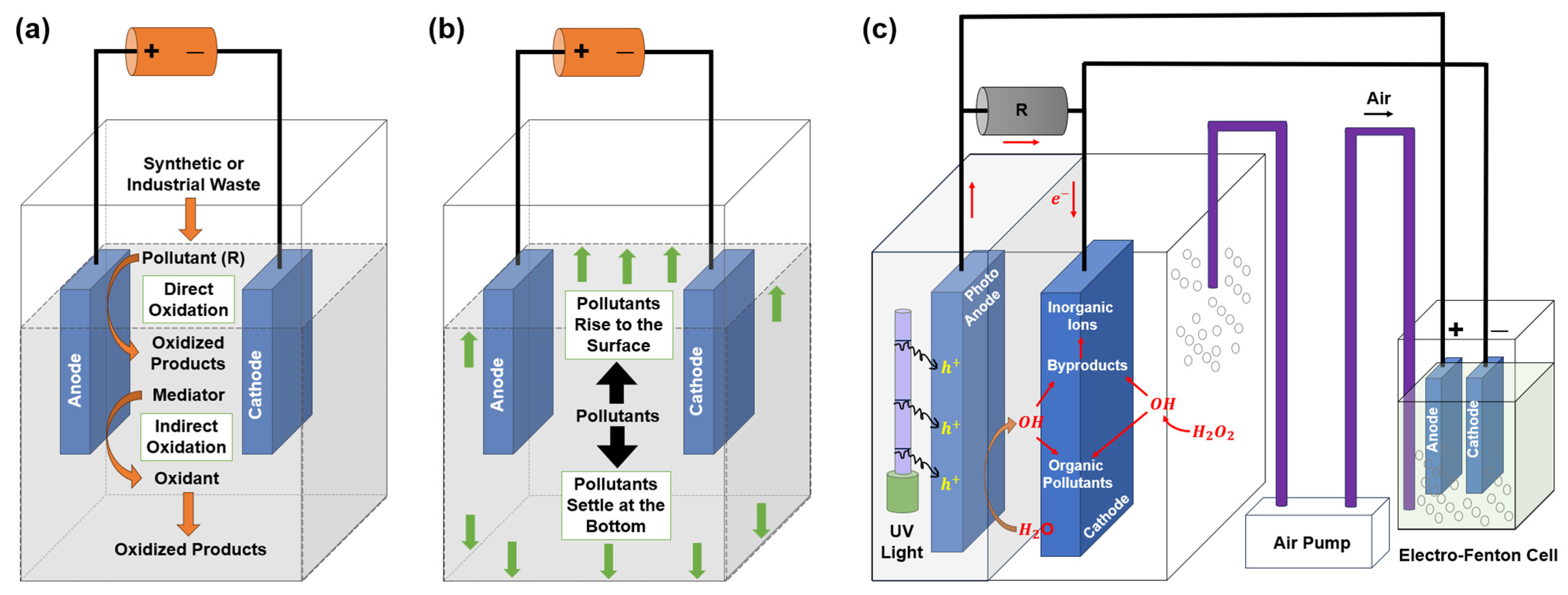
4.2. Electrochemical Degradation Methods
4.2.1. Electro-Oxidation
4.2.2. Electro-Coagulation
4.2.3. Photocatalytic Fuel Cell
4.3. Thermal Degradation Methods
4.3.1. Sonochemical Degradation of PFAS
4.3.2. Subcritical or Supercritical Treatment of PFAS
4.4. Biodegradation Methods
4.4.1. Microbial Degradation of PFAS
4.4.2. Enzymatic Degradation of PFAS
4.5. PFAS Alternatives
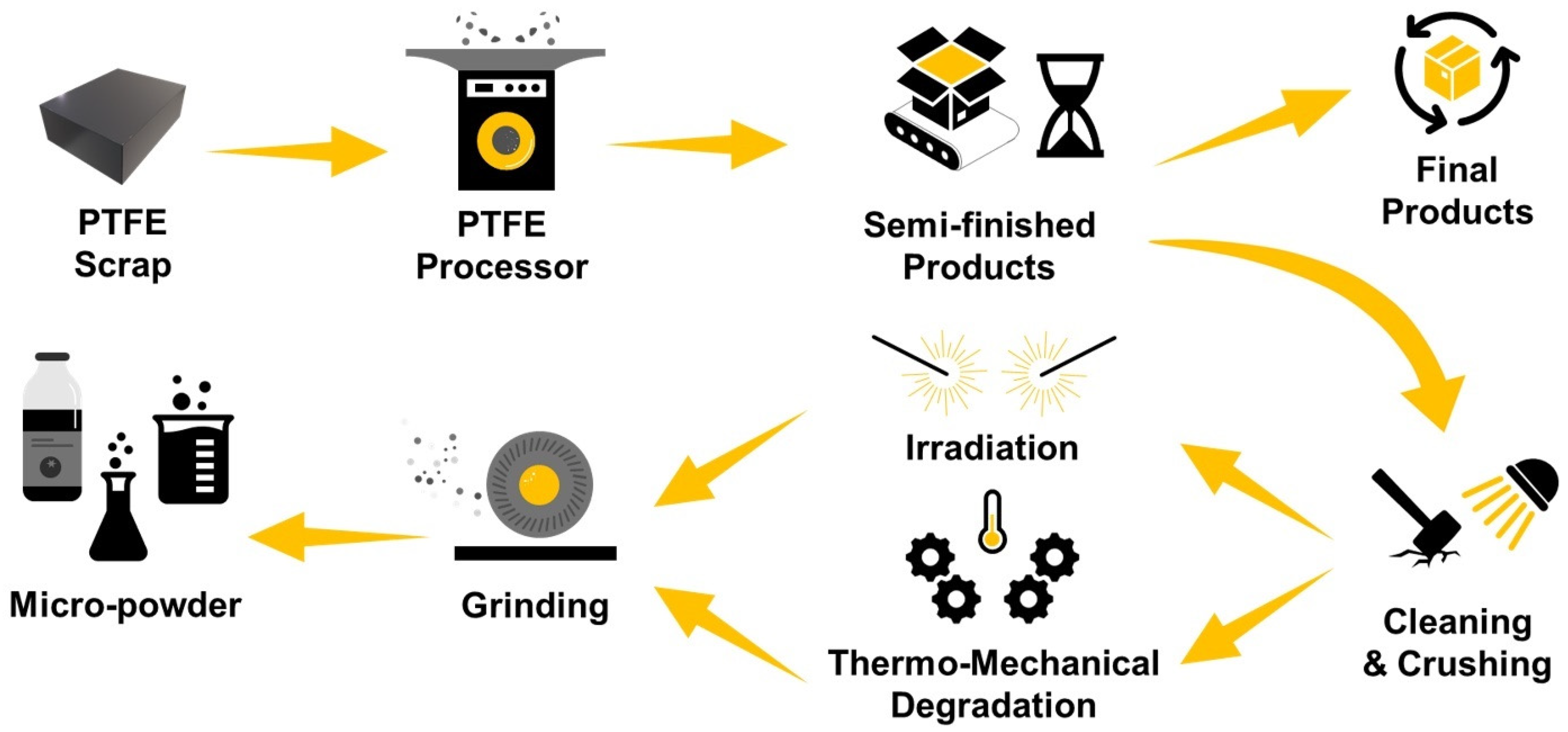
4.6. PFAS Recovery and Recycling
5. Conclusions and Future Outlook
- PFAS has seen extensive use in several industries and applications thanks to their strong C–F bonds and nonpolar nature.
- Their properties make them highly effective and efficient performance enhancers in tribology and lubrication systems, reducing friction coefficients and wear rates.
- These same properties make them resistant to natural degradation and are known to cause many negative health effects through bioaccumulation and environmental contamination as it is transported across various interfaces.
- Rigorous worldwide regulations continue to be introduced around the world in an effort to curtail the use of PFAS as the problem garners more attention given their persistent nature and documented adverse health effects.
