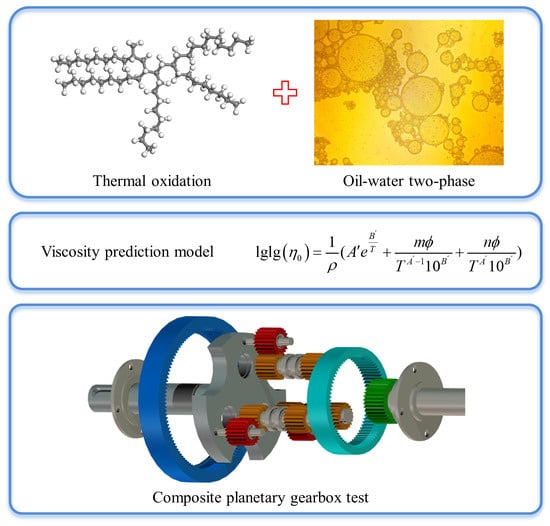
Deterioration Mechanism and Status Prediction of Hydrocarbon Lubricants under High Temperatures and Humid Environments
Abstract
:1. Introduction
2. Materials and Methods
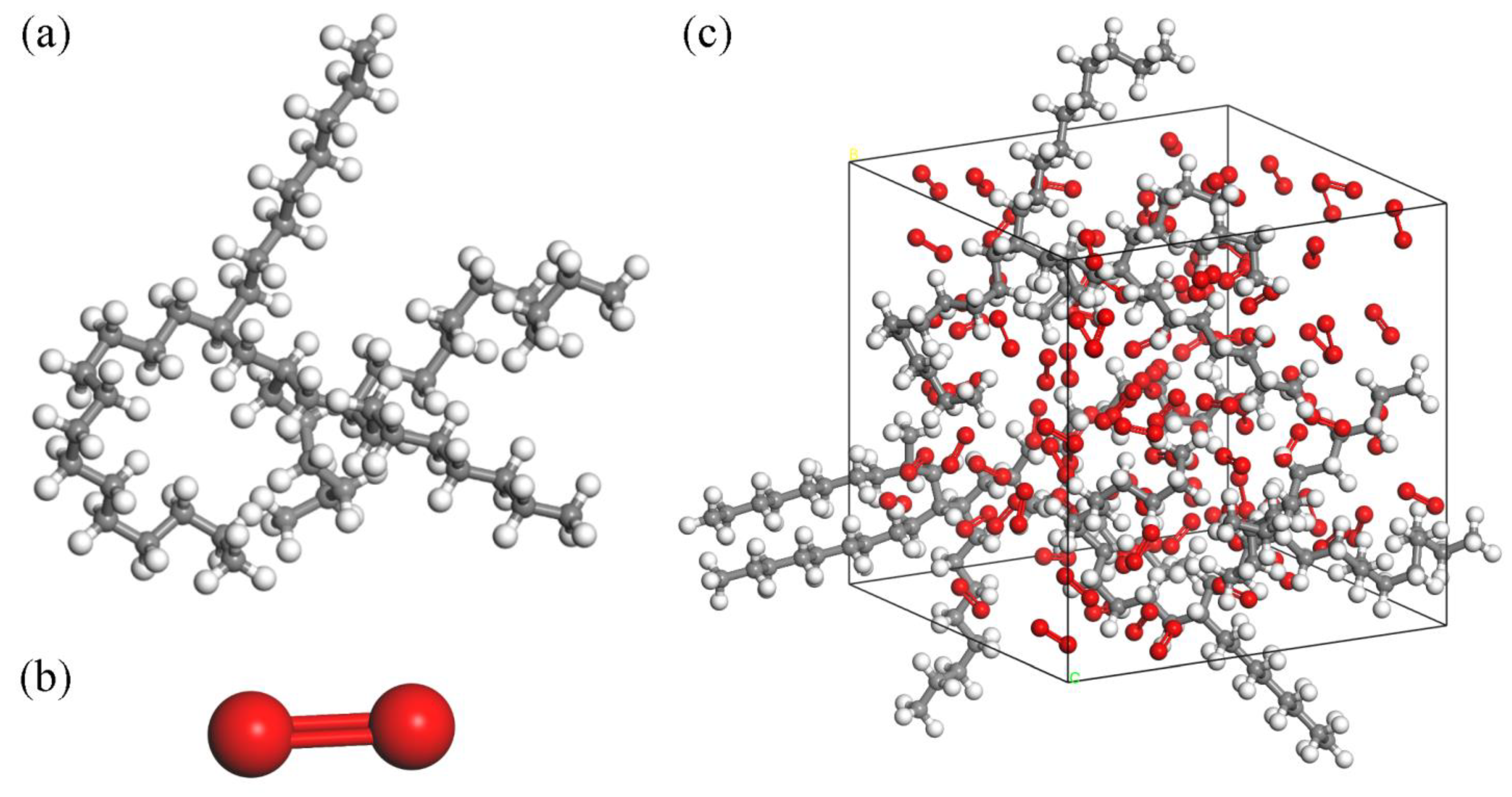
2.1. Thermal Oxidation Model of Lubricants
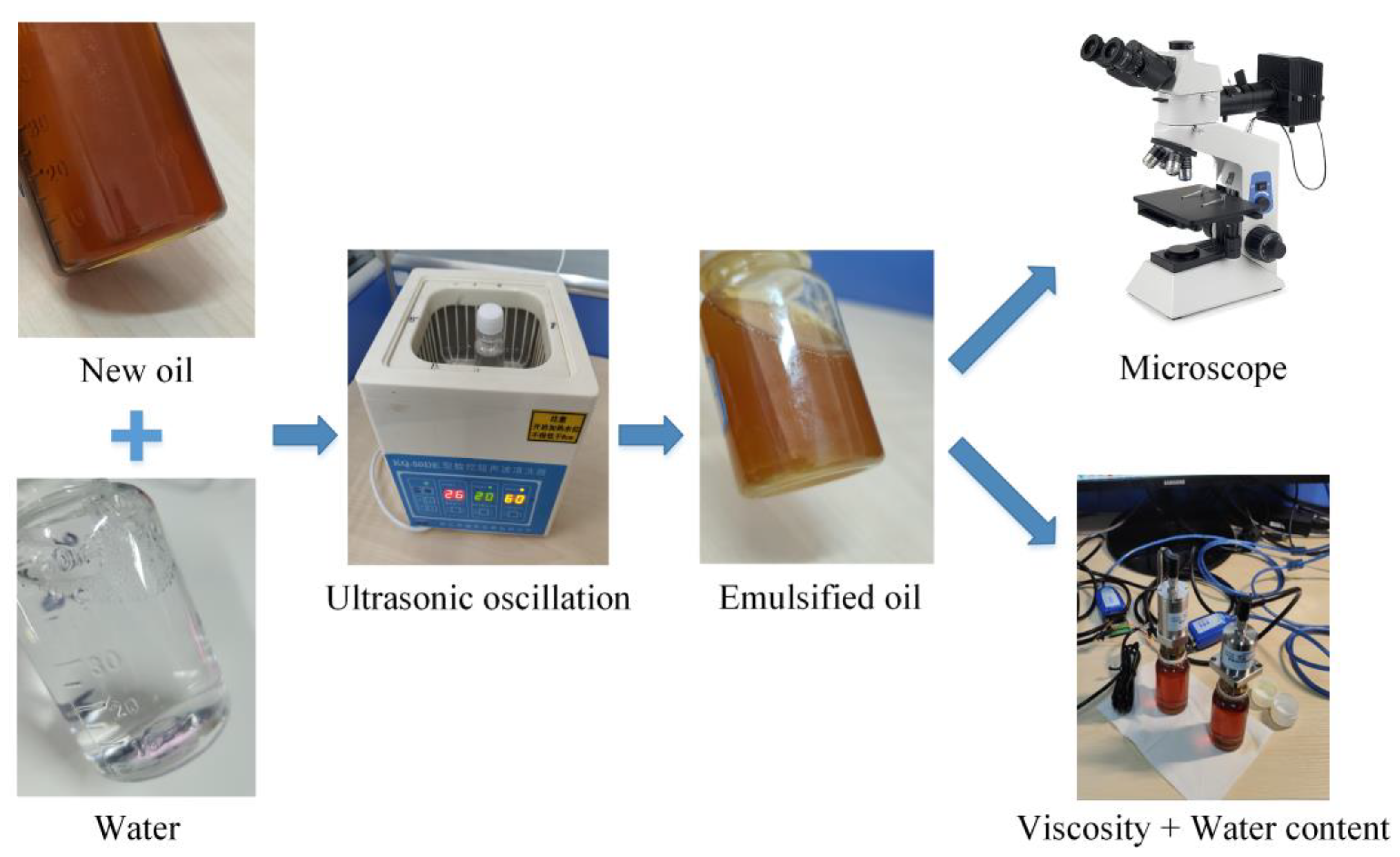
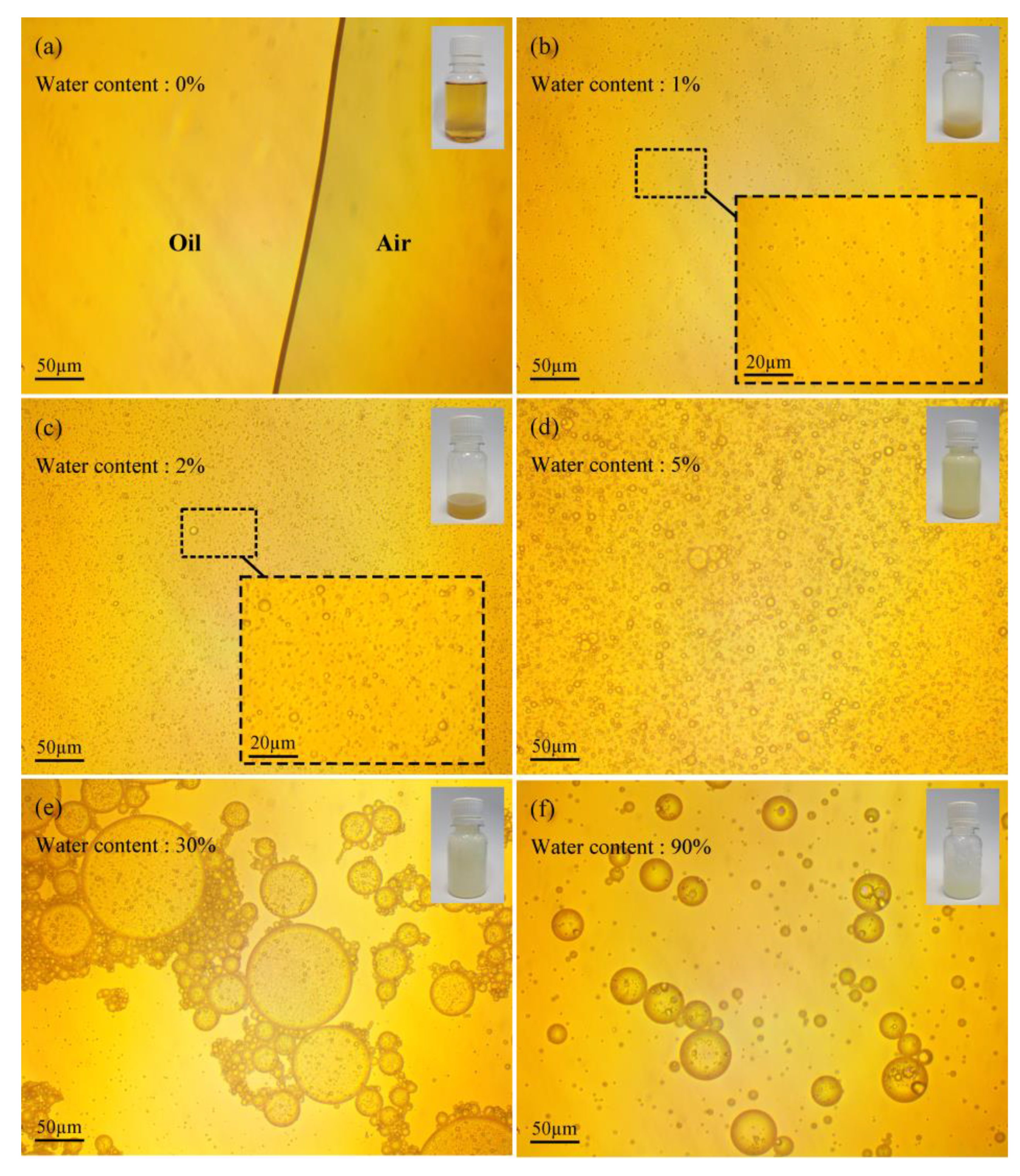
2.2. Analysis of the Micro− and Macro−Morphology of Emulsified Oil
2.3. Viscosity Prediction Model
3. Results and Discussion
3.1. ReaxFF MD Simulation Results of Lubricant
3.2. Microdroplet Morphology of Emulsified Oil
3.3. Viscosity Prediction Model and Safety Threshold Division
4. Wear Experiment
4.1. Wear Test of Compound Planetary Transmission System
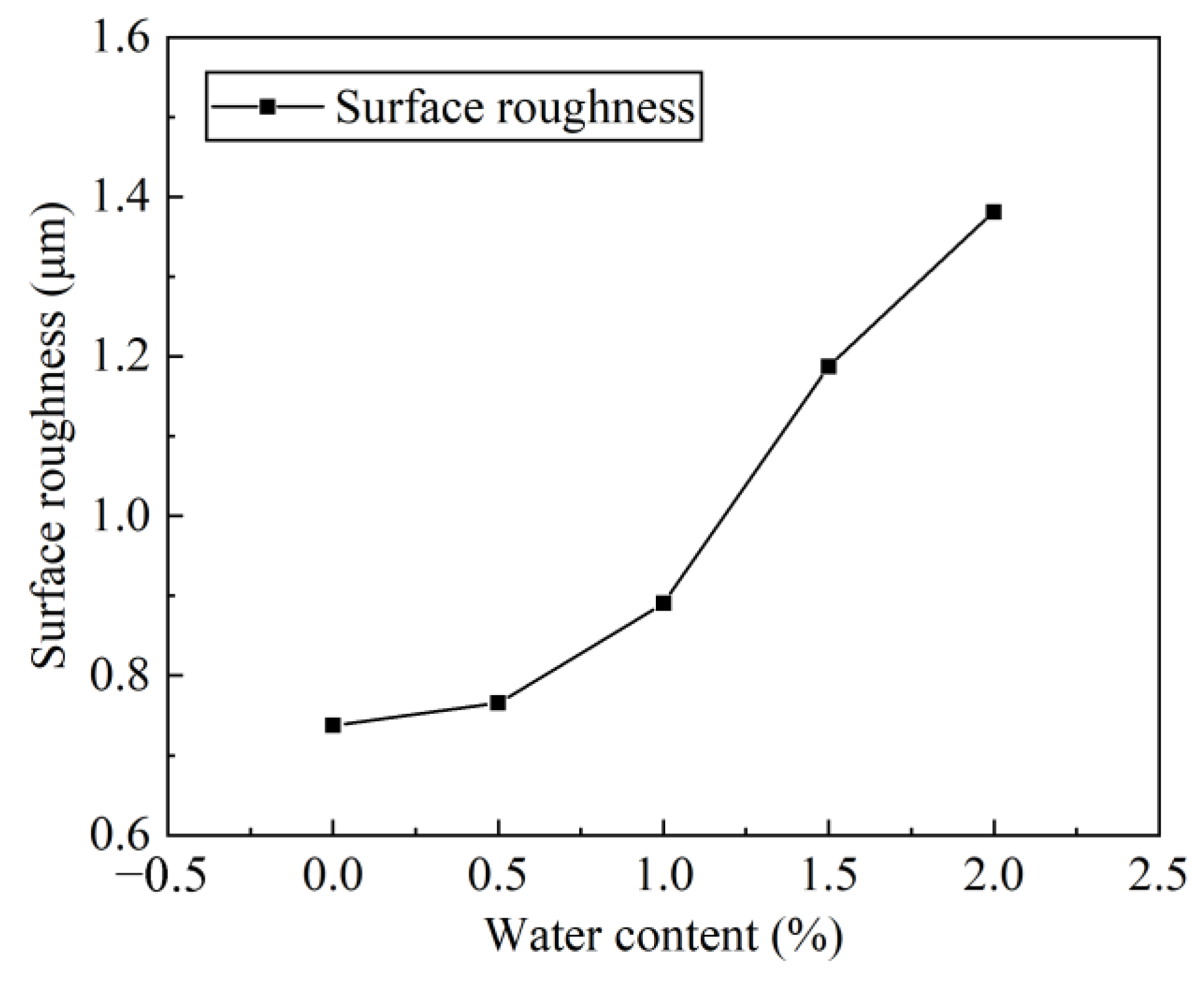
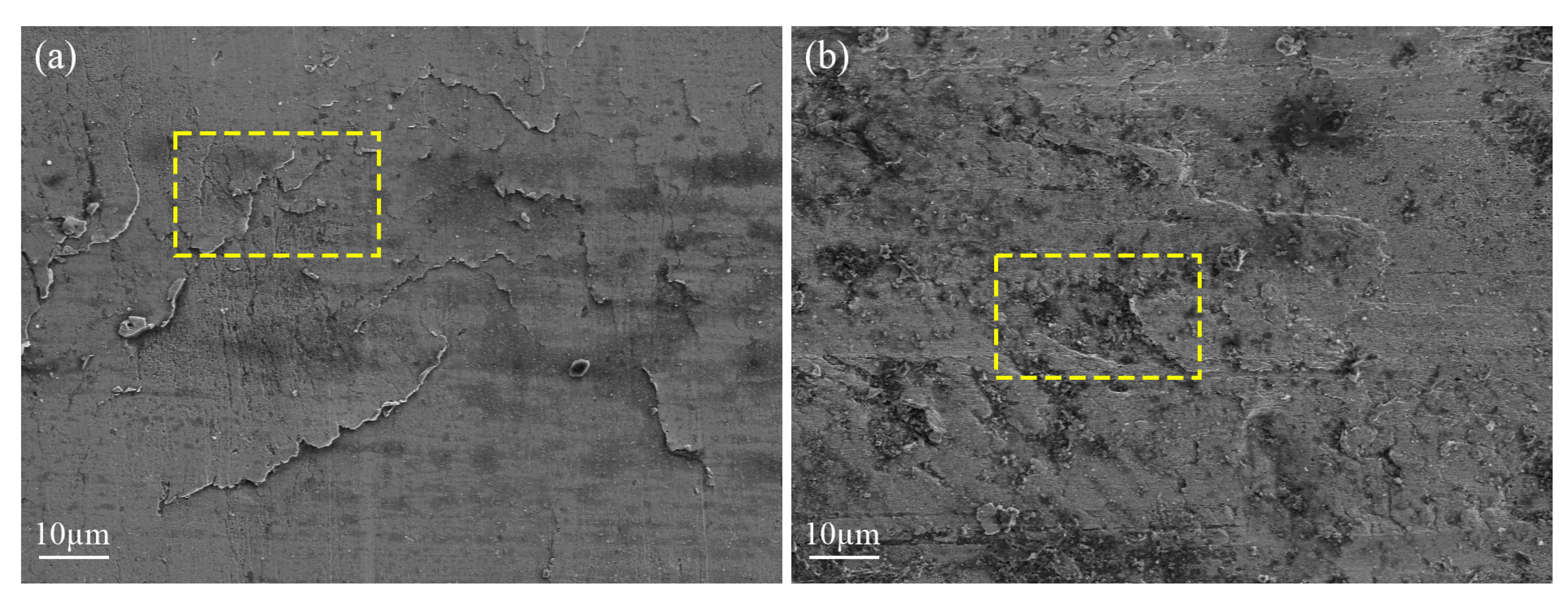
4.2. Characterization Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Dong, G.; Xie, Y.B.; Peng, Z. Prediction of wear trend of engines via on−line wear debris monitoring. Tribol. Int. 2018, 120, 510–519. [Google Scholar] [CrossRef]
- Holmberg, K.; Anderson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Ciulli, E. Vastness of Tribology Research Fields and Their Contribution to Sustainable Development. Lubricants 2024, 12, 33. [Google Scholar] [CrossRef]
