Research Progress of Antioxidant Additives for Lubricating Oils
Abstract
:1. Introduction
2. Oxidation Mechanism of Hydrocarbon Lubricating Oils
3. Peroxide Decomposers
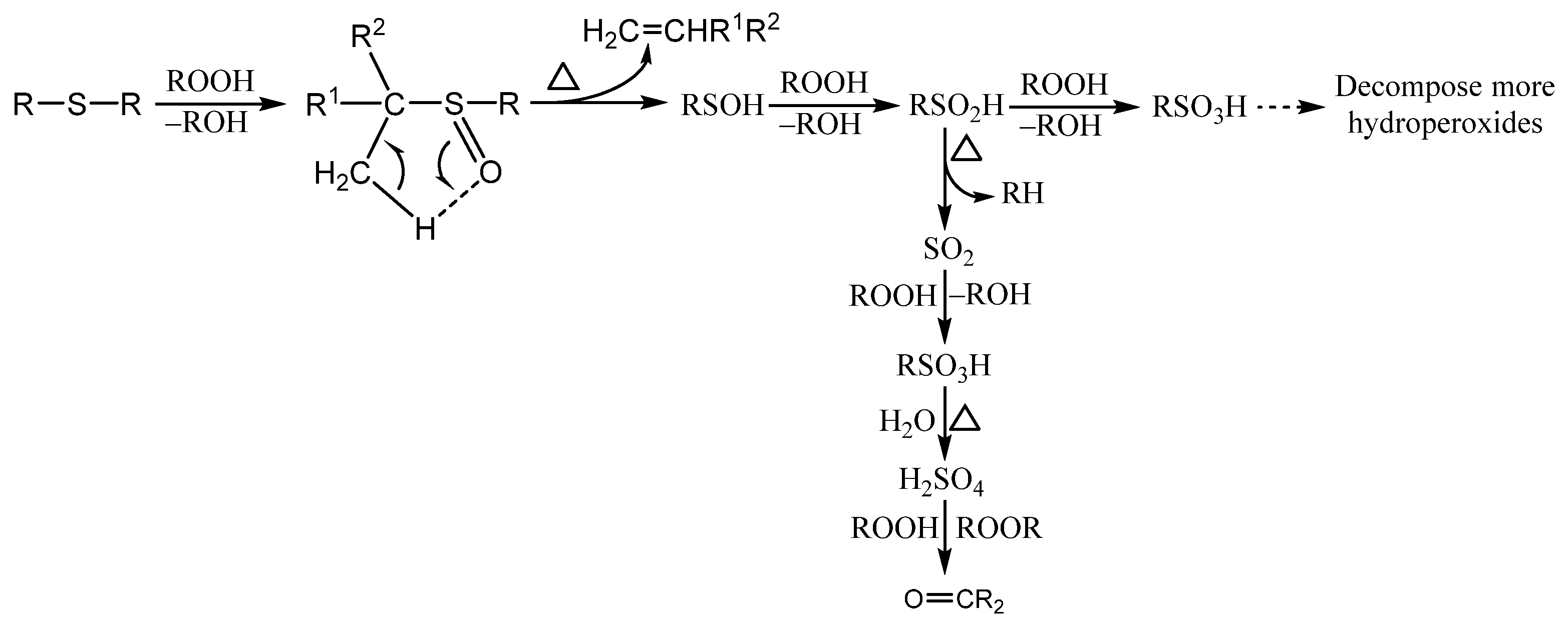
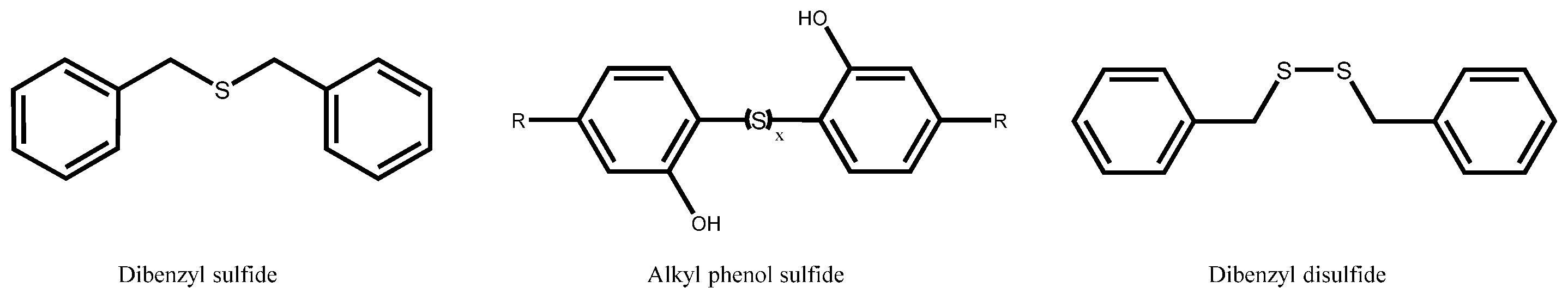
3.1. Organosulfur Compounds
3.2. Organophosphorus Compounds
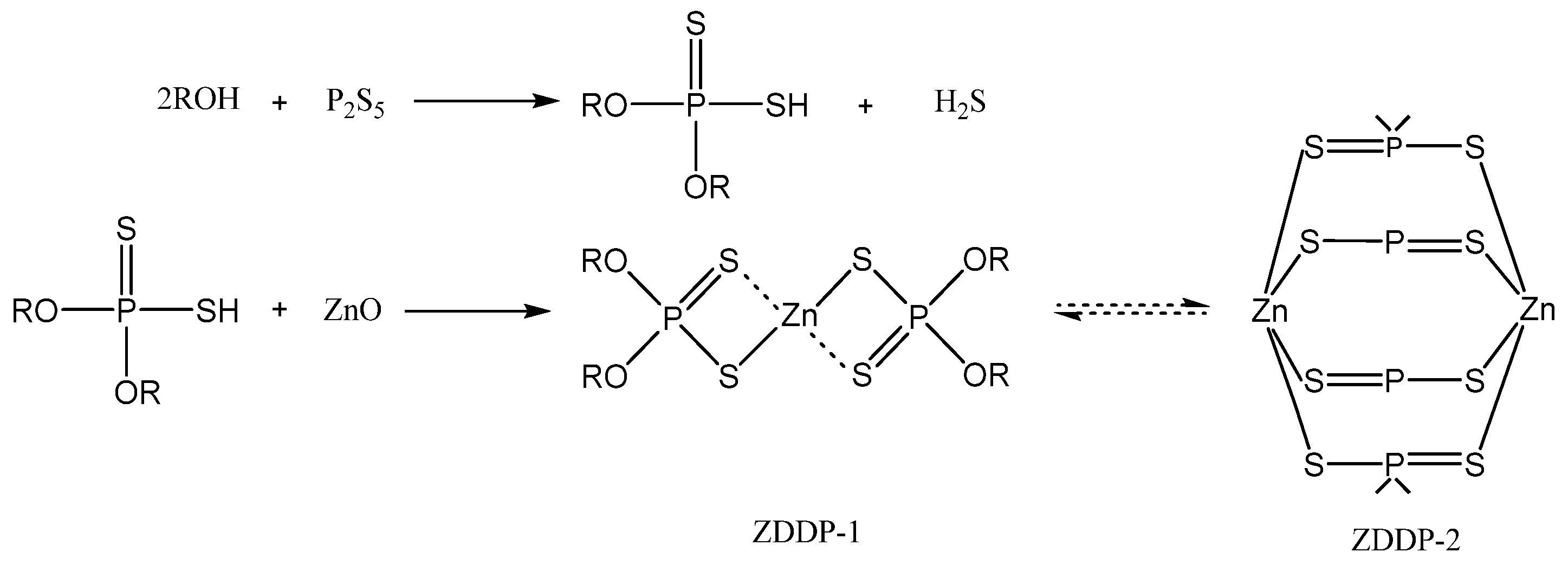
3.3. Sulfur–Phosphorus Compounds
3.4. Sulfur–Nitrogen Compounds
4. Radical Scavengers