- Remediation strategies involve various accelerated degradation techniques and PFAS material recovery through recycling; however, sourcing and utilizing PFAS alternatives remains the most effective method to reduce the world’s PFAS footprint.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| AF | Antifriction |
| AFM | Atomic Force Microscope |
| AOP | Advanced Oxidation Process |
| API | American Petroleum Institute |
| ATR-FTIR | Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy |
| ATSDR | Agency for Toxic Substances and Disease Registry |
| AW | Antiwear |
| BEV | Battery Electric Vehicle |
| CFC | Chlorofluorocarbons |
| CNF | Carbon Nanofiber |
| CNR | Carbon Nanorod |
| COF | Coefficient of Friction |
| EC | Electrocoagulation |
| ECHA | European Chemicals Agency |
| EO | Electro-oxidation |
| EP | Extreme Pressure |
| EPA | Environmental Protection Agency |
| ESIMS/MS | Electrospray Negative Ionization-Tandem Mass Spectrometry |
| EV | Electric Vehicle |
| FCEV | Fuel Cell Electric Vehicle |
| FEC | Fluorobenzene/Fluoroethylene Carbonate |
| FEP | Fluorinated Ethylene Propylene |
| FFKM | Perfluoroelastomer |
| FKM | Fluoroelastomer |
| FKM-O | FKM in Lubricating Oil |
| FT | Fluorotelomer |
| FTAL | Fluorotelomer Aldehyde |
| FTCA | Fluorotelomer Carboxylic Acid |
| FTOH | Fluorotelomer Alcohol |
| FTUCA | Fluorotelomer Unsaturated Carboxylic Acid |
| FVMQ | Fluorosilicone |
| GnPs | Graphene Nanoplatelet |
| HEP | Hydrofluoroethers |
| HFP | Hexafluoropropylene |
| HFPO-TA | Hexafluoropropylene Oxide Trifluoroacetate |
| ICE | Internal Combustion Engine |
| IL | Ionic Liquid |
| L-B104 | 1-butyl-3-methylimidazolium Tetrafluoroborate |
| LD50 | Lethal Dosage 50 |
| L-F104 | 1-butyl-3-methylimidazolium Bis[(trifluoromethyl)sulfony]imide |
| LOAEL | Lower Observed Adverse Effects Level |
| L-P104 | 1-butyl-3-methylimidazolium Hexafluorophosphate |
| MAC | Multialkylated Cyclopentane |
| MAO | Microarc Oxidized |
| MEC | Microbial Electrolysis Cell |
| MEMS | Micro-electromechanical System |
| NEMS | Nano-electromechanical System |
| NL | Nanolubricant |
| NNN | Novel Non-fluoronated Polymer |
| NOAEL | No Observable Adverse Effects Level |
| NP | Nanoparticle |
| NT | Nanotube |
| OBS | Oligomeric Siloxanes |
| OPE | Organophosphate Ester |
| PAO | Polyalphaolefin |
| PASF | Perfluoroalkane Sulfonyl Fluoride |
| PCTFE | Polychlorotrifluoroethylene |
| PDA | Polydopamine |
| PFAA | Perfluoroalkyl Acid |
| PFAS | Per- and Polyfluoroalykyl Substance |
| PFBA | Perfluorobutanoic Acid |
| PFC | Perfluorinated Chemical |
| PFC | Photocatalytic Fuel Cell |
| PFCA | Perfluorocarboxylic Acid |
| PFHpA | Perfluoroheptanoic Acid |
| PFHxA | Perfluorohexanoic Acid |
| PFHxS | Perflurohexanesulfonic Acid |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctane Sulfonic Acid |
| PFOSA | Perfluoroctanesulfonamide |
| PFPE | Perfluoroalkyl Ether |
| PFPeA | Perfluorovaleric Acid |
| PFSA | Perfluorosulfonic Acid |
| PHA | Polyhydroxyalkanoate |
| PLC | Polymers-of-low-concern |
| PMMA | Polymethylmethacrylate |
| POP | Persistent Organic Pollutant |
| PSF | Polysulfone |
| PSIL | Phosphonimum-based Ionic Liquid |
| PTFE | Polytetrafluoroethylene |
| PVDF | Polyvinylidene Fluoride |
| PVF | Polyvinyl Fluoride |
| ROS | Reactive Oxygen Species |
| SA | Slide Angle |
| TFE | Tetrafluoroethylene |
| TFE/P | Tetrafluoroethylene Propylene |
| Tg | Glass Transition Temperature |
| TOP | Total Oxidizable Precursor |
| TPP | Tetraalkyl Phosphonium Perfluorosulfonate |
| UF6 | Uranium Hexafluoride |
| UV | Ultraviolet |
| VDF | Vinylidene Fluoride |
| WCA | Water Contact Angle |
| WSD | Wear Scar Diameter |
| WWTP | Wastewater Treatment Plant |
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; Van Leeuwen, S.P.J. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever Chemicals—Persistent, Bioaccumulative and Mobile. Reviewing the Status and the Need for Their Phase out and Remediation of Contaminated Sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- Renfrew, D.; Pearson, T.W. The Social Life of the “Forever Chemical” PFAS Pollution Legacies and Toxic Events. Environ. Soc. Adv. Res. 2021, 12, 146–163. [Google Scholar] [CrossRef]
- Miner, K.R.; Clifford, H.; Taruscio, T.; Potocki, M.; Solomon, G.; Ritari, M.; Napper, I.E.; Gajurel, A.P.; Mayewski, P.A. Deposition of PFAS ‘Forever Chemicals’ on Mt. Everest. Sci. Total Environ. 2021, 759, 144421. [Google Scholar] [CrossRef] [PubMed]
- Okazoe, T. Overview on the History of Organofluorine Chemistry from the Viewpoint of Material Industry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T. Metal-Fluorocarbon Based Energetic Materials. Propellants Explos. Pyrotech. 2012, 37, 373. [Google Scholar] [CrossRef]
- Plunkett, R.J. Tetrafluoroethylene Polymers. U.S. Patent 2,230,654, 4 February 1941. [Google Scholar]
- Gaines, L.G.T. Historical and Current Usage of Per- and Polyfluoroalkyl Substances (PFAS): A Literature Review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; Dewitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- And Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.M.; Polycarpou, A.A. Tribological Performance of PTFE- and PEEK-Based Coatings under Oil-Less Compressor Conditions. Wear 2012, 296, 638–647. [Google Scholar] [CrossRef]
- Dubey, M.K.; Bijwe, J.; Ramakumar, S.S.V. Nano-PTFE: New Entrant as a Very Promising EP Additive. Tribol. Int. 2015, 87, 121–131. [Google Scholar] [CrossRef]
- Zheng, Q.; Chhattal, M.; Bai, C.; Zheng, Z.; Qiao, D.; Gong, Z.; Zhang, J. Superlubricity of PTFE Triggered by Green Ionic Liquids. Appl. Surf. Sci. 2023, 614, 156241. [Google Scholar] [CrossRef]
- Fan, M.; Jin, Y.; Han, Y.; Ma, L.; Li, W.; Lu, Y.; Zhou, F.; Liu, W. The Effect of Chemical Structure on the Tribological Performance of Perfluorosulfonate ILs as Lubricants for Ti-6Al-4V Tribopairs. J. Mol. Liq. 2021, 321, 114286. [Google Scholar] [CrossRef]
- Rico, E.F.; Minondo, I.; Cuervo, D.G. The Effectiveness of PTFE Nanoparticle Powder as an EP Additive to Mineral Base Oils. Wear 2007, 262, 1399–1406. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Qi, Y.; Cai, W.; Jiang, Z. Self-Lubricating Al2O3/PTFE Composite Coating Formation on Surface of Aluminium Alloy. Surf. Coat. Technol. 2010, 204, 3315–3318. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Kannan, K. Occurrence, Distribution and Human Exposure to 20 Organophosphate Esters in Air, Soil, Pine Needles, River Water, and Dust Samples Collected around an Airport in New York State, United States. Environ. Int. 2019, 131, 105054. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine Disrupting Chemicals in Mixture and Obesity, Diabetes and Related Metabolic Disorders. World J. Biol. Chem. 2017, 8, 108–119. [Google Scholar] [CrossRef] [PubMed]
- USA Lubricants Market Report 2018; Research and Markets: Dublin, Ireland, 2019.
- Wang, Y.; Munir, U.; Huang, Q. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Soil: Sources, Fate, and Remediation. Soil Environ. Health 2023, 1, 100004. [Google Scholar] [CrossRef]
- Su, A.; Rajan, K. A Database Framework for Rapid Screening of Structure-Function Relationships in PFAS Chemistry. Sci. Data 2021, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Frömel, T.; Knepper, T.P. Biodegradation of Fluorinated Alkyl Substances. Rev. Environ. Contam. Toxicol. 2010, 208, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 602040. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; Mutanda, I.; Taolin, J.; Qaria, M.A.; Yang, B.; Zhu, D. A Review of Microbial Degradation of Per- and Polyfluoroalkyl Substances (PFAS): Biotransformation Routes and Enzymes. Sci. Total Environ. 2023, 859, 160010. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Wu, S.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of Perfluoroalkyl Substances (PFAS) in Groundwater by Granular Activated Carbons: Roles of Hydrophobicity of PFAS and Carbon Characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing Ionic Strength and Valency of Cations Enhance Sorption through Hydrophobic Interactions of PFAS with Soil Surfaces. Sci. Total Environ. 2022, 817, 152975. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M.N. Recent Advances on PFAS Degradation via Thermal and Nonthermal Methods. Chem. Eng. J. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the Applications, Environmental Release, and Remediation Technologies of per-and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, T. Lubricating Performance of Polytetrafluoroethylene Hybrid Fabric Composites with Removed Thermosetting Resin at Cryogenic Temperatures. J. Appl. Polym. Sci. 2023, 140, e53935. [Google Scholar] [CrossRef]