- Cao, W.; Han, Z.; Yang, Z.Z.; Wang, N.; Qu, J.X.; Wang, D. Deterioration state diagnosis and wear evolution evaluation of planetary gearbox using vibration and wear debris analysis. Measurement 2022, 193, 110978. [Google Scholar] [CrossRef]
- Airey, J.; Spencer, M.; Greenwood, R.; Simmons, M. The effect of gas turbine lubricant base oil molecular structure on friction. Tribol. Int. 2020, 146, 106052. [Google Scholar] [CrossRef]
- Cao, W.; Han, Z.; Chen, Z.Q.; Jin, Z.L.; Wu, J.J.; Qu, J.X.; Wang, D. The influence of wear volume on surface quality in grinding process based on wear prediction model. Int. J. Adv. Manuf. Technol. 2022, 121, 5793–5809. [Google Scholar] [CrossRef]
- Kaminski, P. Experimental investigation into the effects of fuel dilution on the change in chemical properties of lubricating oil used in fuel injection pump of Pielstick PA4 V185 marine diesel engine. Lubricants 2022, 10, 162. [Google Scholar] [CrossRef]
- Tian, X.; Song, N.; Yang, G.; Zhou, C.H.; Zhang, S.M.; Zhang, P.Y. Organic−Sulfonate Functionalized Graphene as a High Temperature Lubricant for Efficient Antifriction and Antiwear in Water Based Drilling Fluid. Tribol. Lett. 2022, 70, 32. [Google Scholar] [CrossRef]