4.1. Aromatic Amines
4.1.1. Antioxidation Mechanism of Aromatic Amines
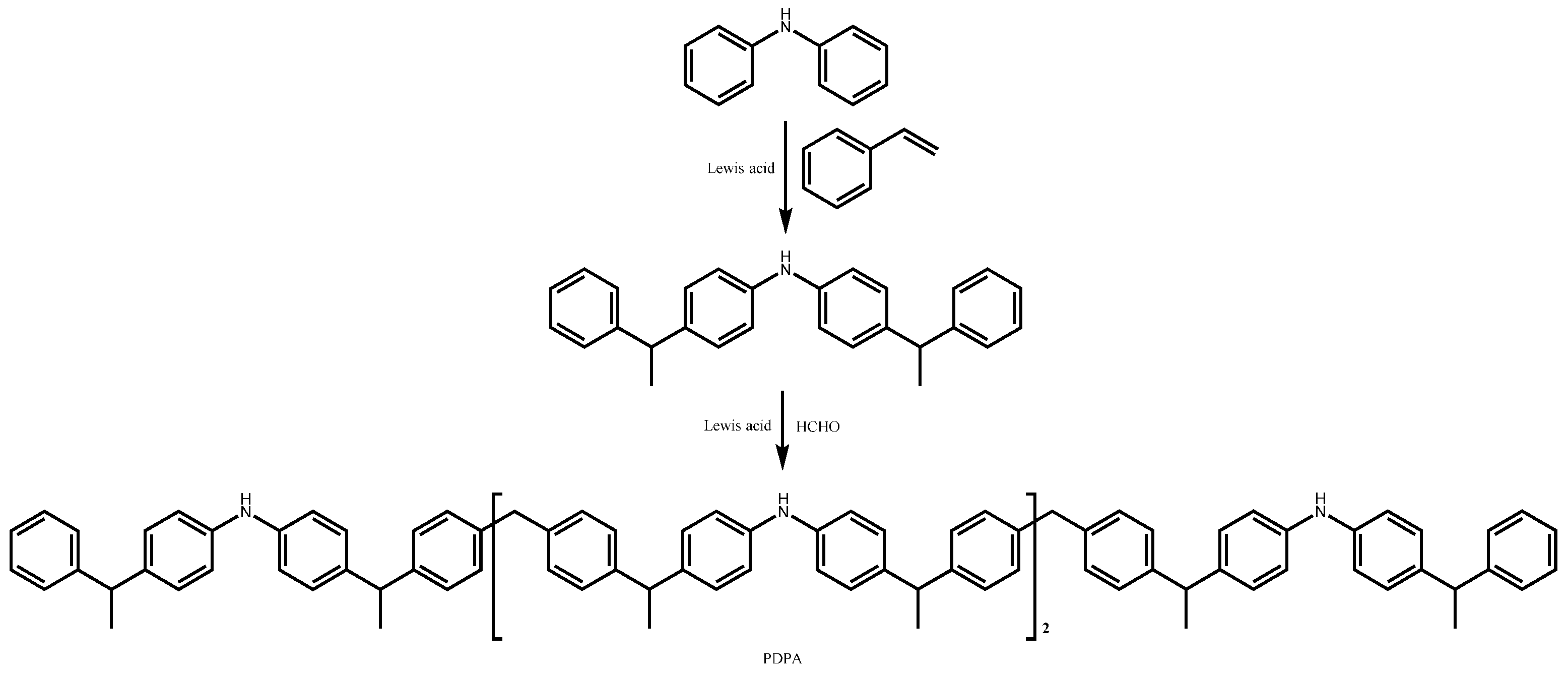
4.1.2. Alkyl Diphenylamine Antioxidants
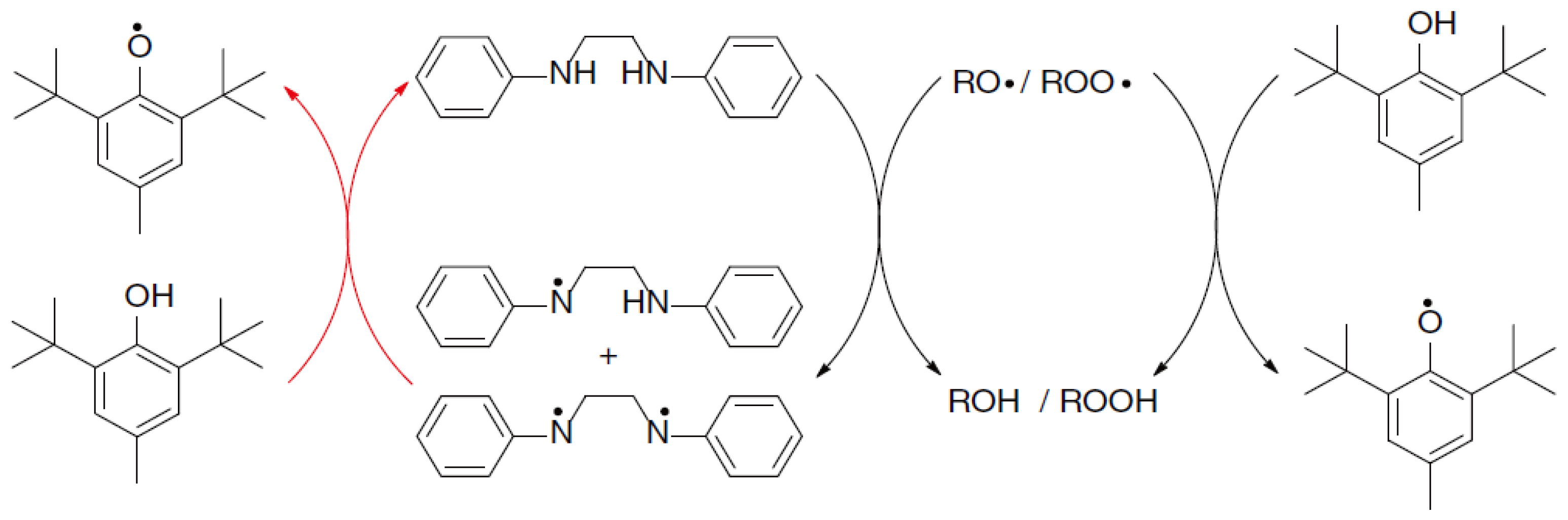
4.2. Hindered Phenols
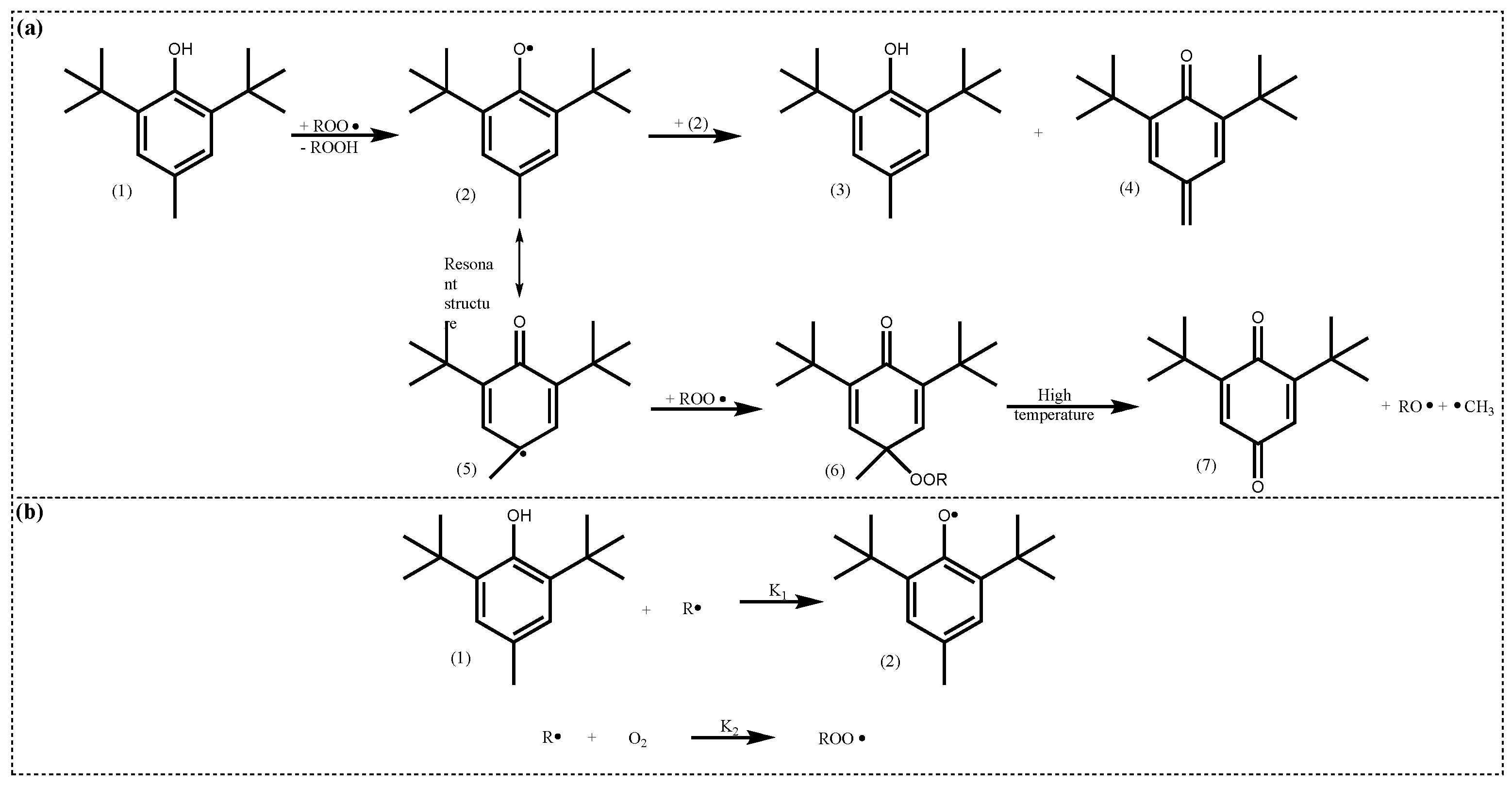
4.2.1. Active Structure and Mechanism of Hindered Phenols
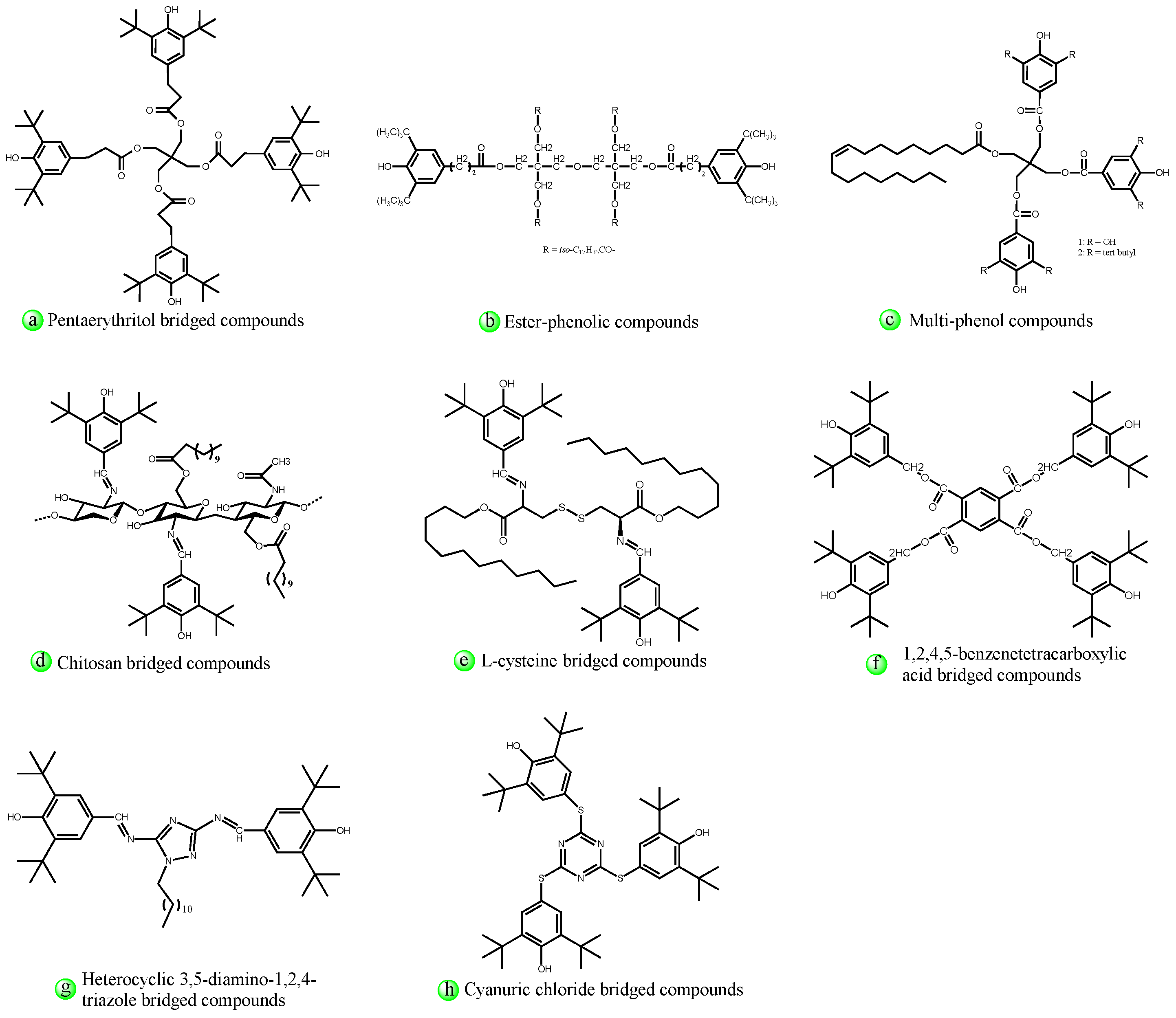
4.2.2. Structural Design of Hindered Phenols
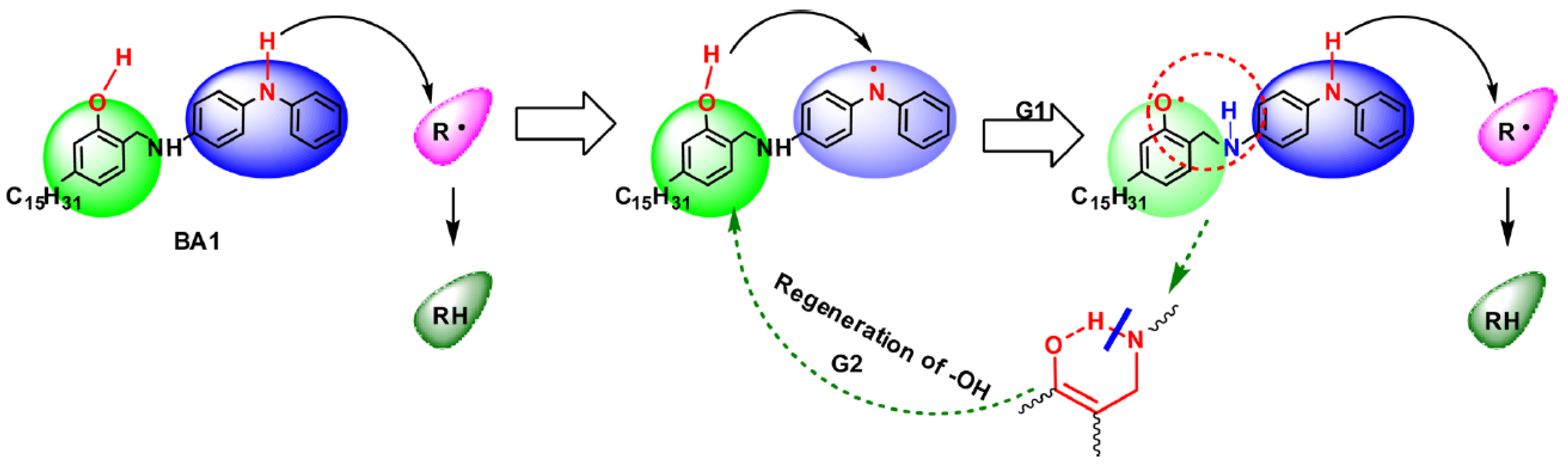
4.3. Phenolic Amine Complex and Its Derivatives
5. Summary and Prospects
- (i)
- Developing toward ashless antioxidants that are sulfur- and phosphorus-free to reduce pollution and oil ash.
- (ii)
- Developing toward multi-phenol antioxidants because of higher antioxidant properties compared to the single phenolic compounds.
- (iii)
- Developing toward macromolecules, such as the alkylation and aromatization of multi-phenols and alkylated aromatic amines.