- Liu, S.B.; Gong, H.; Qian, Y.; Zhao, J.B.; Ye, H.; Zhang, Z. The Friction and Wear Performance of Polytetrafluoroethylene Coating Reinforced with Modified Graphene. Mater. Today Commun. 2022, 31, 103448. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Liu, K.; Xu, J.; Lu, Y.; Ye, J. Mechanochemical Functionality of Graphene Additives in Ultralow Wear Polytetrafluoroethylene Composites. Carbon 2021, 184, 312–321. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y. The Functionalization of Fluoroelastomers: Approaches, Properties, and Applications. RSC Adv. 2016, 6, 53730–53748. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-Chain per- and Polyfluoroalkyl Substances in Aquatic Systems: Occurrence, Impacts and Treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Ohno, N.; Rahman, M.D.Z.; Syusukeyamada; Komiya, H. Effect of Perfluoropolyether Fluids on Life of Thrust Ball Bearings. Tribol. Trans. 2009, 52, 492–500. [Google Scholar] [CrossRef]
- Liang, J.; Helmick, L.S. Tribochemistry of a Pfpae Fluid on M-50 Surfaces by Ftir Spectroscopy. Tribol. Trans. 1996, 39, 705–709. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Panchangam, S.C.; Lo, C.C. The Impact of Semiconductor, Electronics and Optoelectronic Industries on Downstream Perfluorinated Chemical Contamination in Taiwanese Rivers. Environ. Pollut. 2009, 157, 1365–1372. [Google Scholar] [CrossRef]
- Pozo, K.; Moreira, L.B.; Karaskova, P.; Přibylová, P.; Klánová, J.; de Carvalho, M.U.; Maranho, L.A.; de Souza Abessa, D.M. Using Large Amounts of Firefighting Foams Releases Per- and Polyfluoroalkyl Substances (PFAS) into Estuarine Environments: A Baseline Study in Latin America. Mar. Pollut. Bull. 2022, 182, 113938. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, X.; Boiteux, V.; Bach, C.; Rosin, C.; Munoz, J.F. Per- and Polyfluoroalkyl Substances in Firefighting Foam Concentrates and Water Samples Collected near Sites Impacted by the Use of These Foams. Chemosphere 2017, 183, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Alsmeyer, Y.W.; Childs, W.V.; Flynn, R.M.; Moore, G.G.I.; Smeltzer, J.C. Electrochemical Fluorination and Its Applications. In Organofluorine Chemistry; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Ebnesajjad, S. Synthesis and Properties of Monomers of Thermoplastic Fluoropolymers. In Technology of Fluoropolymers; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Dhanumalayan, E.; Joshi, G.M. Performance Properties and Applications of Polytetrafluoroethylene (PTFE)—A Review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Nishioka, A.; Matsumae, K.; Watanabe, M.; Tajima, M.; Owaki, M. Effects of Gamma Radiation on Some Physical Properties of Polytetrafluoroethylene Resin. J. Appl. Polym. Sci. 1959, 2, 114–119. [Google Scholar] [CrossRef]
- Ariawan, A.B.; Ebnesajjad, S.; Hatzikiriakos, S.G. Preforming Behavior of Polytetrafluoroethylene Paste. Powder Technol. 2001, 121, 249–258. [Google Scholar] [CrossRef]
- Yan, L.; Chao, M.; Xiao, J.; Gao, L.; Wieβner, S. Study on Preparation of BaSO4-Containing Polytetrafluoroethylene Granular Powder. Adv. Polym. Technol. 2017, 36, 418–423. [Google Scholar] [CrossRef]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef] [PubMed]
- Aderikha, V.N.; Krasnov, A.P.; Naumkin, A.V.; Shapovalov, V.A. Effects of Ultrasound Treatment of Expanded Graphite (EG) on the Sliding Friction, Wear Resistance, and Related Properties of PTFE-Based Composites Containing EG. Wear 2017, 386–387, 63–71. [Google Scholar] [CrossRef]
- Kirillina, I.V.; Nikiforov, L.A.; Okhlopkova, A.A.; Sleptsova, S.A.; Yoon, C.; Cho, J.H. Nanocomposites Based on Polytetrafluoroethylene and Ultrahigh Molecular Weight Polyethylene: A Brief Review. Bull. Korean Chem. Soc. 2014, 35, 3411–3420. [Google Scholar] [CrossRef]
- Huang, A.; Peng, X.; Turng, L.S. In-Situ Fibrillated Polytetrafluoroethylene (PTFE) in Thermoplastic Polyurethane (TPU) via Melt Blending: Effect on Rheological Behavior, Mechanical Properties, and Microcellular Foamability. Polymer 2018, 134, 263–274. [Google Scholar] [CrossRef]
- Yin, Z.; Tian, B.; Zhu, Q.; Duan, C. Characterization and Application of PVDF and Its Copolymer Films Prepared by Spin-Coating and Langmuir-Blodgett Method. Polymers 2019, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.; Solard, J.; Nong, H.T.T.; Ben Osman, C.; Gomez, A.; Bockelée, V.; Tencé-Girault, S.; Schoenstein, F.; Simón-Sorbed, M.; Carrillo, A.E.; et al. Spin Coating and Micro-Patterning Optimization of Composite Thin Films Based on PVDF. Materials 2020, 13, 1342. [Google Scholar] [CrossRef]
- Cozza, E.S.; Monticelli, O.; Marsano, E.; Cebe, P. On the Electrospinning of PVDF: Influence of the Experimental Conditions on the Nanofiber Properties. Polym. Int. 2013, 62, 41–48. [Google Scholar] [CrossRef]
- He, Z.; Rault, F.; Lewandowski, M.; Mohsenzadeh, E.; Salaün, F. Electrospun PVDF Nanofibers for Piezoelectric Applications: A Review of the Influence of Electrospinning Parameters on the β Phase and Crystallinity Enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric Pvdf Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Y.; Li, Q.; Wei, L.; Guan, S. Influence of Polytetrafluoroethylene (PTFE) Content on Mechanical and Tribological Properties of Poly(Ether Ether Ketone)/PTFE Coatings Prepared by Electrostatic Powder Spraying Technique. High Perform. Polym. 2015, 27, 3–9. [Google Scholar] [CrossRef]
- Weng, R.; Zhang, H.; Liu, X. Spray-Coating Process in Preparing PTFE-PPS Composite Super-Hydrophobic Coating. AIP Adv. 2014, 4, 031327. [Google Scholar] [CrossRef]
- Leivo, E.; Wilenius, T.; Kinos, T.; Vuoristo, P.; Mäntylä, T. Properties of Thermally Sprayed Fluoropolymer PVDF, ECTFE, PFA and FEP Coatings. Prog. Org. Coat. 2004, 49, 69–73. [Google Scholar] [CrossRef]
- Buck, R.C.; Murphy, P.M.; Pabon, M. Chemistry, Properties, and Uses of Commercial Fluorinated Surfactants. In Polyfluorinated Chemicals and Transformation Products; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zhu, H.; Kannan, K. A Pilot Study of Per- and Polyfluoroalkyl Substances in Automotive Lubricant Oils from the United States. Environ. Technol. Innov. 2020, 19, 100943. [Google Scholar] [CrossRef]
- Saleh, S.M.; Alminderej, F.M.; Mohamed, A.M.A. Superhydrophobic and Corrosion Behaviour of PVDF-CeO2 Composite Coatings. Materials 2022, 15, 8674. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Hafezi, M.; Tong, Z.; Qin, L. Preparation and Oil Lubrication of Polyvinylidene Fluoride (PVDF) Nanospheres. Mater. Res. Express 2019, 6, 085093. [Google Scholar] [CrossRef]
- Tansel, B. PFAS Use in Electronic Products and Exposure Risks during Handling and Processing of E-Waste: A Review. J. Environ. Manag. 2022, 316, 115291. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C.M.; et al. Microplastic Ingestion Ubiquitous in Marine Turtles. Glob. Chang. Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef]
- Helmer, R.W.; Reeves, D.M.; Cassidy, D.P. Per- and Polyfluorinated Alkyl Substances (PFAS) Cycling within Michigan: Contaminated Sites, Landfills and Wastewater Treatment Plants. Water Res. 2022, 210, 117983. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, K.; Yu, J.; Zhang, Q.; Zhang, Y.; Valix, M.; Tsang, D.C.W. Challenges in Recycling Spent Lithium-Ion Batteries: Spotlight on Polyvinylidene Fluoride Removal. Glob. Chall. 2023, 7, 2200237. [Google Scholar] [CrossRef] [PubMed]
- Rajeevan, S.; John, S.; George, S.C. Polyvinylidene Fluoride: A Multifunctional Polymer in Supercapacitor Applications. J. Power Sources 2021, 504, 230037. [Google Scholar] [CrossRef]
- Salam, M.A.R.B.A.; Rahman, M.A.; Kabir, M.H.; Alvarado, E.V.; Sadman, T.; Mahamud, R.; Cano, L.; Ashraf, A. Testing and Modeling of an in Situ Shear Exfoliated 2D Nanocomposite Coating Casing Material for the Suppression of Li-Ion Battery Fires in Electric Vehicles. MRS Adv. 2023, 8, 953–959. [Google Scholar] [CrossRef]
- McMillan, R.; Slegr, H.; Shu, Z.X.; Wang, W. Fluoroethylene Carbonate Electrolyte and Its Use in Lithium Ion Batteries with Graphite Anodes. J. Power Sources 1999, 81–82, 20–26. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Zhong, W.; Ge, Z.; Li, L.; Lei, S.; Wu, Q.; Zhang, H.; Cheng, S.; Xie, J. Non-Flammable Fluorobenzene-Diluted Highly Concentrated Electrolytes Enable High-Performance Li-Metal and Li-Ion Batteries. J. Colloid Interface Sci. 2022, 619, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Zhang, H. Nafion/PTFE Composite Membranes for Fuel Cell Applications. J. Membr. Sci. 2003, 212, 213–223. [Google Scholar] [CrossRef]
- Benipal, N.; Qi, J.; Gentile, J.C.; Li, W. Direct Glycerol Fuel Cell with Polytetrafluoroethylene (PTFE) Thin Film Separator. Renew. Energy 2017, 105, 647–655. [Google Scholar] [CrossRef]
- Scheel, K.-C.; Püschner, M. PFAS in Automotive Technologies of the Future; Verband der Automobilindustrie e.V.: Berlin, Germany, 2021. [Google Scholar]
- Bulson, E.E.; Remucal, C.K.; Hicks, A.L. End-of-Life Circulation of PFAS in Metal Recycling Streams: A Sustainability-Focused Review. Resour. Conserv. Recycl. 2023, 194, 106978. [Google Scholar] [CrossRef]
- Wickersham, L.C.; Mattila, J.M.; Krug, J.D.; Jackson, S.R.; Wallace, M.A.G.; Shields, E.P.; Halliday, H.; Li, E.Y.; Liberatore, H.K.; Farrior, S.; et al. Characterization of PFAS Air Emissions from Thermal Application of Fluoropolymer Dispersions on Fabrics. J. Air Waste Manag. Assoc. 2023, 73, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.E.; Gheisari, R.; Polycarpou, A.A. Tribology Review of Blended Bulk Polymers and Their Coatings for High-Load Bearing Applications. Tribol. Int. 2019, 129, 92–111. [Google Scholar] [CrossRef]