- Nagendramma, P.; Kaul, S.; Bisht, R.P.S. Study of synthesised ecofriendly and biodegradable esters: Fire resistance and lubricating properties. Lubr. Sci. 2010, 22, 103–110. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Lin, B.C.; Huang, Q.X.; Ma, Z.Y.; Chi, Y.; Yan, J.H. Micro− and mesoporous−enriched carbon materials prepared from a mixture of petroleum−derived oily sludge and biomass. Fuel Process. Technol. 2018, 171, 140–147. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V. Market Potential of Industrial Technologies for Production of Synthetic Bases of Motor Oils. Russ. J. Gen. Chem. 2021, 91, 1243–1259. [Google Scholar] [CrossRef]
- Liu, X.; Yamaguchi, R.; Umehara, N.; Deng, X.; Kousaka, H.; Murashima, M. Clarification of high wear resistance mechanism of ta−CNx coating under poly alpha−olefin (PAO) lubrication. Tribol. Int. 2017, 105, 193–200. [Google Scholar] [CrossRef]
- Wang, W.W.; Li, P.; Sheng, S.Z.; Tian, H.T.; Zhang, X. Influence of Hydrocarbon Base Oil Molecular Structure on Lubricating Properties in Nano−scale Thin Film. Tribol. Lett. 2019, 67, 111. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhao, Y.; Ma, K.; Wang, Q. Friction behavior and wear protection ability of selected base lubricants. Friction 2016, 4, 72–83. [Google Scholar] [CrossRef]
- Wu, N.; Zong, Z.M.; Fei, Y.W.; Ma, J.; Guo, F. Thermal oxidation stability of poly−α−olefin lubricating oil. Asia−Pac. J. Chem. Eng. 2017, 12, 813–817. [Google Scholar] [CrossRef]
- Ouyang, W.; Yan, Z.; Zhou, X.; Luo, B.; Wang, B.; Huang, J.A. Thermal Hydrodynamic Model for Emulsified Oil−Lubricated Tilting−Pad Thrust Bearings. Lubricants 2023, 11, 529. [Google Scholar] [CrossRef]
- Harika, E.; Bouyer, J.; Fillon, M.; Hélène, M. Effects of Water Contamination of Lubricants on Hydrodynamic Lubrication: Rheological and Thermal Modeling. J. Tribol. 2013, 135, 1115–1124. [Google Scholar] [CrossRef]
- Wang, Z.N.; Li, J.J.; Liu, Y.H.; Luo, J.B. Synthesis and characterizations of zwitterionic copolymer hydrogels with excellent lubrication behavior. Tribol. Int. 2019, 143, 106026. [Google Scholar] [CrossRef]
- Liang, H.; Guo, D.; Ma, L.R.; Luo, J.B. Increased Film Thickness of Oil−in−Water (O/W) Emulsions at High Speed. Tribol. Lett. 2017, 65, 68. [Google Scholar] [CrossRef]