- (iv)
- Developing toward composite antioxidants. This is because these composites can effectively improve the oxidation resistance of lubricating oil at high temperatures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gou, R.; Chen, J. Tribological properties of graphene/MoS2 combinations as lubricant additives for polycrystalline diamond compact. Ceram. Int. 2024, 50, 12713–12723. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X. Effect of morphology on tribological properties of Fe3O4 as lubricant additive: Nanospheres, nanowires and nanosheets. Tribol. Int. 2024, 191, 109201. [Google Scholar] [CrossRef]
- Georges, J.M.; Martin, J.M.; Mathia, T.; Kapsa, P.; Meille, G.; Montes, H. Mechanism of boundary lubrication with zinc dithiophosphate. Wear 1979, 53, 9–34. [Google Scholar] [CrossRef]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 4–41. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. 2D nanomaterials as lubricant additive: A review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Jia, T.; Yu, Y.; Liu, Q.; Yang, Y.; Zou, J.-J.; Zhang, X.; Pan, L. Theoretical and experimental study on the inhibition of jet fuel oxidation by diarylamine. Chin. J. Chem. Eng. 2023, 56, 225–232. [Google Scholar] [CrossRef]
- Martini, A.; Ramasamy, U.S.; Len, M. Review of viscosity modifier lubricant additives. Tribol. Lett. 2018, 66, 58. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Bin Ali, M.A.; Kamangar, S.; Cho, H.M.; Akram, N. An investigation on the influence of aluminium oxide nano-additive and honge oil methyl ester on engine performance, combustion and emission characteristics. Renew. Energy 2020, 146, 2291–2307. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Zhang, M.; Wang, X. Oxidative degradation of synthetic ester and its influence on tribological behavior. Tribol. Int. 2013, 64, 16–23. [Google Scholar] [CrossRef]
- Maleville, X.; Faure, D.; Legros, A.; Hipeaux, J.C. Oxidation of mineral base oils of petroleum origin: The relationship between chemical composition, thickening, and composition of degradation products. Lubr. Sci. 1996, 9, 1–60. [Google Scholar] [CrossRef]
- Hamblin, P.C.; Rohrbach, P. Piston deposit control using metal-free additives. Lubr. Sci. 2001, 14, 1–23. [Google Scholar] [CrossRef]
- Hu, C.; You, G.; Liu, J.; Du, S.; Zhao, X.; Wu, S. Study on the mechanisms of the lubricating oil antioxidants: Experimental and molecular simulation. J. Mol. Liq. 2021, 324, 115099. [Google Scholar] [CrossRef]
- Salah, H.; Elkatory, M.R.; Fattah, M.A. Novel zinc-polymer complex with antioxidant activity for industrial lubricating oil. Fuel 2021, 305, 121536. [Google Scholar] [CrossRef]
- Mills, R.; Wu, G.Z. Synthesis and evaluation of novel prodrugs of foscarnet and dideoxycytidine with a universal carrier compound comprising a chemiluminescent and a photochromic conjugate. J. Pharm. Sci. 2004, 93, 1320–1336. [Google Scholar] [CrossRef]
- Rasberger, M. Oxidative degradation and stabilisation of mineral oil based lubricants. In Chemistry and Technology of Lubricants; Springer: Berlin/Heidelberg, Germany, 1997; pp. 98–143. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Lersch, M.; Tilset, M. Mechanistic aspects of c-h activation by pt complexes. Chem. Rev. 2005, 105, 2471–2526. [Google Scholar] [CrossRef]
- Perrin, C.L.; Chang, K.-L. The complete mechanism of an aldol condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Wang, W.-W. Radical scavenging efficiencies of modified and microwave-treated multiwalled carbon nanotubes. Carbon 2014, 79, 354–362. [Google Scholar] [CrossRef]
- Yan, S.; Wu, G. Thermo-induced chain scission and oxidation of isosorbide-based polycarbonates: Degradation mechanism and stabilization strategies. Polym. Degrad. Stab. 2022, 202, 110028. [Google Scholar] [CrossRef]
- Kobbe, W.H. Sulphur-Containing Oil Composition and Method of Making the Same. U.S. Patent 1844400, 9 February 1932. [Google Scholar]
- Palmer, R.C.; Powers, P.O. Sulfurized Terpene Oil and Process of Preparing The Same. U.S. Patent 1926687, 12 September 1933. [Google Scholar]
- Knowles, E.C.; McCoy, F.C.; Patterson, J.A. Lubricating Oil and Method of Lubricating. U.S. Patent 2417305, 11 March 1947. [Google Scholar]
- Lincoln, B.H.; Steiner, W.L.; Byrkit, G.D. Sulphur Containing Lubricant. U.S. Patent 2218132, 15 October 1940. [Google Scholar]
- Lincoln, B.H.; Byrkit, G.D.; Steiner, W.L. Method for the Synthesis of Sulphur-Bearing Derivatives of High Molecular Weight. U.S. Patent 2348080, 15 October 1944. [Google Scholar]
- Haas, F.C. Nonsludging Lubricating Oil for Internal-Combustion Engines. U.S. Patent 2162398, 13 June 1939. [Google Scholar]
- Koelewijn, P.; Berger, H. Mechanism of the antioxidant action of dialkyl sulfoxides. Recl. Trav. Chim. Pays-Bas 1972, 91, 1275–1286. [Google Scholar] [CrossRef]
- Bridgewater, A.J.; Sexton, M.D. Mechanism of antioxidant action: Reactions of alkyl and aryl sulfides with hydroperoxides. J. Chem. Soc. Perkin Trans. 1978, 6, 530–536. [Google Scholar] [CrossRef]
- Mikeska, L.A.; Lieber, E. Preparation of Phenol Sulfides. U.S. Patent 2139321, 6 December 1938. [Google Scholar]
- Mikeska, L.A.; Cohen, C.A. Stabilizing Mineral Lubricating Oils. U.S. Patent 2139766, 13 December 1938. [Google Scholar]
- Mikeska, L.A.; Lieber, E. Stabilized Lubricating Composition. U.S. Patent 2174248, 26 September 1939. [Google Scholar]
- Mikeska, L.A.; Lieber, E. Polymerization and Condensation Products of Phenol Sulfides. U.S. Patent 2239534, 22 April 1941. [Google Scholar]
- Askew, H.F.; Jayne, G.J.J.; Elliott, J.S. Lubricant Compositions. U.S. Patent 3882031, 6 May 1975. [Google Scholar]
- Oumar-Mahamat, H.; Horodysky, A.G.; Jeng, A. Dihydrobenzothiophenes as Antioxidant and Antiwear additives. U.S. Patent 5514289, 7 May 1996. [Google Scholar]
- Brown, A.L. Treatment of Hydrocarbon Oils. U.S. Patent 1234862, 31 July 1917. [Google Scholar]
- Hall, F.W.; Towne, C.C. Method of Lubrication. U.S. Patent 2257601, 30 September 1941. [Google Scholar]
- Ashburn, H.V.; Alsop, W.G. Lubricating Oil. U.S. Patent 2221162, 12 November 1940. [Google Scholar]
- Musher, S. Lubricating Oil and Method of Making the Same. U.S. Patent 2223941, 3 December 1940. [Google Scholar]
- Loane, C.M.; Gaynor, J.W. Lubricant. U.S. Patent 2322859, 29 June 1943. [Google Scholar]
- Moran, R.C.; Kozacik, A.P. Mineral Oil Composition. U.S. Patent 2151300, 21 March 1939. [Google Scholar]
- Li, W.M.; Wang, Q.R.; Wang, X.B.; Liu, W.M. Preparation and properties study of hindered phenol derivatives as multifunctional lubricating additive. Lubr. Oil 2012, 27, 30–34. [Google Scholar] [CrossRef]
- Messina, N.V.; Donald, R.S. Stabilized Flulds. U.S. Patent 3556999, 19 January 1971. [Google Scholar]
- Meyers, D. Method of Lubricating Compression Cylinders Used in the Manufacture of High-Pressure Polyethylene. U.S. Patent 6172014, 9 January 2001. [Google Scholar]
- Dong, J.; Migdal, C.A. Stabilized Lubricant Compositions. U.S. Patent 7829511, 9 November 2010. [Google Scholar]