- Kianfar, P.; Bongiovanni, R.; Ameduri, B.; Vitale, A. Electrospinning of Fluorinated Polymers: Current State of the Art on Processes and Applications. Polym. Rev. 2023, 63, 127–199. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Perez, G.; Goss, J.A.; Beckford, S.; Zou, M. Tribological Properties of PDA + PTFE Coating in Oil-Lubricated Condition. Appl. Surf. Sci. 2020, 534, 147627. [Google Scholar] [CrossRef]
- Inderherbergh, J. Polyvinylidene Fluoride (PVDF) Appearance, General Properties and Processing. Ferroelectrics 1991, 115, 295–302. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wedel, A.; Danz, R. Charge Storage and Its Dynamics in Porous Polytetrafluoroethylene (PTFE) Film Electrets. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 102–108. [Google Scholar] [CrossRef]
- Light, D.N.; Wilcox, J.R. Process Considerations in the Fabrication of Fluoropolymer Printed Circuit Boards. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1995, 18, 118–126. [Google Scholar] [CrossRef]
- Catanese, J.; Cooke, D.; Maas, C.; Pruitt, L. Mechanical Properties of Medical Grade Expanded Polytetrafluoroethylene: The Effects of Internodal Distance, Density, and Displacement Rate. J. Biomed. Mater. Res. 1999, 48, 187–192. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- McCook, N.L.; Burris, D.L.; Dickrell, P.L.; Sawyer, W.G. Cryogenic Friction Behavior of PTFE Based Solid Lubricant Composites. Tribol. Lett. 2005, 20, 109–113. [Google Scholar] [CrossRef]
- Thomas, P. The Use of Fluoropolymers for Non-Stick Cooking Utensils. JOCCA Surf. Coat. Int. 1998, 81, 604–609. [Google Scholar] [CrossRef]
- Storgårds, E.; Simola, H.; Sjöberg, A.M.; Wirtanen, G. Hygiene of Gasket Materials Used in Food Processing Equipment Part 2: Aged Materials. Food Bioprod. Process. Trans. Inst. Chem. Eng. Part C 1999, 77, 146–155. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Zhang, F.; Wang, Y.; Xing, W. Amphiphobic Polytetrafluoroethylene Membranes for Efficient Organic Aerosol Removal. ACS Appl. Mater. Interfaces 2016, 8, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lopez, J.; Alpuche-Aviles, M.A.; Bard, A.J. Selective Insulation with Poly(Tetrafluoroethylene) of Substrate Electrodes for Electrochemical Background Reduction in Scanning Electrochemical Microscopy. Anal. Chem. 2008, 80, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, L.; Xie, G.; Guo, Y.; Si, L.; Luo, J. Friction and Wear Behavior of PTFE Coatings Modified with Poly (Methyl Methacrylate). Compos. B Eng. 2019, 172, 316–322. [Google Scholar] [CrossRef]
- Miller, C.; Choudhury, D.; Zou, M. The Effects of Surface Roughness on the Durability of Polydopamine/PTFE Solid Lubricant Coatings on NiTiNOL 60. Tribol. Trans. 2019, 62, 919–929. [Google Scholar] [CrossRef]
- Mohammadpourfazeli, S.; Arash, S.; Ansari, A.; Yang, S.; Mallick, K.; Bagherzadeh, R. Future Prospects and Recent Developments of Polyvinylidene Fluoride (PVDF) Piezoelectric Polymer; Fabrication Methods, Structure, and Electro-Mechanical Properties. RSC Adv. 2023, 13, 370–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, K.; Chai, B.; Qiao, S.; Huang, Z.; Jiang, P.; Huang, X. Core-Shell Structured Silk Fibroin/PVDF Piezoelectric Nanofibers for Energy Harvesting and Self-Powered Sensing. Nano Mater. Sci. 2022, 4, 126–132. [Google Scholar] [CrossRef]
- Lee, H.; Bhushan, B. Nanotribology of Polyvinylidene Difluoride (PVDF) in the Presence of Electric Field. J. Colloid Interface Sci. 2011, 360, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, M.; Wermelinger, J.; Setz, W.; Müller, D. Suitability of Polyvinylidene Fluoride (PVDF) Piping in Pharmaceutical Ultrapure Water Applications. PDA J. Pharm. Sci. Technol. 1996, 50, 246–251. [Google Scholar] [PubMed]
- Liu, R.; Yuan, B.; Zhong, S.; Liu, J.; Dong, L.; Ji, Y.; Dong, Y.; Yang, C.; He, W. Poly(Vinylidene Fluoride) Separators for Next-generation Lithium Based Batteries. ano Sel. 2021, 2, 2308–2345. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, D. Numerical Analysis of Piezoelectric Signal of PVDF Membrane Flapping Wing in Flight. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012090. [Google Scholar] [CrossRef]
- Ahmad, Z.; Prasad, A.; Prasad, K. A Comparative Approach to Predicting Effective Dielectric, Piezoelectric and Elastic Properties of PZT/PVDF Composites. Phys. B Condens. Matter 2009, 404, 3637–3644. [Google Scholar] [CrossRef]
- Hussein, A.A.; Dawood, N.M.; Al-Kawaz, A.E. Corrosion Protection of 316L Stainless Steel by (PVDF/HA) Composite Coating Using a Spinning Coating Technique. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69, e136810. [Google Scholar] [CrossRef]
- Remskar, M.; Jelenc, J.; Visic, B.; Varlec, A.; Cesarek, M.; Krzan, A. Friction Properties of Polyvinylidene Fluoride with Added MoS2 Nanotubes. Phys. Status Solidi A Appl. Mater. Sci. 2013, 210, 2314–2319. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Dysart, A.D.; Shaffer, S.J.; Pol, V.G.; Stacke, L.E.; Sadeghi, F. Novel Tertiary Dry Solid Lubricant on Steel Surfaces Reduces Significant Friction and Wear under High Load Conditions. Carbon 2017, 123, 7–17. [Google Scholar] [CrossRef]
- Clausi, M.; Grasselli, S.; Malchiodi, A.; Bayer, I.S. Thermally Conductive PVDF-Graphene Nanoplatelet (GnP) Coatings. Appl. Surf. Sci. 2020, 529, 147070. [Google Scholar] [CrossRef]
- Park, M.S.; Sung, H.S.; Park, C.H.; Han, T.S.; Kim, J.H. High Tribology Performance of Poly(Vinylidene Fluoride) Composites Based on Three-Dimensional Mesoporous Magnesium Oxide Nanosheets. Compos. B Eng. 2019, 163, 224–235. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, M.S.; Lee, C.S.; Han, T.S.; Kim, J.H. Wear-Resistant Carbon Nanorod-Embedded Poly(Vinylidene Fluoride) Composites with Excellent Tribological Performance. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105721. [Google Scholar] [CrossRef]
- Wang, H.G.; Ren, J.F.; Jian, L.Q.; Pan, B.L.; Zhang, J.Y.; Yang, S.R. Friction and Wear Behavior of Polyamide 66/Poly(Vinylidene Fluoride) Blends. J. Macromol. Sci. Part B Phys. 2008, 47, 701–711. [Google Scholar] [CrossRef]
- Liang, L.; Ma, Y.; Ji, X.; Ma, J.; Zhang, W.; Song, L. The Sustainable Recycling of Polyvinylidene Fluoride Membrane for Tribological Application. J. Appl. Polym. Sci. 2023, 140, e54287. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, E.; Yuan, R.; Gao, D.; Zhang, X.; Zhu, Y. A Robust Superhydrophobic PVDF Composite Coating with Wear/Corrosion-Resistance Properties. Appl. Surf. Sci. 2015, 332, 518–524. [Google Scholar] [CrossRef]
- Qu, M.; Yao, Y.; He, J.; Ma, X.; Feng, J.; Liu, S.; Hou, L.; Liu, X. Tribological Study of Polytetrafluoroethylene Lubricant Additives Filled with Cu Microparticles or SiO2 Nanoparticles. Tribol. Int. 2017, 110, 57–65. [Google Scholar] [CrossRef]
- Kumar Dubey, M.; Bijwe, J.; Ramakumar, S.S.V. PTFE Based Nano-Lubricants. Wear 2013, 306, 80–88. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Li, J.; Dong, G. Preparation and Lubricating Properties of Poly(Vinylidene-Fluoride) Particles Wrapped by Reduced Graphene Oxide. Tribol. Int. 2018, 127, 351–360. [Google Scholar] [CrossRef]
- Zeng, Q. Superlow Friction and Diffusion Behaviors of a Steel-Related System in the Presence of Nano Lubricant Additive in PFPE Oil. J. Adhes. Sci. Technol. 2019, 33, 1001–1018. [Google Scholar] [CrossRef]
- Fan, X.; Li, W.; Li, H.; Zhu, M.; Xia, Y.; Wang, J. Probing the Effect of Thickener on Tribological Properties of Lubricating Greases. Tribol. Int. 2018, 118, 128–139. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Liu, Y.; Wang, L. The Effect of Electron Irradiation on the Tribological Property of Perfluoropolyether Grease in Vacuum. J. Fluor. Chem. 2015, 175, 114–120. [Google Scholar] [CrossRef]
- Gupta, T.C.S.M.; Kumar, A.; Prasad, B. Sustainable Lubrication: Low Molecular Weight PTFE Micro-Particles as Extreme Pressure Additives for Heavy Duty Grease Applications. Ind. Lubr. Tribol. 2021, 73, 1209–1218. [Google Scholar] [CrossRef]
- Papay, A.G. Antiwear and Extreme-Pressure Additives in Lubricants. Lubr. Sci. 1998, 10, 209–224. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z.; et al. Extreme Pressure and Antiwear Additives for Lubricant: Academic Insights and Perspectives. Int. J. Adv. Manuf. Technol. 2022, 120, 1–27. [Google Scholar] [CrossRef]
- Saini, V.; Bijwe, J.; Seth, S.; Ramakumar, S.S.V. Role of Base Oils in Developing Extreme Pressure Lubricants by Exploring Nano-PTFE Particles. Tribol. Int. 2020, 143, 106071. [Google Scholar] [CrossRef]
- Gumprecht, W.H. Pr-143—A New Class of High-Temperature Fluids. ASLE Trans. 1966, 9, 24–30. [Google Scholar] [CrossRef]
- Caporiccio, G.; Flabbi, L.; Marchionni, G.; Viola, G.T. The Properties and Applications of Perfluoropolyether Lubricants. J. Synth. Lubr. 1989, 6, 133–149. [Google Scholar] [CrossRef]