- Dittes, N.; Sjodahl, M.; Pettersson, A.; Lang, D.F. Corrosion sensor for water contaminated grease. Tribol. Trans. 2020, 63, 891–896. [Google Scholar] [CrossRef]
- Lu, W.L.; Zhai, W.Z.; Zhang, P.; Zhou, M.Z.; Liu, X.J.; Zhou, L.P. Effect of different levels of free water in oil on the fretting wear of nickel−aluminum bronze based composites. Wear 2017, 390–391, 376–384. [Google Scholar] [CrossRef]
- Qin, H.; Doll, G.L. Effects of water contamination on micropitting and rolling contact fatigue of bearing steels. ASME J. Tribol. 2023, 145, 011501. [Google Scholar] [CrossRef]
- Dawczyk, J.; Morgan, N.; Russo, J.; Spikes, H. Film Thickness and Friction of ZDDP Tribofilms. Tribol. Lett. 2019, 67, 34. [Google Scholar] [CrossRef]
- Meng, Y.G.; Xu, J.; Ma, L.R.; Jin, Z.M.; Prakash, B.; Ma, T.B.; Wang, W.Z. A review of advances in tribology in 2020–2021. Friction 2022, 10, 1443–1595. [Google Scholar] [CrossRef]
- Noureddini, H.; Teoh, B.C.; Clements, L.D. Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1189–1191. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukherjee, P.S.; Mishra, N.M. Interrelationship among viscosity, temperature and age of lubricant. Ind. Lubr. Tribol. 