Comparative Study of Tribological Behavior of Electroless Ni–B, Ni–B–Mo, and Ni–B–W Coatings at Room and High Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of Electroless Nickel Boron and Its Variants
2.2. Characterization of Nickel Boron and Its Variants
2.3. Tribological Characterization of Nickel Boron and Its Variants
3. Results and Discussion
3.1. Composition, Structure, and Surface Morphology of Nickel Boron and Its Variants
3.2. Microhardness of Nickel Boron and Its Variants
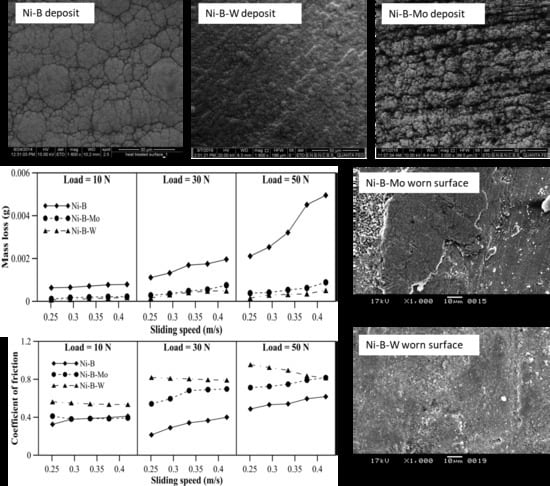
3.3. Tribological Behavior of Nickel Boron and Its Variants at Room Temperature
3.4. Tribological Behavior of Nickel Boron and Its Variants at 100 °C
3.5. Tribological Behavior of Nickel Boron and Its Variants at 300 °C
3.6. Tribological Behavior of Nickel Boron and Its Variants at 500 °C
3.7. Effect of Temperature on Tribology of Nickel Boron and Its Variants
3.8. Selection of the Coatings Based on Tribological Behavior
4. Conclusions
- EDX analysis of the as-deposited coatings reveals that the deposits lie in the mid-B range (within 5–6 wt % B).
- To improve the mechanical properties of the coatings, they are heat treated. XRD analysis of the heat treated coatings indicates the precipitation of crystalline Ni (111) and boride phases.
- SEM micrograph of the heat treated coatings show that the surface is characterized by nodulated self-lubricating structures. Ni–B–Mo coatings present a coarse morphology with visible cellular boundaries; whereas Ni–B–W coatings have a densely nodulated morphology. The surface of Ni–B coating resembles cauliflower like morphology, which is common in borohydride reduced coatings.
- Because of precipitation of hard boride phases, a high microhardness of the coatings is observed on heat treatment. Ni–B–W coatings exhibit the highest microhardness followed by Ni–B and Ni–B–Mo coatings. Because of the coarse structure, Ni–B–Mo coatings have lower microhardness compared with Ni–B or Ni–B–W.
- The heat treated coatings are subjected to tribological tests at room and elevated temperatures of 100 °C, 300 °C, and 500 °C. For the temperature range considered, Ni–B–W coatings exhibit the highest wear resistance. Based on COF, Ni–B coatings exhibit lower values at room temperature, Ni–B–Mo at 100 °C, and Ni–B at 300 °C.
- At the highest value of test temperature, that is, 500 °C, almost all the three coatings exhibit similar values of COF. Although if one coating among the three has to be selected, Ni–B–W would be a suitable option.
- Ni–B–W coatings show higher COF at room temperature or 100 °C, but it improves at 300 °C or 500 °C. This is due to the fact that oxides of tungsten tend to be brittle at room temperatures, but have a lubricating effect at high temperatures.
- The wear mechanism also varies at different temperatures for Ni–B, Ni–B–Mo,or Ni–B–W coatings. In fact, the tribological behavior, wear mechanisms, and microstructural changes are inter-related.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sahoo, P.; Das, S.K. Tribology of electroless nickel coatings—A review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Duari, S.; Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Tribological performance optimization of electroless nickel coatings under lubricated condition. In Design and Optimization of Mechanical Engineering Products; Kumar, K., Davim, J.P., Eds.; IGI Global: Hershey, PA, USA, 2018; pp. 250–280. [Google Scholar]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Electroless nickel coatings for high temperature applications. In Composites and Advanced Materials for Industrial Applications; Kumar, K., Davim, J.P., Eds.; IGI Global: Hershey, PA, USA, 2018; pp. 297–331. [Google Scholar]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Zangari, G. Study of the electroless deposition process of Ni-P-based ternary alloys. J. Electrochem. Soc. 2003, 150, C777–C786. [Google Scholar] [CrossRef]
- Zhang, W.X.; Huang, N.; He, J.G.; Jiang, Z.H.; Jiang, Q.; Lian, J.S. Electroless deposition of Ni–W–P coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2007, 253, 5116–5121. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Anandan, C.; Rajam, K.S. Morphological study of ternary Ni–Cu–P alloys by atomic force microscopy. Appl. Surf. Sci. 2005, 250, 88–97. [Google Scholar] [CrossRef]