- Snyder, C.E.; Gschwender, L.J. Fluoropolymers in Fluid and Lubricant Applications. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 383–386. [Google Scholar] [CrossRef]
- Nyberg, E.; Schneidhofer, C.; Pisarova, L.; Dörr, N.; Minami, I. Ionic Liquids as Performance Ingredients in Space Lubricants. Molecules 2021, 26, 1013. [Google Scholar] [CrossRef]
- Jung, Y.; Yeo, C. Mechano-Chemical Properties and Tribological Performance of Thin Perfluoropolyether (PFPE) Lubricant Film under Environmental Contaminants. Lubricants 2023, 11, 306. [Google Scholar] [CrossRef]
- Gleirscher, M.; Wolfberger, A.; Schlögl, S.; Hołyńska, M.; Hausberger, A. Accelerated Thermo-Catalytic Degradation of Perfluoropolyether (PFPE) Lubricants for Space Applications. Lubricants 2023, 11, 81. [Google Scholar] [CrossRef]
- Tao, Z.; Bhushan, B. Bonding, Degradation, and Environmental Effects on Novel Perfluoropolyether Lubricants. Wear 2005, 259, 1352–1361. [Google Scholar] [CrossRef]
- Wolfberger, A.; Hausberger, A.; Schlögl, S.; Hołyńska, M. Assessment of the Chemical Degradation of PFPE Lubricants and Greases for Space Applications: Implications for Long-Term on-Ground Storage. CEAS Space J. 2021, 13, 377–388. [Google Scholar] [CrossRef]
- Sinha, S.K.; Kawaguchi, M.; Kato, T.; Kennedy, F.E. Wear Durability Studies of Ultra-Thin Perfluoropolyether Lubricant on Magnetic Hard Disks. Tribol. Int. 2003, 36, 217–225. [Google Scholar] [CrossRef]
- He, Y.; Fujikawa, Y.; Zhang, H.; Fukuzawa, K.; Mitsuya, Y. Evaluations of Tribological Characteristics of PFPE Lubricants on DLC Surfaces of Magnetic Disks. Tribol. Lett. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Gow, G. Lubricating Grease. In Chemistry and Technology of Lubricants; Mortier, R.M., Fox, M.F., Orszulik, S.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 411–432. ISBN 978-1-4020-8662-5. [Google Scholar]
- Derosa, T.F.; Kaufman, B.J.; Sung, R.L.; Russo, J.M. Dissolution of Perfluoroalkyl Oligomers in Lubricating Oil for Enhancing Wear Resistance and Fuel Economy. J. Appl. Polym. Sci. 1994, 51, 1339–1346. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, Y.; Dong, X.; Baqar, M.; Fang, B.; Chen, H.; Sun, H. Nontarget Identification of Novel Per- and Polyfluoroalkyl Substances (PFAS) in Soils from an Oil Refinery in Southwestern China: A Combined Approach with TOP Assay. Environ. Sci. Technol. 2023, 57, 20194–20205. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.M.; Ye, C.; Phillips, B.S.; Zabinski, J.S.; Liu, X.; Liu, W.; Shreeve, J.M. Polyethylene Glycol Functionalized Dicationic Ionic Liquids with Alkyl or Polyfluoroalkyl Substituents as High Temperature Lubricants. J. Mater. Chem. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Wang, B.; Moran, C.; Lin, D.; Tang, H.; Gage, E.; Li, L. Nanometer-Thick Fluorinated Ionic Liquid Films as Lubricants in Data-Storage Devices. ACS Appl. Nano Mater. 2019, 2, 5260–5265. [Google Scholar] [CrossRef]
- Lertola, A.C.; Wang, B.; Li, L. Understanding the Friction of Nanometer-Thick Fluorinated Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 11681–11685. [Google Scholar] [CrossRef]
- Blanco, D.; González, R.; Viesca, J.L.; Fernández-González, A.; Bartolomé, M.; Hernández Battez, A. Antifriction and Antiwear Properties of an Ionic Liquid with Fluorine-Containing Anion Used as Lubricant Additive. Tribol. Lett. 2017, 65, 66. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, C.; Guo, Y.; Zhang, R.; Lin, L.; Yang, D.; Zhou, F.; Liu, W. An Investigation on the Friction and Wear Properties of Perfluorooctane Sulfonate Ionic Liquids. Tribol. Lett. 2016, 63, 11. [Google Scholar] [CrossRef]
- Romanova, N.V.; Shafigullin, L.N.; Buyatova, S.G. Study of the Local and Imported Rubber Products Made from Fluoroelastomer. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1079, 022027. [Google Scholar] [CrossRef]
- Améduri, B. Fluoropolymers as Unique and Irreplaceable Materials: Challenges and Future Trends in These Specific Per or Poly-Fluoroalkyl Substances. Molecules 2023, 28, 7564. [Google Scholar] [CrossRef] [PubMed]
- Kalfayan, S.H.; Mazzeo, A.A.; Silver, R.H. Long-Term Aging of Elastomers. Chemical Stress Relaxation of Fluorosilicone Rubber and Other Studies. JPL Quart. Tech. Rev. 1971, 1, 38–47. [Google Scholar]
- Hull, D.; Eggers, R.; Wellner, S. TFE/P Based Elastomers Expand Seal/Lubricant Compatibility; SAE Technical Paper 920709; SAE International: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J.R. Comparison of PVDF and PVDF-TFE-P as Binders for Electrode Materials Showing Large Volume Changes in Lithium-Ion Batteries. J. Electrochem. Soc. 2003, 150, A1073–A1078. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Pourrahimi, A.M.; Sjöstedt, C.; Bellander, M.; Hedenqvist, M.S.; Gedde, U.W. Degradation of Fluoroelastomers in Rapeseed Biodiesel at Different Oxygen Concentrations. Polym. Degrad. Stab. 2017, 136, 10–19. [Google Scholar] [CrossRef]
- Wu, F.; Chen, B.; Pan, M. Degradation of the Sealing Silicone Rubbers in a Proton Exchange Membrane Fuel Cell at Cold Start Conditions. Int. J. Electrochem. Sci. 2020, 15, 3013–3028. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; He, A. Insights into the Effects of High-Temperature Lubricating Oils on the Aging Behavior and Degradation Mechanism of Fluoroelastomers. Polym. Eng. Sci. 2023, 63, 2371–2384. [Google Scholar] [CrossRef]
- Wang, Q.L.; Pei, J.K.; Li, G.; He, X.; Niu, Y.H.; Li, G.X. Accelerated Aging Behaviors and Mechanism of Fluoroelastomer in Lubricating Oil Medium. Chin. J. Polym. Sci. (Engl. Ed.) 2020, 38, 853–866. [Google Scholar] [CrossRef]
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and Health Effects of Lubricant Oils Emitted into the Environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. [Google Scholar] [CrossRef]
- Diphare, M.J.; Pilusa, J.; Muzenda, E.; Mollagee, M. A Review of Waste Lubricating Grease Management. In Proceedings of the 2nd International Conference on Environment, Agriculture and Food Sciences (ICEAFS’2013), Kuala Lumpur, Malaysia, 6–7 May 2013; Available online: https://api.semanticscholar.org/CorpusID:38915437 (accessed on 22 January 2024).
- Brusseau, M.L.; Anderson, R.H.; Guo, B. PFAS Concentrations in Soils: Background Levels versus Contaminated Sites. Sci. Total. Environ. 2020, 740, 140017. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Shyu, D.J.H.; Domínguez, R.; Lorenzo, J.M.; Pereira, J.A.M.; Câmara, J.S. Polystyrene Microplastic Particles in the Food Chain: Characteristics and Toxicity. A Review. Sci. Total. Environ. 2023, 892, 164531. [Google Scholar] [CrossRef] [PubMed]
- Smalling, K.L.; Romanok, K.M.; Bradley, P.M.; Morriss, M.C.; Gray, J.L.; Kanagy, L.K.; Gordon, S.E.; Williams, B.M.; Breitmeyer, S.E.; Jones, D.K.; et al. Per- and Polyfluoroalkyl Substances (PFAS) in United States Tapwater: Comparison of Underserved Private-Well and Public-Supply Exposures and Associated Health Implications. Environ. Int. 2023, 178, 108033. [Google Scholar] [CrossRef] [PubMed]
- MDH. MDH Evaluation of Point-of-Use Water Treatment Devices for Perfluorochemical Removal; Minnesota Department of Health: St. Paul, MN, USA, 2008.
- Wang, P.; Zhang, M.; Li, Q.; Lu, Y. Atmospheric Diffusion of Perfluoroalkyl Acids Emitted from Fluorochemical Industry and Its Associated Health Risks. Environ. Int. 2021, 146, 106247. [Google Scholar] [CrossRef] [PubMed]
- Schlummer, M.; Sölch, C.; Meisel, T.; Still, M.; Gruber, L.; Wolz, G. Emission of Perfluoroalkyl Carboxylic Acids (PFCA) from Heated Surfaces Made of Polytetrafluoroethylene (PTFE) Applied in Food Contact Materials and Consumer Products. Chemosphere 2015, 129, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per- and Polyfluoroalkyl Substances (PFASs) in Water, Soil and Plants in Wetlands and Agricultural Areas in Kampala, Uganda. Sci. Total. Environ. 2018, 631–632, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, L.L.; Wojcinski, Z.W. PTFE (Polytetrafluoroethylene; Teflon®). Encycl. Toxicol. 2024, 7, 1001–1006. [Google Scholar] [CrossRef]
- Gaber, N.; Bero, L.; Woodruff, T.J. The Devil They Knew: Chemical Documents Analysis of Industry Influence on PFAS Science. Ann. Glob. Health 2023, 89, 37. [Google Scholar] [CrossRef]
- Angela, K. Oliver National Health and Nutrition Examination Survey (NHANES); National Center for Health Statistics: Hyattsville, MD, USA, 2022. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. ATSDR Toxicological Profiles. 2023. Available online: https://www.atsdr.cdc.gov/toxprofiledocs/index.html (accessed on 25 January 2024).
- Boston University School of Public Health. Toxicology. 2019. Available online: https://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH717-QuantCore/PH717-Module2-ExposureAssessment/PH717-Module2-ExposureAssessment6.html (accessed on 25 January 2024).
- U.S. Department of Health and Human Services and Agency for Toxic Substances and Disease Registry. Toxological Profile for Perfluoroalkyls. 2021. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 25 January 2024).