2006, 58, 50–55. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, K.W. The validity of the Reynolds equation in spool valve analysis considering cavitation. Friction 2016, 4, 266–276. [Google Scholar] [CrossRef]
- Roy, S.; Pedersen, H.; Sinha, S.; Hansen, A. The Co−Moving Velocity in Immiscible Two−Phase Flow in Porous Media. Transp. Porous. Med. 2022, 143, 69–102. [Google Scholar] [CrossRef]
- Gan, Z.T.; Yao, T.; Zhang, M.; Hu, J.Q.; Liao, X.X.; Shen, Y.L. Effect of Temperature on the Composition of a Synthetic Hydrocarbon Aviation Lubricating Oil. Materials 2020, 13, 1606. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, M.; Besser, C.; Allmaier, G.; Dörr, N. Oxidation Products of Ester−Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry. Appl. Sci. 2017, 7, 396. [Google Scholar] [CrossRef]
- Gopal, K.N.; Raj, R.T.K. Effect of pongamia oil methyl ester−diesel blend on lubricating oil degradation of di compression ignition engine. Fuel 2016, 165, 105–114. [Google Scholar] [CrossRef]
- Chen, M.Q.; He, M.X.; Li, M.; Qu, Q.H. Application of Interval Selection Methods in Quantitative Analysis of Water Content in Engine Oil by Terahertz Spectroscopy. Spectrosc. Spect. Anal. 2021, 41, 1393–1397. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.N.; Su, H.G.; Li, W.M.; Lou, W.J.; Wang, X.B. Synthesis and evaluation of a protic ionic liquid as a multifunctional lubricant additive. Friction 2020, 8, 568–576. [Google Scholar] [CrossRef]
- Li, Y.B.; He, T.S.; Hu, Z.M.; Zhang, Y.Q.; Luo, Q.; Pu, W.F.; Zhao, J.Z. Study on the mathematical model for predicting settling of water−in−oil emulsion. J. Petrol. Sci. Eng. 2021, 206, 109070. [Google Scholar] [CrossRef]
- Chang, H.C.; Borghesani, P.; Smith, W.A.; Peng, Z.X. Application of surface replication combined with image analysis to investigate wear evolution on gear teeth−A case study. Wear 2019, 430, 355–368. [Google Scholar] [CrossRef]





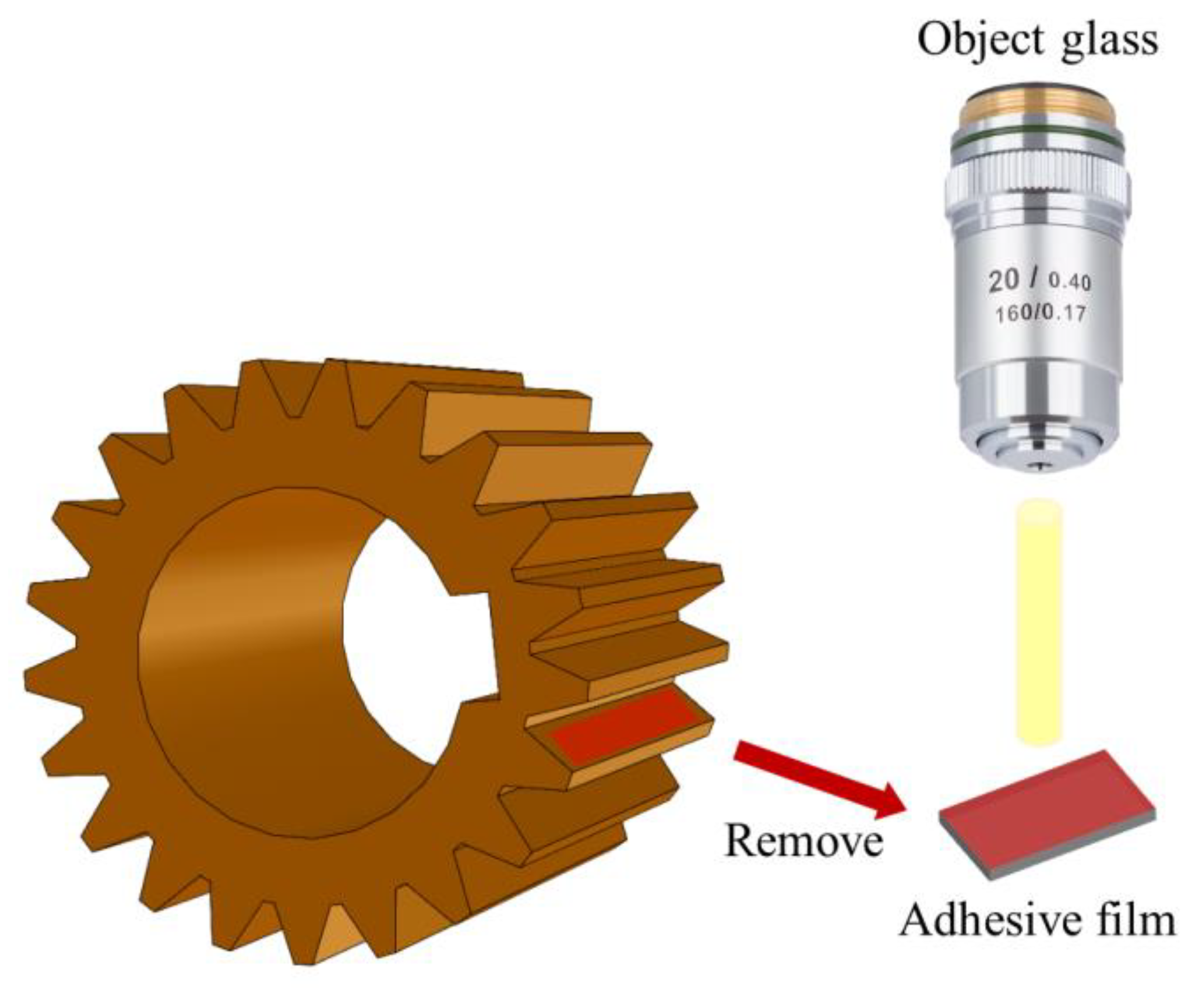
| Product No. | Kinematic Viscosity (40 °C) | Kinematic Viscosity (100 °C) | Viscosity Index | Flash Point (Opening) | Pour Point |
|---|---|---|---|---|---|
| KunLun GL−5 85W/90 | 182.1 mm2/s | 16.5 mm2/s | 96 | 230 °C | −20 °C |
| Water Content | Stabilization Time | Threshold Time | Safety Threshold |
| 0% | 160 min | 115 min | 142.89 mm2/s |
| 1% | 160 min | 100 min | 197.85 mm2/s |