- Holt, A.; Mulqueen, G. Stabilizing Compositions for Lubricating Oils. U.S. Patent 20030171227, 11 September 2003. [Google Scholar]
- Durr, A.M., Jr.; Krenowicz, R.A. Turbine Oil Compositions. U.S. Patent 3923672, 2 December 1975. [Google Scholar]
- Cohen, S.C. Synergistic Antioxidant System for Severely Hydrocracked Lubricating Oils. U.S. Patent 5124057, 23 June 1992. [Google Scholar]
- Chen, Y.; Renner, P.; Liang, H. A review of current understanding in tribochemical reactions involving lubricant additives. Friction 2023, 11, 489–512. [Google Scholar] [CrossRef]
- Armstrong, D.R.; Ferrari, E.S.; Roberts, K.J.; Adams, D. An investigation into the molecular stability of zinc di-alkyl-di-thiophosphates (zddps) in relation to their use as anti-wear and anti-corrosion additives in lubricating oils. Wear 1997, 208, 138–146. [Google Scholar] [CrossRef]
- Fox, M.F.; Pawlak, Z.; Picken, D.J. Inverse micelles and solubilization of proton donors in hydrocarbon formulations. Tribol. Int. 1991, 24, 341–349. [Google Scholar] [CrossRef]
- Rivier, G. Metal Dithiophosphates and Their Use as Additives for Lubricating Oils. U.S. Patent 4288335, 8 September 1981. [Google Scholar]
- Schroeck, C.W. Metal Salts of Lower Dialkylphosphorodithioic Acids. U.S. Patent 4466895, 21 August 1984. [Google Scholar]
- Clason, D.L.; Schroeck, C.W. Mixed Metal Salts and Lubricants and Functional Fluids Containing Them. U.S. Patent 4308154, 29 December 1981. [Google Scholar]
- Asseff, P.A. Lubricant. U.S. Patent 2261047, 28 October 1941. [Google Scholar]
- Cook, E.W.; Thomas, W.D., Jr. Lubricating Compositions. U.S. Patent 2368000, 23 January 1945. [Google Scholar]
- Sarin, R.; Tuli, D.K.; Sureshbabu, A.V.; Misra, A.K.; Rai, M.M.; Bhatnagar, A.K. Molybdenum dialkylphosphorodithioates: Synthesis and performance evaluation as multifunctional additives for lubricants. Tribol. Int. 1994, 27, 379–386. [Google Scholar] [CrossRef]
- Ghanbari, B.; Khailli, A.A.; Taheri, Z.; Mohajerani, B.; Soleymani Jamarani, M. The effect of fullerene c60 and its amine derivative on the zddp antioxidative functionality. Fuller. Nanotub. Carbon Nanostruct. 2007, 15, 439–443. [Google Scholar] [CrossRef]
- Wang, S.; Yu, S.; Huang, B.; Feng, J.; Liu, S. Unique synergism between zinc dialkyldithiophosphates and schiff base bridged phenolic diphenylamine antioxidants. Tribol. Int. 2020, 145, 106134. [Google Scholar] [CrossRef]
- Wang, J.; He, T.X.; Song, C.Y.; Li, X.Q.; Chen, B.S. Engine oil degradation induced by biodiesel: Effect of methyl oleate on the performance of zinc dialkyldithiophosphate. ACS Omega 2019, 4, 16166–16170. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, J.S.; Ren, T.H. Tribological study of a novel borate ester containing s, p with high hydrolytic stability as a multifunctional lubricating additive. Tribol. Trans. 2008, 51, 160–165. [Google Scholar] [CrossRef]
- Jin, Y.; Duan, H.; Cheng, B.; Wei, L.; Tu, J.; Liu, J.; Li, J. Synthesis of a multi-phenol antioxidant and its compatibility with alkyl diphenylamine and zddp in ester oil. Tribol. Lett. 2019, 67, 58. [Google Scholar] [CrossRef]
- Hester, W.F. Fungicidal Compositions Suitable for Use on Plants or Seeds. U.S. Patent 2317765, 27 April 1943. [Google Scholar]
- Hu, J.-Q.; Wei, X.-Y.; Dai, G.-L.; Fei, Y.-W.; Liu, C.-C.; Zong, Z.-M.; Yao, J.-B. Synergistic antioxidation of organic molybdenum complex with dithiocarbamate antioxidant evaluated by differential scanning calorimetry and thin film micro oxidation test. Thermochim. Acta 2007, 453, 21–26. [Google Scholar] [CrossRef]
- Chesluk, R.P.; Askew, J.D., Jr.; Henderson, C.C. Oxidation-Inhibited Lubricating Oil. U.S. Patent 4125479, 14 November 1978. [Google Scholar]
- Yao, J.B. The application of ashless thiocarbamate as lubricant antioxidation and ep additive. Lubr. Oil 2005, 20, 41–44. [Google Scholar] [CrossRef]
- Doe, L.A. Antioxidant Synergists for Lubricating Compositions. U.S. Patent 4880551, 14 November 1989. [Google Scholar]
- Nakazato, M.; Magarifuchi, J.; Isozaki, Y. Low Phosphorus Engine Oil Compositions and Additive Compositions. U.S. Patent 5629272, 13 May 1997. [Google Scholar]
- DeVries, L.; King, J.M. Antioxidant Combinations of Molybdenum Complexes and Aromatic Amine Compounds. U.S. Patent 4370246, 25 January 1983. [Google Scholar]
- Arai, K.; Tomizawa, H. Lubricating Oil Composition. U.S. Patent 5605880, 25 February 1994. [Google Scholar]
- Tomizawa, H. Lubricating Oil Composition for Internal Combustion Engines. U.S. Patent 5688748, 18 November 1997. [Google Scholar]
- Yao, J.B. Recent development of antiwear and extreme pressure-resistant additives for lubricating oils and greases. Lubr. Oil 2006, 21, 29–37. [Google Scholar] [CrossRef]
- Hoffman, D.M.; Feher, J.J.; Farmer, H.H. Lubricating Compositions Containing 5,5’-Dithiobis(1,3,4-Thiadiazole-2-Thiol). U.S. Patent 4517103, 14 May 1985. [Google Scholar]
- Salomon, M.F. N-Substituted Thio Alkyl Phenothiazines. U.S. Patent 5034019, 23 July 1991. [Google Scholar]
- Styer, J.; Guinther, G. Fuel economy beyond ilsac GF-5: Correlation of modern engine oil tests to real world performance. SAE Int. J. Fuels Lubr. 2012, 5, 1025–1033. [Google Scholar] [CrossRef]
- Hu, J.Q.; Wei, X.Y.; Yao, J.B.; Han, L.; Zong, Z.M. Evaluation of molybdate ester as a synergist for arylamine antioxidant in lubricants. Tribol. Int. 2006, 39, 1469–1473. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, L.; Zheng, K.; Cui, Y.; Zhang, S.; Yu, L.; Zhang, P. Synthesis of ploy(p-methoxyphenol) and evaluation of its antioxidation behavior as an antioxidant in several ester oils. Tribol. Int. 2015, 88, 95–99. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Valgimigli, L.; Gigmes, D.; Tordo, P. Bond dissociation energies of the n−h bond and rate constants for the reaction with alkyl, alkoxyl, and peroxyl radicals of phenothiazines and related compounds. J. Am. Chem. Soc. 1999, 121, 11546–11553. [Google Scholar] [CrossRef]
- Asadauskas, S.J.; Grigucevičienė, A.; Leinartas, K.; Bražinskienė, D. Application of three-electrode electrolytic cell to evaluate thin films of vegetable and mineral oils. Tribol. Int. 2011, 44, 557–564. [Google Scholar] [CrossRef]
- Zhan, W.; Tu, J.S.; Qian, X.Z.; Li, J.; Liu, J. Synthesis of butyl-octyl-diphenylamine as lubricant antioxidant additive by ionic liquids. Int. J. Adv. Manuf. Technol. 2018, 96, 1647–1653. [Google Scholar] [CrossRef]
- Bolsman, T.A.B.M.; Blok, A.P.; Frijns, J.H.G. Catalytic inhibition of hydrocarbon autoxidation by secondary amines and nitroxides. Recl. Des Trav. Chim. Des Pays-Bas 1978, 97, 310–312. [Google Scholar] [CrossRef]
- Jensen, R.K.; Korcek, S.; Zinbo, M.; Gerlock, J.L. Regeneration of amine in catalytic inhibition of oxidation. J. Org. Chem. 1995, 60, 5396–5400. [Google Scholar] [CrossRef]
- Holubec, Z.M. Lubricant Compositions. U.S. Patent 3876550, 8 April 1975. [Google Scholar]