- Alexander, B.H.; Olsen, G.W.; Burris, J.M.; Mandel, J.H.; Mandel, J.S. Mortality of Employees of a Perfluorooctanesulphonyl Fluoride Manufacturing Facility. Occup. Environ. Med. 2003, 60, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Tinker, S.; Shankar, A.; Ducatman, A. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with Uric Acid among Adults with Elevated Community Exposure to PFOA. Environ. Health Perspect. 2010, 118, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Consonni, D.; Straif, K.; Symons, J.M.; Tomenson, J.A.; Van Amelsvoort, L.G.P.M.; Sleeuwenhoek, A.; Cherrie, J.W.; Bonetti, P.; Colombo, I.; Farrar, D.G.; et al. Cancer Risk among Tetrafluoroethylene Synthesis and Polymerization Workers. Am. J. Epidemiol. 2013, 178, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Budtz-Jørgensen, E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017, 125, 077018. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Cutler, A.J.; Jeddy, Z.; Northstone, K.; Kato, K.; Hartman, T.J. Maternal Serum Concentrations of Perfluoroalkyl Substances and Birth Size in British Boys. Int. J. Hyg. Environ. Health 2019, 222, 889–895. [Google Scholar] [CrossRef]
- Kang, J.S.; Ahn, T.G.; Park, J.W. Perfluorooctanoic Acid (PFOA) and Perfluooctane Sulfonate (PFOS) Induce Different Modes of Action in Reproduction to Japanese Medaka (Oryzias latipes). J. Hazard. Mater. 2019, 368, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Liu, S.; Lai, H.; Tu, W. Comparative Chronic Toxicities of PFOS and Its Novel Alternatives on the Immune System Associated with Intestinal Microbiota Dysbiosis in Adult Zebrafish. J. Hazard. Mater. 2022, 425, 127950. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a Mixture of Legacy, Alternative, and Replacement per- and Polyfluoroalkyl Substances (PFAS) Results in Sex-Dependent Modulation of Cholesterol Metabolism and Liver Injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef] [PubMed]
- Teunen, L.; Bervoets, L.; Belpaire, C.; De Jonge, M.; Groffen, T. PFAS Accumulation in Indigenous and Translocated Aquatic Organisms from Belgium, with Translation to Human and Ecological Health Risk. Environ. Sci. Eur. 2021, 33, 39. [Google Scholar] [CrossRef]
- Yu, S.; Feng, W.R.; Liang, Z.M.; Zeng, X.Y.; Bloom, M.S.; Hu, G.C.; Zhou, Y.; Ou, Y.Q.; Chu, C.; Li, Q.Q.; et al. Perfluorooctane Sulfonate Alternatives and Metabolic Syndrome in Adults: New Evidence from the Isomers of C8 Health Project in China. Environ. Pollut. 2021, 283, 117078. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Per- and Polyfluoroalkyl Substances (PFAS)—ECHA. 2023. Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 25 January 2024).
- Stockholm Convention. Stockholm Convention Overview. 2019. Available online: https://chm.pops.int/TheConvention/Overview/tabid/3351/Default.aspx (accessed on 25 January 2024).
- Stockholm Convention. PFASs Listed under the Stockholm Convention. 2023. Available online: https://chm.pops.int/Implementation/IndustrialPOPs/PFAS/Overview/tabid/5221/Default.aspx (accessed on 25 January 2024).
- United States Environmental Protection Agency. PFAS Strategic Roadmap: EPA’s Commitments to Action 2021–2024. 2021. Available online: https://www.epa.gov/system/files/documents/2021-10/pfas-roadmap_final-508.pdf (accessed on 25 January 2024).
- European Council. European Green Deal. 2023. Available online: https://www.consilium.europa.eu/en/policies/green-deal/ (accessed on 25 January 2024).
- European Council. European Green Deal: Commission Proposes Rules for Cleaner Air and Water. 2022. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_22_6278 (accessed on 25 January 2024).
- Glüge, J.; London, R.; Cousins, I.T.; DeWitt, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; et al. Information Requirements under the Essential-Use Concept: PFAS Case Studies. Environ. Sci. Technol. 2021, 56, 6232–6242. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Vierke, L.; et al. The Concept of Essential Use for Determining When Uses of PFASs Can Be Phased Out. Environ. Sci. Process. Impacts 2019, 21, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical Oxidation Remediation of Real Wastewater Effluents—A Review. Process Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical Advanced Oxidation Processes for Wastewater Treatment: Advances in Formation and Detection of Reactive Species and Mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Gamal El-Din, M. Insight into In-Situ Radical and Non-Radical Oxidative Degradation of Organic Compounds in Complex Real Matrix during Electrooxidation with Boron Doped Diamond Electrode: A Case Study of Oil Sands Process Water Treatment. Appl. Catal. B Environ. 2020, 279, 119366. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, O.; Mousset, E.; Olvera-Vargas, H.; Lefebvre, O. Electrochemical Treatment of Highly Concentrated Wastewater: A Review of Experimental and Modeling Approaches from Lab- to Full-Scale. Crit. Rev. Environ. Sci. Technol. 2022, 52, 240–309. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods. An Updated Review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical Oxidation of Organic Pollutants for the Wastewater Treatment: Direct and Indirect Processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Sivagami, K.; Sharma, P.; Karim, A.V.; Mohanakrishna, G.; Karthika, S.; Divyapriya, G.; Saravanathamizhan, R.; Kumar, A.N. Electrochemical-Based Approaches for the Treatment of Forever Chemicals: Removal of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) from Wastewater. Sci. Total. Environ. 2023, 861, 160440. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Kumar, A.; Syam Babu, D.; Scaria, J.; Suresh Kumar, M. Treatment of Mixed Industrial Wastewater by Electrocoagulation and Indirect Electrochemical Oxidation. Chemosphere 2020, 251, 126437. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Cañizares, P.; Carmona, M.; Lobato, J.; Martínez, F.; Rodrigo, M.A. Electrodissolution of Aluminum Electrodes in Electrocoagulation Processes. Ind. Eng. Chem. Res. 2005, 44, 4178–4185. [Google Scholar] [CrossRef]
- Kartikaningsih, D.; Shih, Y.J.; Huang, Y.H. Boron Removal from Boric Acid Wastewater by Electrocoagulation Using Aluminum as Sacrificial Anode. Sustain. Environ. Res. 2016, 26, 150–155. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of Textile Wastewaters by Electrocoagulation Using Iron and Aluminum Electrodes. J. Hazard. Mater. 2003, 100, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.H.; Hossain, M.Z.; Jalil, M.A.; Ghosh, S.; Hossain, M.M.; Ali, M.A.; Khandaker, M.U.; Jana, D.; Rahman, M.M.; Hossain, M.K.; et al. The Efficacy of Rare-Earth Doped V2O5 Photocatalyst for Removal of Pollutants from Industrial Wastewater. Opt. Mater. 2024, 147, 114724. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Boffito, D.C. Mechanisms and Pathways of PFAS Degradation by Advanced Oxidation and Reduction Processes: A Critical Review. Chem. Eng. J. 2022, 450, 138352. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Degradation of Pharmaceuticals by Ultrasound-Based Advanced Oxidation Process. Environ. Chem. Lett. 2016, 14, 259–290. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Ibrahim, S. Influence of Ultrasound Power on Acoustic Streaming and Micro-Bubbles Formations in a Low Frequency Sono-Reactor: Mathematical and 3D Computational Simulation. Ultrason. Sonochem. 2015, 24, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ince, N.H.; Tezcanli, G.; Belen, R.K.; Apikyan, G. Ultrasound as a Catalyzer of Aqueous Reaction Systems: The State of the Art and Environmental Applications. Appl. Catal. B Environ. 2001, 29, 167–176. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Arias Espana, V.A.; Mallavarapu, M.; Naidu, R. Treatment Technologies for Aqueous Perfluorooctanesulfonate (PFOS) and Perfluorooctanoate (PFOA): A Critical Review with an Emphasis on Field Testing. Environ. Technol. Innov. 2015, 4, 168–181. [Google Scholar] [CrossRef]
- Ciawi, E.; Rae, J.; Ashokkumar, M.; Grieser, F. Determination of Temperatures within Acoustically Generated Bubbles in Aqueous Solutions at Different Ultrasound Frequencies. J. Phys. Chem. B 2006, 110, 13656–13660. [Google Scholar] [CrossRef]
- Hori, H.; Nagaoka, Y.; Yamamoto, A.; Sano, T.; Yamashita, N.; Taniyasu, S.; Kutsuna, S.; Osaka, I.; Arakawa, R. Efficient Decomposition of Environmentally Persistent Perfluorooctanesulfonate and Related Fluorochemicals Using Zerovalent Iron in Subcritical Water. Environ. Sci. Technol. 2006, 40, 1049–1054. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lo, S.L.; Chiueh, P.T.; Chang, D.G. Efficient Decomposition of Perfluorocarboxylic Acids in Aqueous Solution Using Microwave-Induced Persulfate. Water Res. 2009, 43, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Nagaoka, Y.; Sano, T.; Kutsuna, S. Iron-Induced Decomposition of Perfluorohexanesulfonate in Sub- and Supercritical Water. Chemosphere 2008, 70, 800–806. [Google Scholar] [CrossRef]
- Hori, H.; Murayama, M.; Sano, T.; Kutsuna, S. Decomposition of Perfluorinated Ion-Exchange Membrane to Fluoride Ions Using Zerovalent Metals in Subcritical Water. Ind. Eng. Chem. Res. 2010, 49, 464–471. [Google Scholar] [CrossRef]