| 2% | 160 min | 115 min | 201.52 mm2/s |
| 3% | 160 min | 115 min | 201.82 mm2/s |
| 4% | 160 min | 120 min | 232.79 mm2/s |
| 5% | 160 min | 115 min | 234.82 mm2/s |
| Model No. | Accuracy | Hardness | Dimensional Changes | Working Temperature | Setting Time |
|---|---|---|---|---|---|
| MICROSET 101 RF | 0.1 μm | 28–30 Shore A | <0.1% | −10 °C–180 °C | 5 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Cao, W.; Jin, Z.; Wang, Y.; Ding, L.; Maqsood, M.; Wang, D. Deterioration Mechanism and Status Prediction of Hydrocarbon Lubricants under High Temperatures and Humid Environments. Lubricants 2024, 12, 116. https://doi.org/10.3390/lubricants12040116
Su R, Cao W, Jin Z, Wang Y, Ding L, Maqsood M, Wang D. Deterioration Mechanism and Status Prediction of Hydrocarbon Lubricants under High Temperatures and Humid Environments. Lubricants. 2024; 12(4):116. https://doi.org/10.3390/lubricants12040116
Chicago/Turabian StyleSu, Rui, Wei Cao, Zili Jin, Yifan Wang, Letian Ding, Muhammad Maqsood, and Dong Wang. 2024. "Deterioration Mechanism and Status Prediction of Hydrocarbon Lubricants under High Temperatures and Humid Environments" Lubricants 12, no. 4: 116. https://doi.org/10.3390/lubricants12040116