- Jian-Qiang, H.; Yang, S.-Z.; Zhang, J.-J.; Guo, L.; Xu, X. Synthesis and anti-oxidative properties of poly(diphenylamine) derivative as lubricant antioxidant. Pet. Chem. 2019, 59, 1037–1042. [Google Scholar] [CrossRef]
- Miao, C.; Yu, D.; Huang, L.; Zhang, S.; Yu, L.; Zhang, P. Synthesis of 1,3,5-tris(phenylamino) benzene derivatives and experimental and theoretical investigations of their antioxidation mechanism. Ind. Eng. Chem. Res. 2016, 55, 1819–1826. [Google Scholar] [CrossRef]
- Shah, R.; Poon, J.-F.; Haidasz, E.A.; Pratt, D.A. Temperature-dependent effects of alkyl substitution on diarylamine antioxidant reactivity. J. Org. Chem. 2021, 86, 6538–6550. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Dong, J. Evaluation of sodium stearate as a synergist for arylamine antioxidants in synthetic lubricants. Thermochim. Acta 1995, 262, 157–163. [Google Scholar] [CrossRef]
- Yao, J.B.; Dong, J.X. Antioxidation synergism between alkali metal salts and arylamine compounds in synthetic lubricants. Tribol. Trans. 1996, 39, 498–500. [Google Scholar] [CrossRef]
- Yao, J.B. Evaluation of sodium acetylacetonate as a synergist for arylamine antioxidants in synthetic lubricants. Tribol. Int. 1997, 30, 795–799. [Google Scholar] [CrossRef]
- Hu, J.Q.; Wei, X.Y.; Dai, G.L.; Liu, C.C.; Fu, Y.; Zong, Z.M.; Yao, J.B. Study demonstrating enhanced oxidation stability when arylamine antioxidants are combined with organic molybdenum complexes. Tribol. Trans. 2007, 50, 205–210. [Google Scholar] [CrossRef]
- Hu, J.Q.; Wang, X.L.; Dai, G.L.; Fei, Y.W.; Wei, X.Y.; Zong, Z.M. Evaluation on synergistic antioxidation of molybdenum dialkydithiocarbamate with arylamine antioxidant. Ind. Lubr. Tribol. 2011, 63, 78–83. [Google Scholar] [CrossRef]
- Cai, T.; Liu, D.; Zhao, L.; Ye, M.; Liu, S. In situ tribochemical sulfurization of polyisobutylene-based molybdenum species for enhanced tribo-performance. Tribol. Int. 2019, 136, 556–569. [Google Scholar] [CrossRef]
- Hu, J.-Q.; Zhang, J.-J.; Guo, L.; Miao, C.-Q.; Yang, S.-Z.; Ma, J.; Xu, X.; Xie, F. Synthesis of styrenated sulfur- and phosphorus-free organic titanate and evaluation of its tribological and antioxidant properties as an additive in poly-α-olefin. Ind. Eng. Chem. Res. 2019, 58, 1754–1759. [Google Scholar] [CrossRef]
- Chao, M.R.; Li, W.M.; Wang, X.B. Antioxidant synergism between synthesised alkylated diphenylamine and dilauryl thiodipropionate in polyolefin base fluid. J. Therm. Anal. Calorim. 2014, 117, 925–933. [Google Scholar] [CrossRef]
- Chao, M.; Fan, R.; Zhang, L.; Wang, X.; Shu, Q.; Gao, J.; Chen, L.; Gong, P.; Shen, D. Synthesis and antioxidant properties of a novel arylamine antioxidant. Bull. Korean Chem. Soc. 2021, 42, 1440–1445. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (bht): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, T.; Ohkatsu, Y. Effect of para-substituents of phenolic antioxidants. Polym. Degrad. Stab. 2001, 71, 445–452. [Google Scholar] [CrossRef]
- Lawandy, S.N.; Shehata, A.B.; Younan, A.F. Acrylamides as phenolic antioxidants for acrylqnitrile-butadiene rubber compounds. Polym.-Plast. Technol. Eng. 1996, 35, 813–825. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Valgimigli, L.; Johansson, H.; Engman, L. Organochalcogen substituents in phenolic antioxidants. Org. Lett. 2010, 12, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Nazarbahjat, N.; Nordin, N.; Abdullah, Z.; Abdulla, M.A.; Yehye, W.A.; Halim, S.N.; Kee, C.H.; Ariffin, A. New thiosemicarbazides and 1,2,4-triazolethiones derived from 2-(ethylsulfanyl) benzohydrazide as potent antioxidants. Molecules 2014, 19, 11520–11537. [Google Scholar] [CrossRef] [PubMed]
- Costil, R.; Sterling, A.J.; Duarte, F.; Clayden, J. Atropisomerism in diarylamines: Structural requirements and mechanisms of conformational interconversion. Angew. Chem. Int. Ed. 2020, 59, 18670–18678. [Google Scholar] [CrossRef]
- Aparicio, S.; Alcalde, R. On the structure of liquid methyl salicylate: The role of intramolecular hydrogen bonding. Eur. J. Chem. 2010, 1, 162–167. [Google Scholar] [CrossRef]
- Kajiyama, T.; Ohkatsu, Y. Effect of meta-substituents of phenolic antioxidants-proposal of secondary substituent effect. Polym. Degrad. Stab. 2002, 75, 535–542. [Google Scholar] [CrossRef]
- Boozer, C.E.; Hammond, G.S.; Hamilton, C.E.; Sen, J.N. Air oxidation of hydrocarbons.1 ii. The stoichiometry and fate of inhibitors in benzene and chlorobenzene. J. Am. Chem. Soc. 1955, 77, 3233–3237. [Google Scholar] [CrossRef]
- Suzuki, A.; Ulfiati, R.; Masuko, M. Evaluation of antioxidants in rapeseed oils for railway application. Tribol. Int. 2009, 42, 987–994. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, G.; Eli, W. Synthesis and characterization of novel liquid ester-phenolic antioxidant based on dipentaerythritol. Lubr. Sci. 2013, 25, 209–216. [Google Scholar] [CrossRef]
- Singh, R.K.; Kukrety, A.; Singh, A.K. Study of novel ecofriendly multifunctional lube additives based on pentaerythritol phenolic ester. ACS Sustain. Chem. Eng. 2014, 2, 1959–1967. [Google Scholar] [CrossRef]
- Singh, R.K.; Kukrety, A.; Chatterjee, A.K.; Thakre, G.D.; Bahuguna, G.M.; Saran, S.; Adhikari, D.K.; Atray, N. Use of an acylated chitosan schiff base as an ecofriendly multifunctional biolubricant additive. Ind. Eng. Chem. Res. 2014, 53, 18370–18379. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, S.; Saxena, R.C.; Thakre, G.D.; Atray, N.; Ray, S.S. Study of cystine schiff base esters as new environmentally benign multifunctional biolubricant additives. J. Ind. Eng. Chem. 2015, 26, 149–156. [Google Scholar] [CrossRef]
- Singh, R.K.; Kukrety, A.; Sharma, O.P.; Baranwal, S.; Atray, N.; Ray, S.S. Study of a novel phenolic-ester as antioxidant additive in lube, biodiesel and blended diesel. J. Ind. Eng. Chem. 2016, 37, 27–31. [Google Scholar] [CrossRef]
- Faujdar, E.; Singh, R.K. Study on alkylated schiff base of a triazole with 3, 5-di-tert-butyl-4-hydroxybenzaldehyde as a novel multifunctional lubricant additive. Fuel 2021, 302, 121158. [Google Scholar] [CrossRef]
- Higgins, C.L.; Filip, S.V.; Afsar, A.; Hayes, W. Synthesis, characterisation, and performance evaluation of tri-armed phenolic antioxidants. Tetrahedron Lett. 2020, 61, 152127. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, C.; Zhang, Y.; Zhang, S.; Zhang, P. Dbhp-functionalized zno nanoparticles with improved antioxidant properties as lubricant additives. Langmuir 2019, 35, 4342–4352. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.L.; Filip, S.V.; Afsar, A.; Hayes, W. Evaluation of thermal and oxidative stability of three generations of phenolic based novel dendritic fuel and lubricant additives. React. Funct. Polym. 2019, 142, 119–127. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; Ma, Y.; Wang, L.; Zhu, J.; Liu, S. Structure, thermal stability, antioxidant activity and dft studies of trisphenols and related phenols. Inorganica Chim. Acta 2017, 468, 159–170. [Google Scholar] [CrossRef]