- Yi, L.B.; Chai, L.Y.; Xie, Y.; Peng, Q.J.; Peng, Q.Z. Isolation, Identification, and Degradation Performance of a PFOA-Degrading Strain. Genet. Mol. Res. 2016, 15, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium Sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Sima, M.; Long, Y.; Messenger, C.; Jaffé, P.R. Anaerobic Degradation of Perfluorooctanoic Acid (PFOA) in Biosolids by Acidimicrobium sp. Strain A6. J. Hazard. Mater. 2022, 424, 127699. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Urigüen, M.; Shuai, W.; Huang, S.; Jaffé, P.R. Biodegradation of PFOA in Microbial Electrolysis Cells by Acidimicrobiaceae sp. Strain A6. Chemosphere 2022, 292, 133506. [Google Scholar] [CrossRef] [PubMed]
- Key, B.D.; Howell, R.D.; Criddle, C.S. Defluorination of Organofluorine Sulfur Compounds by Pseudomonas sp. Strain D2. Environ. Sci. Technol. 1998, 32, 2283–2287. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lim, H.J.; Na, S.H.; Choi, B.I.; Shin, D.S.; Chung, S.Y. Biodegradation of Perfluorooctanesulfonate (PFOS) as an Emerging Contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Dinglasan, M.J.A.; Ye, Y.; Edwards, E.A.; Mabury, S.A. Fluorotelomer Alcohol Biodegradation Yields Poly- and Perfluorinated Acids. Environ. Sci. Technol. 2004, 38, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Wang, N.; Mcdonald, T.; Chu, K.H. Biodefluorination and Biotransformation of Fluorotelomer Alcohols by Two Alkane-Degrading Pseudomonas Strains. Biotechnol. Bioeng. 2012, 109, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Szostek, B.; Buck, R.C.; Folsom, P.W.; Sulecki, L.M.; Capka, V.; Berti, W.R.; Gannon, J.T. Fluorotelomer Alcohol Biodegradation—Direct Evidence That Perfluorinated Carbon Chains Breakdown. Environ. Sci. Technol. 2005, 39, 7516–7528. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Szostek, B.; Buck, R.C.; Folsom, P.W.; Sulecki, L.M.; Gannon, J.T. 8-2 Fluorotelomer Alcohol Aerobic Soil Biodegradation: Pathways, Metabolites, and Metabolite Yields. Chemosphere 2009, 75, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Su, Q.; Zhou, Z.; Liao, X.; Zou, J.; Yuan, B.; Sun, W. Anaerobic Biodegradation of 8:2 Fluorotelomer Alcohol in Anaerobic Activated Sludge: Metabolic Products and Pathways. Chemosphere 2018, 200, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Li, L.Y.; Grace, J.R. Aerobic Biotransformation of Fluorotelomer Compounds in Landfill Leachate-Sediment. Sci. Total. Environ. 2020, 713, 136547. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Takabe, Y.; Yamamoto, K.; Matsumura, C.; Nishimura, F. Biodegradation Property of 8:2 Fluorotelomer Alcohol (8:2 FTOH) under Aerobic/Anoxic/Anaerobic Conditions. J. Water Environ. Technol. 2016, 14, 177–190. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, K.; Li, Z.; Ren, C.; Chen, J.; Lin, Y.H.; Liu, J.; Men, Y. Microbial Cleavage of C-F Bonds in Two C6Per- And Polyfluorinated Compounds via Reductive Defluorination. Environ. Sci. Technol. 2020, 54, 14393–14402. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Che, S.; Ren, C.; Jin, B.; Tian, Z.; Liu, J.; Men, Y. Microbial Defluorination of Unsaturated Per- and Polyfluorinated Carboxylic Acids under Anaerobic and Aerobic Conditions: A Structure Specificity Study. Environ. Sci. Technol. 2022, 56, 4894–4904. [Google Scholar] [CrossRef] [PubMed]
- Goldman, P. The Enzymatic Cleavage of The Carbon-Fluorine Bond in Fluoroacetate. J. Biol. Chem. 1965, 240, 3434–3438. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.E.X.; Denman, S.E.; Hugenholtz, P.; McSweeney, C.S. Amino Acid and Peptide Utilization Profiles of the Fluoroacetate-Degrading Bacterium Synergistetes Strain MFA1 Under Varying Conditions. Microb. Ecol. 2016, 71, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.E.X.; Khan, S.; Davis, C.K.; Denman, S.E.; McSweeney, C.S. Fluoroacetate in Plants—A Review of Its Distribution, Toxicity to Livestock and Microbial Detoxification. J. Anim. Sci. Biotechnol. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.D. Biodegradation and Biotransformation of Organofluorine Compounds. Biotechnol. Lett. 2010, 32, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.J.; Kwon, S.W.; Seo, D.C.; Kim, J.H.; Jang, Y.S. Enzymatic Defluorination of Fluorinated Compounds. Appl. Biol. Chem. 2019, 62, 62. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Du, L.; Zhang, Q.; Wang, W. Catalytic Mechanism of C-F Bond Cleavage: Insights from QM/MM Analysis of Fluoroacetate Dehalogenase. Catal. Sci. Technol. 2016, 6, 73–80. [Google Scholar] [CrossRef]
- Dickman, R.A.; Aga, D.S. A Review of Recent Studies on Toxicity, Sequestration, and Degradation of per- and Polyfluoroalkyl Substances (PFAS). J. Hazard. Mater. 2022, 436, 129120. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The Overlooked Short- and Ultrashort-Chain Poly- and Perfluorinated Substances: A Review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, S.; Obare, S.O.; Zhang, L. Addressing Short-Chain PFAS Contamination in Water with Nanofibrous Adsorbent/Filter Material from Electrospinning. Acc. Chem. Res. 2023, 56, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Talha, M.; Ma, Y.; Xu, M.; Wang, Q.; Lin, Y.; Kong, X. Recent Advancements in Corrosion Protection of Magnesium Alloys by Silane-Based Sol-Gel Coatings. Ind. Eng. Chem. Res. 2020, 59, 19840–19857. [Google Scholar] [CrossRef]
- Chen, F.; Teniola, O.R.; Ogueri, K.S.; Laurencin, C.T. Recent Trends in the Development of Polyphosphazenes for Bio-Applications. Regen. Eng. Transl. Med. 2023, 9, 202–223. [Google Scholar] [CrossRef]
- Ma, W.; Lopez, G.; Ameduri, B.; Takahara, A. Fluoropolymer Nanoparticles Prepared Using Trifluoropropene Telomer Based Fluorosurfactants. Langmuir 2020, 36, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, A.; Liu, Z.; Ohsuna, T.; Terasaki, O.; Kuroda, K. Self-Assembly of Designed Oligomeric Siloxanes with Alkyl Chains into Silica-Based Hybrid Mesostructures. J. Am. Chem. Soc. 2005, 127, 14108–14116. [Google Scholar] [CrossRef] [PubMed]
- Suresh Babu, D.; Mol, J.M.C.; Buijnsters, J.G. Experimental Insights into Anodic Oxidation of Hexafluoropropylene Oxide Dimer Acid (GenX) on Boron-Doped Diamond Anodes. Chemosphere 2022, 288, 132417. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.U.; Khan, Z.A. Friction and Wear Performance Analysis of Hydrofluoroether-7000 Refrigerant. Tribol. Int. 2019, 139, 36–54. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.A.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications—A Review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouya, M.; Vahabi, H.; Barletta, M.; Laheurte, P.; Langlois, V. Additive Manufacturing of Polyhydroxyalkanoates (PHAs) Biopolymers: Materials, Printing Techniques, and Applications. Mater. Sci. Eng. C 2021, 127, 112216. [Google Scholar] [CrossRef] [PubMed]
- Nowak, T.; Mazela, B.; Olejnik, K.; Peplińska, B.; Perdoch, W. Starch-Silane Structure and Its Influence on the Hydrophobic Properties of Paper. Molecules 2022, 27, 3136. [Google Scholar] [CrossRef] [PubMed]
- Albright, V.; Penarete-Acosta, D.; Stack, M.; Zheng, J.; Marin, A.; Hlushko, H.; Wang, H.; Jayaraman, A.; Andrianov, A.K.; Sukhishvili, S.A. Polyphosphazenes Enable Durable, Hemocompatible, Highly Efficient Antibacterial Coatings. Biomaterials 2021, 268, 120586. [Google Scholar] [CrossRef] [PubMed]
- Peshoria, S.; Nandini, D.; Tanwar, R.K.; Narang, R. Short-Chain and Long-Chain Fluorosurfactants in Firefighting Foam: A Review. Environ. Chem. Lett. 2020, 18, 1277–1300. [Google Scholar] [CrossRef]
- He, J.; Zhou, L.; Soucek, M.D.; Wollyung, K.M.; Wesdemiotis, C. UV-Curable Hybrid Coatings Based on Vinylfunctionlized Siloxane Oligomer and Acrylated Polyester. J. Appl. Polym. Sci. 2007, 105, 2376–2386. [Google Scholar] [CrossRef]
- Peng, B.X.; Li, F.; Mortimer, M.; Xiao, X.; Ni, Y.; Lei, Y.; Li, M.; Guo, L.H. Perfluorooctanoic Acid Alternatives Hexafluoropropylene Oxides Exert Male Reproductive Toxicity by Disrupting Blood-Testis Barrier. Sci. Total. Environ. 2022, 846, 157313. [Google Scholar] [CrossRef] [PubMed]
- Vinš, V.; Aminian, A.; Celný, D.; Součková, M.; Klomfar, J.; Čenský, M.; Prokopová, O. Surface Tension and Density of Dielectric Heat Transfer Fluids of HFE Type-Experimental Data at 0.1 MPa and Modeling with PC-SAFT Equation of State and Density Gradient Theory. Int. J. Refrig. 2021, 131, 956–969. [Google Scholar] [CrossRef]
- Ovaskainen, L.; Rodriguez-Meizoso, I.; Birkin, N.A.; Howdle, S.M.; Gedde, U.; Wågberg, L.; Turner, C. Towards Superhydrophobic Coatings Made by Non-Fluorinated Polymers Sprayed from a Supercritical Solution. J. Supercrit. Fluids 2013, 77, 134–141. [Google Scholar] [CrossRef]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Kumar, P.S.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (Phas) and Pha-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef] [PubMed]
- Richard, R. Thomas Fluorinated Surfactant. In Chemistry and Technology of Surfactants; Farn, R.J., Ed.; Wiley: Hoboken, NJ, USA, 2006; Volume 1, pp. 227–235. [Google Scholar]
- Cirisano, F.; Ferrari, M. Superhydrophobicity and Durability in Recyclable Polymers Coating. Sustainability 2021, 13, 8244. [Google Scholar] [CrossRef]
- Améduri, B.; Hori, H. Recycling and the End of Life Assessment of Fluoropolymers: Recent Developments, Challenges and Future Trends. Chem. Soc. Rev. 2023, 52, 4208–4247. [Google Scholar] [CrossRef] [PubMed]
- pro-K Fluoropolymergroup. Recycling of Fluoropolymers. 2018. Available online: https://www.pro-kunststoff.de/fachwissen/recycling-of-fluoropolymers.html (accessed on 25 January 2024).