- Alexsandra de Sousa Rios, M.; Sales, F.A.M.; Mazzetto, S.E. Study of antioxidant properties of 5-n-pentadecyl-2-tert-amylphenol. Energy Fuels 2009, 23, 2517–2522. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, G.; Wang, Y.; Eli, W. Synthesis and characterisation of antioxidant-modified esters of dipentaerythritol as lubricating base oil. Lubr. Sci. 2013, 25, 329–337. [Google Scholar] [CrossRef]
- Faujdar, E.; Singh, R.K. Methyl oleate derived multifunctional additive for polyol based lubricants. Wear 2021, 466–467, 203550. [Google Scholar] [CrossRef]
- Nath, A.R.; Yehye, W.A.; Zulkifli, N.W.M.; Rafie Johan, M. Ester of thiolated butylated hydroxytoluene: Potential antioxidant for synthetic lubricant oil. Thermochim. Acta 2018, 670, 7–12. [Google Scholar] [CrossRef]
- Chao, M.; Li, W.; Chen, L.; Wang, X. Hindered phenol derivative as a multifunctional additive in lithium complex grease. Ind. Eng. Chem. Res. 2015, 54, 6605–6610. [Google Scholar] [CrossRef]
- Yu, S.; Feng, J.; Cai, T.; Liu, S. Schiff base bridged phenolic diphenylamines for highly efficient and superior thermostable lubricant antioxidants. Ind. Eng. Chem. Res. 2017, 56, 4196–4204. [Google Scholar] [CrossRef]
- Czochara, R.; Kusio, J.; Symonowicz, M.; Litwinienko, G. Fullerene c60 derivatives as high-temperature inhibitors of oxidative degradation of saturated hydrocarbons. Ind. Eng. Chem. Res. 2016, 55, 9887–9894. [Google Scholar] [CrossRef]
- Czochara, R.; Kusio, J.; Litwinienko, G. Fullerene c60 conjugated with phenols as new hybrid antioxidants to improve the oxidative stability of polymers at elevated temperatures. RSC Adv. 2017, 7, 44021–44025. [Google Scholar] [CrossRef]
- Bolbukh, Y.; Kuzema, P.; Tertykh, V.; Laguta, I. Thermal degradation of polyethylene containing antioxidant and hydrophilic/hydrophobic silica. J. Therm. Anal. Calorim. 2008, 94, 727–736. [Google Scholar] [CrossRef]
- Gensler, R.; Plummer, C.J.G.; Kausch, H.H.; Kramer, E.; Pauquet, J.R.; Zweifel, H. Thermo-oxidative degradation of isotactic polypropylene at high temperatures: Phenolic antioxidants versus has. Polym. Degrad. Stab. 2000, 67, 195–208. [Google Scholar] [CrossRef]
- Yu, S.; Liu, S. Multifunctional antioxidants with high activity at elevated temperatures based on intramolecular synergism. Eur. J. Org. Chem. 2018, 2018, 381–385. [Google Scholar] [CrossRef]
- Higgins, C.L.; Filip, S.V.; Afsar, A.; Hayes, W. Increasing the antioxidant capability via the synergistic effect of coupling diphenylamine with sterically hindered phenol. Tetrahedron 2019, 75, 130759. [Google Scholar] [CrossRef]
- Wang, S.P.; Yu, S.S.; Feng, J.X.; Liu, S.G. Multifunctional lubricant additive based on difluoroboron derivatives of a diphenylamine antioxidant. RSC Adv. 2019, 9, 35059–35067. [Google Scholar] [CrossRef]
- Wang, S.; Yu, S.; Feng, J.; Liu, S. A highly efficient antioxidant based on boron and a schiff base bridged phenolic diphenylamine: Synthesis, crystal structure and thermal and antioxidant properties. Acta Crystallogr. Sect. C 2019, 75, 1274–1279. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, Y.; Yang, G.; Zhang, S.; Yu, L.; Zhang, P. Enzymatic oligomerization of p-methoxyphenol and phenylamine providing poly(p-methoxyphenol-phenylamine) with improved antioxidant performance in ester oils. Ind. Eng. Chem. Res. 2016, 55, 12703–12709. [Google Scholar] [CrossRef]
- Miao, C.; Ma, Z.; Yu, L.; Tang, W.; Li, G.; Chen, G.; Cui, M. Controllable synthesis and synergistic antioxidation mechanism of poly(p-methoxyphenol-phenylamine) in biodegradable vegetable-based lubricating oils. Ind. Eng. Chem. Res. 2022, 61, 15784–15795. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, H.; Yue, S.; Liu, S. One-pot synthesis of cardanol-derived high-efficiency antioxidants based on intramolecular synergism. ACS Sustain. Chem. Eng. 2017, 5, 3399–3408. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, J.; Yang, L.; Du, D.; Tang, C. Differences analysis of water molecular diffusion behaviors in vegetable oil and mineral oil under temperature field. J. Mol. Liq. 2021, 323, 115030. [Google Scholar] [CrossRef]
- Xu, Y.; Geng, J.; Peng, Y.; Liu, Z.; Yu, J.; Hu, X. Lubricating mechanism of fe3o4@mos2 core-shell nanocomposites as oil additives for steel/steel contact. Tribol. Int. 2018, 121, 241–251. [Google Scholar] [CrossRef]
- Fan, M.; Ai, J.; Hu, C.; Du, X.; Zhou, F.; Liu, W. Naphthoate based lubricating oil with high oxidation stability and lubricity. Tribol. Int. 2019, 138, 204–210. [Google Scholar] [CrossRef]
- Xue, W.; Shi, L.; Chen, X.; Qiu, M.; Zhou, C.; Liu, H.; Li, S.; Sun, Y. The direct synthesis of a bio-lubricant by the oligomerization of methyllinoleate via castor oil. Green Chem. 2019, 21, 6658–6666. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Zhu, J.; Yu, H.; Liu, Y.; Shi, P.; Wang, S.; Liu, S. Synthesis and application of highly efficient multifunctional vegetable oil additives derived from biophenols. J. Clean. Prod. 2020, 242, 118274. [Google Scholar] [CrossRef]






| Lubricant Type | Phosphite Ester | Supplementary Antioxidants | Reference |
|---|---|---|---|
| Hydraulic fluids | Trialkyl phosphites | Secondary aminic and hindered phenolic | [42] |
| Compressor oils | Tributyl phosphite, triphenyl phosphite, and tridecyl phosphite, etc. | As above | [43] |
| Automotive and industrial lubricants | Triphenyl phosphite, dilauryl phosphite, diisodecyl pentaerythritol diphosphate, etc. | As above | [44] |
| Automotive and industrial lubricants | Triaryl phosphites, alkyl aryl phosphites, and acid dialkyl phosphites, etc. | As above | [45] |
| Steam turbine oils | Triphenyl phosphite | Alkylated diphenylamine | [46] |
| Hydraulic fluids and steam turbine oils | Steric hindered tributyl phosphite, bis(butylphenyl pentaerythritol) diphosphite | (3,5-Di-t-butyl)4-hydroxybenzyl isocyanurate | [47] |
| Additive Types | Additive Contents | Based Oils | Performances | Advantage | Disadvantage | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Sulfide fatty oils | - | Itself | Improved antioxidant performances | Simple preparation | The high S contents corrode metals and limit their large-scale application | [26] |
| 2 | Dihydrobenzothiophenes | 1.0 wt.% | Mineral oils | Antioxidant and antiwear performances | Better thermostability than alkyl sulfide | [34] | |
| 3 | Heterocyclic sulfide | 2.0 wt.% | Naphtha | Increased OIT values | Having both anticorrosive and extreme-pressure performances | [33] | |
| 4 | Natural phospholipid compounds | 0.01–2.0 wt.% | Motor oils | Decreased paint film and improved the oxidation resistance of oils | Naturally degradable | The high S and P contents damage mechanical equipment, ternary catalysts, and environment | [36] |
| 5 | ZDDP | 0.4 wt.% | Naphtha | Good antioxidant performance | Having both thermal stability and oil solubility | [55] | |
| 6 | MoDTP | 0.2 wt.% | Mineral oils | Antioxidation properties comparable to commercial ZDDP | Having both anti-friction and anti-wear performances | [56] | |