| Substrate | Coating | Thickness (μm) | Test Parameters | Performance | Refs |
|---|---|---|---|---|---|
| Aluminum | Al2O3 + PTFE | 33 | GCr15 Steel Ball ⌀ = 15 mm Force = 2 N RPM = 150 rpm | μ = 0.13 | [15] |
| 7050 Aluminum Alloy | PTFE/PMMA | 13.3 | GCr15 Steel ⌀ = 4.68 mm Load = 3 N Speed = 8.4 mm/s | μ = 0.069 ΔW = 1.04 × 10−6 mm3/N m | [89] |
| 60 NiTi | PDA + PTFE | 1.3 | Si3N4 Ball ⌀ = 6.35 mm Load = 2 N Speed = 10 mm/s | μ = 0.096 | [90] |
| Cast Iron | PDA + PTFE | 45 | Chrome-Steel Ball Load = 10 N Speed = 10 mm/s | μ = 0.05 | [77] |
| Gray Cast Iron | Pyrrolidone + PTFE | 20 | 52100 Steel Wrist Pins ⌀ = 8 mm Load = 445 N Speed = 0.22 m/s | μ = 0.043 W = 1.23 × 10−6 mm3/N m | [10] |
| MoS2 + PTFE | 20 | μ = 0.044 W = 3.76 × 10−7 mm3/N m |
| Substrate | Coating | Thickness (μm) | Test Parameters | Performance | Refs |
|---|---|---|---|---|---|
| Aluminum | 40 wt.% Graphene Nanoplatelet + PVDF | 15–20 | AISI52100 Steel Ball ⌀ = 9.5 mm Load = 5 N Speed = 24 mm/s | μ = 0.10 | [101] |
| AISI 316 | 2 wt.% of MoS2 + PVDF | - | AISI 316 Stainless steel ⌀ = 6 mm Load = 223 g Speed = 5 mm/s | μ = 0.10 | [99] |
| Stainless Steel | 85.5 wt. % Graphene + 9.5 wt.%. Zinc Oxide + 5 wt.% PVDF | 10 | Stainless Steel ball ⌀ = 6.3 mm Load = 10 N | μ = 0.08 | [100] |
| Composite | Test Parameters | Performance | Refs |
|---|---|---|---|
| 5.0% MgO + PVDF | Load = 3 N Speed = 10 mm/s | μ = 0.091 W = 1.2 × 10−5 mm3/N m | [102] |
| 10 wt.% Carbon Nanorod + PVDF | Stainless Steel Ball ⌀ = 6 mm Load = 3 N Speed = 10 mm/s | μ = 0.03 W = 3.70 × 10−5 mm3/N m | [103] |
| 50 wt.% Polyamide 66 + PVDF | 52100 Steel Ring Load = 200 N Speed = 0.43 m/s | μ = 0.49 W = 1.5 × 10−5 mm3/N m | [104] |
| 20 wt.% PAO/PSF + Recycled PVDF | GCr15 Steel Ball ⌀ = 6 mm Load = 10 N Speed = 0.075 mm/s | μ = 0.077 W = 2.34 × 10−6 mm3/N m | [105] |
| Medium | Additive Type | Size and Concentration | Test Parameters | Performance | Refs. |
|---|---|---|---|---|---|
| White Oil | PVDF Nanospheres | 100 nm 0.1 wt.% | AISI52100 Steel Ball (⌀ = 9.5 mm) AISI 52100 Steel Disks Load = 5 N Frequency = 2.5 Hz | μ = 0.12 w = 1 × 10−6 mm3/N m | [61] |
| KH570 modified PVDF Nanospheres | 100 nm 0.1 wt.% | μ = 0.11 w = 0.25 × 10−6 mm3/N m | |||
| PTFE filled with Cu | 1.5–5 μm 0.3 wt.% | GCr15 Steel (⌀ = 12.7 mm) Load = 392 N Speed = 1450 rpm/min | μ = 0.07 WSD = 0.55 mm | [107] | |
| PTFE filled with SiO2 | 1.5–5 μm 0.5 wt.% | μ = 0.05 WSD = 0.55 mm | |||
| 150 N Group II Base Oil | PTFE Nano | 30–50 nm 8 wt.% | AISI 52100 Steel Ball (∅ = 10 mm) AISI 52100 Steel Block Load = 100 N Speed = 50 mm/s | μ = 0.11 WSD = 0.77 mm | [108] |
| Paraffin Oil | 0.25 wt.% RGO + 0.25 wt.% PVDF | 180 nm | AISI 52100 Steel Ball (∅ = 9.5 mm) AISI 52100 Steel Block Load = 5 N Speed = 24 mm/s | μ = 0.13 w = 27 × 10−8 mm3/N m | [109] |
| 0.5 wt.% RGO/PVDF Composite | 500 nm | μ = 0.10 w = 0.48 × 10−8 mm3/N m | |||
| PFPE Oil | None | N/A | Steel Ball Diamond-like Carbon Films Load = 10 N Speed = 50 mm/s | μ = 0.13 WSD = None | [110] |
| BN Nano | 80 nm 0.2 wt.% | μ = 0.07 WSD = 423 μm | |||
| WS2 Nano | 50 nm 0.2 wt.% | μ = 0.03 WSD = 405 μm | |||
| MoS2 Nano | 50 nm 0.2 wt.% | μ = 0.02 WSD = 472 μm | |||
| MAC Grease | PTFE Thickener | 4 μm 25 wt.% | AISI 52100 Steel Ball (⌀ = 10 mm) AISI 52100 Steel Disks Load = 1400 N (max.) Frequency = 25 Hz | μ = 0.12 at T = 25 °C μ = 0.14 at T = 150 °C | [111] |
| PFPE Grease | PTFE Thickener | - | 2Cr13 Stainless Steel Ball (⌀ = 9 mm) 2Cr13 Stainless Steel Disk Load = 30 N Speed = 400 mm/s | μ = 0.2 at 2 × 10−3 Pa vacuum | [112] |
| Lithium Complex Grease | Recycled PTFE Micro | 4 μm 25 wt.% | AISI 52100 Steel Ball (∅ = 12.7 mm) Load = 392 N Speed = 1200 rpm | μ = 0.07 WSD = 571 μm | [113] |
| Medium | Ionic Liquid | Test Parameters | Performance | Refs. |
|---|---|---|---|---|
| Liquid | [DMA][OA] | PTFE Ball 304 SS Disc Load = 5 N Frequency = 5 Hz | μ = 0.008 | [12] |
| [DMA][DA] | μ = 0.007 | |||
| Liquid | C6F17SO3P4444 | AISI5200 Steel Ball (10 mm) Ti-6Al-4V Alloy Disc Load = 5 N Frequency = 25 Hz | μ = 0.0.66 | [13] |
| C8F17SO3P4444 | μ = 0.070 | |||
| C8F17SO3P8888 | μ = 0.073 | |||
| Nanofilm | [Bmim][FAP] | Stainless Steel Sphere (2 mm) Load = 10 mN Speed = 0.20 cm/s | μ = 0.150 | [131] |
| PFPE Z-tetraol | μ = 0.100 | |||
| EMIM FAP | μ = 0.15 | [132] | ||
| HMIM FAP | μ = 0.19 | |||
| BMPL FAP | μ = 0.19 | |||
| Additive in Diester Oil | [P66614][NTf2] Concentration: 0.25 wt.% | Disc and Ball: AISI 52100 steel Load = 80 N Frequency = 15 Hz | μ = 0.075 | [133] |
| PFAS Alternative | Chemical Formula | Applications | Refs. |
|---|---|---|---|
| Silanes | SinH2n+2 | Coatings, adhesives, lubricants, electronics | [227,235] |
| Polyphosphazenes | [NP(R1)(R2)]n | Fire-resistant materials, membranes, adhesives | [228,236] |
| Telomer-based fluoropolymers (C6O4) | C₆F₁₂O₄ | Coatings, membranes, lubricants, fire retardants | [229,237] |
| Oligomeric siloxanes (OBS) | (R2SiO)n | Coatings, adhesives, cosmetics, electronics | [230,238] |
| Hexafluoropropylene oxide trifluoroacetate (HFPO-TA) | C₃F₆O₃ | Solvents, cleaning agents, electronics | [231,239] |
| Hydrofluoroethers (HEPs) | C₂F₆O | Solvents, cleaning agents, electronics | [232,240] |
| Novel nonfluorinated polymers (NNN) | Variable | Coatings, lubricants, textiles, membranes | [233,241] |
| Polyhydroxyalkanoates (PHAs) | [-O(CH2)m CHOH-]n | Biodegradable plastics, coatings, adhesives | [234,242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, D.; Bons, J.; Kumar, A.; Kabir, M.H.; Liang, H. Forever Chemicals, Per-and Polyfluoroalkyl Substances (PFAS), in Lubrication. Lubricants 2024, 12, 114. https://doi.org/10.3390/lubricants12040114
Dias D, Bons J, Kumar A, Kabir MH, Liang H. Forever Chemicals, Per-and Polyfluoroalkyl Substances (PFAS), in Lubrication. Lubricants. 2024; 12(4):114. https://doi.org/10.3390/lubricants12040114
Chicago/Turabian StyleDias, Darrius, Jake Bons, Abhishek Kumar, M. Humaun Kabir, and Hong Liang. 2024. "Forever Chemicals, Per-and Polyfluoroalkyl Substances (PFAS), in Lubrication" Lubricants 12, no. 4: 114. https://doi.org/10.3390/lubricants12040114