| 7 | ZDDP+ phenolic diphenylamine | 0.64 wt.% + 5 μmol/g | Poly-α-olefin | synergism between them and improved antioxidant performance | Having both anti-wear and extreme pressure performance | Relatively high S dosage | [58] |
| 8 | ZDDP + methyl oleate | 0–2.0 wt.% + 0–20 wt.% | Mineral oils | Improved antioxidation ability of ZDDP | Reduced P and S dosage to a certain extent | Impaired anti-wear capacity of ZDDP | [59] |
| 9 | ZDDP+ multi-phenol compounds | 0.5 wt.% + 1.0 wt.% | Triisodecyl trimellitate | Higher thermal stability and antioxidation ability than commercial phenols | Reduced P and S dosage to a certain extent | THA alone does not improve its tribological performance | [61] |
| 10 | Organic molybdenum complex + methylene bis(di-n-butyldithiocarbamate) | 1.0 wt.% + 0.5 wt.% | Poly-α-olefin derivatives | Increased IOT, OIT values, and antioxidation performance | Synergistic effect | Unclear in the pattern and structure of the coordination of Mo with N or S | [63] |
| 11 | Methylene bis(dialkyl dithiocarbamate) + 4-methyl-2,6-ditertiary butyl phenol | 0.1–4.0 wt.% + 0.01–2.0 wt.% | Paraffinic oils | Improved antioxidation performance | Synergistic effect | Relatively high S dosage | [64] |
| 12 | 2,5-dithiobis(1,3,4-thiadiazole-2-thiol) | 0.1–10.0 wt.% | Lithium 12-hydroxystearate greases | Improved antioxidation performance | Having extreme-pressure performance | Relatively high S dosage | [72] |
| 13 | N-substituted phenothiazine derivatives | 5.0 wt.% | SAE 30 motor oils | Improved antioxidation performance | Having both anticorrosive and extreme-pressure performance | Relatively high S dosage | [73] |
| Additive Types | Additive Contents | Based Oils | Performances and Benefits | Ref. | |
|---|---|---|---|---|---|
| 1 | Poly(diphenylamine) derivatives | 0.5~0.8 wt.% | Pentaerythritol ester oils | Reduced the total acid value of base oils, improved IOT values, and antioxidant performances | [83] |
| 2 | 1,3,5-tris(phenylamino) benzene | 0.5 wt.% | Synthetic ester oils | High-temperature antioxidants (210 °C) Improved IOT values and antioxidant performances | [84] |
| 3 | Dioctyldiphenylamine + sodium stearate | 1.0 wt.% + 0.06 wt.% | Pentaerythritol ester oils | Synergistic effect Reduced the acid value and viscosity of oils | [86] |
| 4 | Dioctyldiphenylamine + perfluorobutyric acid salts | 1.0 wt.% + 0.06 wt.% | Pentaerythritol ester oils | Synergistic effect Reduced the acid value and viscosity of oils | [87] |
| 5 | Dioctyldiphenylamine + acetylacetone | 1.0 wt.% + 0.06 wt.% | Pentaerythritol ester oils | Synergistic effect Reduced the acid value and viscosity of oils | [88] |
| 6 | Dioctyldiphenylamine + molybdate esters | 1.0 wt.% + 0.5 wt.% | Poly-α-olefin | Synergistic effect Improved the IOT and OIT values | [75] |
| 7 | Dioctyldiphenylamine + organic molybdenum complexes | 1.0 wt.% + 0.5–1.0 wt.% | Poly-α-olefin | Synergistic effect Reduced the acid value, oxidation, and deposition of oils | [89] |
| 8 | Dioctyldiphenylamine + molybdenum dithiocarbamate | 1.0 wt.% + 0.5 wt.% | Poly-α-olefin | Synergistic effect Reduced the oxidation and deposition of oils | [90] |
| 9 | Dioctyldiphenylamine + organic titanates | 0.5 wt.% + 0.5–3.0 wt.% | Poly-α-olefin | Synergistic effect Reduced the oxidation rates of oils | [92] |
| 10 | Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) | 0.95 wt.% | Rapeseed oils | Improved the OIT values and antioxidation performances | [104] |
| 11 | Ester-phenolic compounds | 0.5 wt.% | Mineral oils | Decreased volatility Improved the OIT values and thermal stability | [105] |
| 12 | Acylated chitosan Schiff base | 3000 ppm | N-butyl palmitate/stearate oils | Improved RBOT time and thermo-oxidative stability Decreased friction coefficient and wear diameter | [107] |
| 13 | Triazole derivatives | 5000 ppm | Polyol oils | Improved antioxidant and anti-corrosion performances | [110] |
| 14 | 2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenylthio)-1,3,5-triazine | 1 wt.% | Triisodecyl trimellitate ester oils | Improved antioxidant and thermal stability, as well as friction-reducing and anti-wear performances | [61] |
| 15 | Dendrons-like 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic ester chain | 0.5 wt.% | Hydrocarbon oils | Good solubility Better thermal stability and antioxidant performances than Irganox L135 and L57 | [113] |
| 16 | Mo-Tga {(S)-2-(18-methoxy-18-oxooctadecan-9-ylthio)acetic acid} | 0.5 wt.% | Polyol oils | Improved antioxidant ability as well as friction-reducing, anti-wear, and -corrosion performances | [117] |
| 17 | Esters of thiolated Butylated hydroxytoluene | 1.5–3 wt.% | Trimethylolpropane trioleate | Reducing oxidation rates of oils Better oxidation stability than BHT and Irganox 1076 | [118] |
| 18 | 3-(3,5-di-tert-butyl-4-hydroxy-phenyl)-propionic acid 2-(4-meth yl-thiazol-5-yl)-ethyl ester | 1–5 wt.% | Lithium complex greases | Improved antioxidant ability of oils Better antioxidant, anti-friction, and anti-wear properties than traditional ZDDP | [119] |
| 19 | Phenolic diphenylamine | 5 μmol/g | Poly-α-olefin and mineral oils | Intramolecular synergistic effect Improved the OIT values of oils Better antioxidant properties than BHT and Irganox 1076, etc. | [120] |
| 20 | S-containing phenolic diphenylamine | 5 μmol/g | Poly-α-olefin | Intramolecular synergistic effect Higher OIT values and antioxidant properties than BHT, Irganox 1076, DPA, and ODA | [125] |
| 21 | Difluoroboron derivatives | 0–10 μmol/g | 150 N containing 4 wt.% dispersant PIBSI | Improved antioxidant ability of oils Better antioxidant, extreme pressure, and anti-wear properties than diphenylamine and ZDDP | [127] |
| 22 | Poly(p-methoxyphenol-phenylamine) | 0.5 wt.% | Di-iso-octyl sebacate and petrochemical diester | Intramolecular synergistic effect Higher OIT values and antioxidant properties than T512, BHT, and BHA | [129] |
| 23 | Bio-based phenolic diphenylamine | 5 μmol/g | Dipentaeythritol ester | Intramolecular synergistic effect Higher free radical scavenging activities than commercial antioxidants | [131] |
| 24 | Bio-based multifunctional compounds | 5 μmol/g | Vegetable oils | Intramolecular synergistic effect Higher thermal stability and radical scavenging activity than commercial antioxidants Better anti-wear activities than ZDDP | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, D.; Wang, Y.; Liu, H.; Yan, J.; Lin, H.; Han, S. Research Progress of Antioxidant Additives for Lubricating Oils. Lubricants 2024, 12, 115. https://doi.org/10.3390/lubricants12040115
Xia D, Wang Y, Liu H, Yan J, Lin H, Han S. Research Progress of Antioxidant Additives for Lubricating Oils. Lubricants. 2024; 12(4):115. https://doi.org/10.3390/lubricants12040115
Chicago/Turabian StyleXia, Deping, Yonggang Wang, Hui Liu, Jincan Yan, Hualin Lin, and Sheng Han. 2024. "Research Progress of Antioxidant Additives for Lubricating Oils" Lubricants 12, no. 4: 115. https://doi.org/10.3390/lubricants12040115