- Georgieva, J.; Armyanov, S. Electroless deposition and some properties of Ni–Cu–P and Ni–Sn–P coatings. J. Solid State Electrochem. 2007, 11, 869–876. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Selvakumar, S.; Stephen, A. Electroless Ni–Co–P ternary alloy deposits: Preparation and characteristics. Surf. Coat. Technol. 2003, 172, 298–307. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Rajam, K.S. Electroless deposition of Ni–Cu–P, Ni–W–P and Ni–W–Cu–P alloys. Surf. Coat. Technol. 2005, 195, 154–161. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Grips, V.W.; Rajam, K.S. Electrochemical studies on electroless ternary and quaternary Ni–P based alloys. Electrochim. Acta 2006, 52, 1064–1074. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Jahan, S.M.; Jain, A.; Rajam, K.S. Structure and phase transformation behavior of electroless Ni–P alloys containing tin and tungsten. J. Alloys Compd. 2007, 436, 319–327. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Effect of heat treatment on microstructure and corrosion resistance of Ni-B-W-Mo coating deposited by electroless method. Surf. Rev. Lett. 2017, 1950023. [Google Scholar] [CrossRef]
- Palaniappa, M.; Seshadri, S.K. Friction and wear behavior of electroless Ni–P and Ni–W–P alloy coatings. Wear 2008, 265, 735–740. [Google Scholar] [CrossRef]
- Liu, H.; Lv, Y.Y.; Liu, Z.; Thompson, G.E. Dry sliding wear behaviour and structural characteristics of laser-annealed electroless Ni–P/Ni–Mo–P duplex coatings. Tribol. Int. 2016, 103, 343–351. [Google Scholar] [CrossRef]
- Tien, S.K.; Duh, J.G. Thermal reliability of electroless Ni–P–W coating during the aging treatment. Thin Solid Films 2004, 469, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, L.V.; Barba, A.; Bolarin, A.; Sanchez, F. Age hardening of Ni–P–Mo electroless deposit. Surf. Eng. 2006, 22, 58–62. [Google Scholar] [CrossRef]
- Liu, H.; Yao, H.L.; Thompson, G.E.; Liu, Z.; Harrison, G. Correlation between structure and properties of annealed electroless Ni–W–P coatings. Surf. Eng. 2015, 31, 412–419. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Tian, Z.; Yuan, M.; Ma, X. Effect of copper content on the properties of electroless Ni–Cu–P coatings prepared on magnesium alloys. Appl. Surf. Sci. 2015, 356, 289–293. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P and Ni-P-Al2O3 nanocomposite coatings and their corrosion and wear resistance. J. Mater. Eng. Perform. 2013, 22, 176–183. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W.; He, Y. A novel electroless plating of Ni–P–TiO2 nano-composite coatings. Surf. Coat. Technol. 2010, 204, 2493–2498. [Google Scholar] [CrossRef]
- Farzaneh, A.; Mohammadi, M.; Ehteshamzadeh, M.; Mohammadi, F. Electrochemical and structural properties of electroless Ni–P–SiC nanocomposite coatings. Appl. Surf. Sci. 2013, 276, 697–704. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electroless Ni–B coatings: Preparation and evaluation of hardness and wear resistance. Surf. Coat. Technol. 2005, 190, 115–121. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Priyadarshi, A.; Kumar, V.; Manikandanath, N.T.; Kumar, P.P.; Ravisankar, B. Hardness and wear behaviour of electroless Ni–B coatings. Mater. Sci. Technol. 2016, 32, 1654–1665. [Google Scholar] [CrossRef]
- Çelik, İ.; Karakan, M.; Bülbül, F. Investigation of structural and tribological properties of electroless Ni–B coated pure titanium. Proc. IMechE Part J J. Eng. Tribol. 2016, 230, 57–63. [Google Scholar] [CrossRef]
- Dervos, C.T.; Novakovic, J.; Vassiliou, P. Vacuum heat treatment of electroless Ni–B coatings. Mater. Lett. 2004, 58, 619–623. [Google Scholar] [CrossRef]
- Correa, E.; Zuleta, A.A.; Guerra, L.; Gómez, M.A.; Castaño, J.G.; Echeverría, F.; Liu, H.; Skeldon, P.; Thompson, G.E. Tribological behavior of electroless Ni–B coatings on magnesium and AZ91D alloy. Wear 2013, 305, 115–123. [Google Scholar] [CrossRef]
- Aydeniz, A.I.; Göksenli, A.; Dil, G.; Muhaffel, F.; Calli, C.; Yüksel, B. Electroless Ni–B–W coatings for improving hardness, wear and corrosion resistance. Mater. Technol. 2013, 47, 803–806. [Google Scholar]
- Serin, I.G.; Göksenli, A. Effect of annealing temperature on hardness and wear resistance of electroless Ni–B–Mo coatings. Surf. Rev. Lett. 2015, 22, 1550058. [Google Scholar] [CrossRef]
- Ghaderi, M.; Rezagholizadeh, M.; Heidary, A.; Monirvaghefi, S.M. The effect of Al2O3 nanoparticles on tribological and corrosion behavior of electroless Ni–B–Al2O3 composite coating. Prot. Met. Phys. Chem. Surf. 2016, 52, 854–858. [Google Scholar] [CrossRef]
- Rezagholizadeh, M.; Ghaderi, M.; Heidary, A.; Monirvaghefi, S.M. The effect of B4C nanoparticles on the corrosion and tribological behavior of electroless Ni–B–B4C composite coatings. Surf. Eng. Appl. Electrochem. 2015, 51, 18–24. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Cao, L.; Zhang, M.; Gao, J.; Qi, C. Corrosion and tribological performance of PTFE-coated electroless nickel boron coatings. Surf. Coat. Technol. 2016, 307, 316–323. [Google Scholar] [CrossRef]
- Riddle, Y.W.; Bailerare, T.O. Friction and wear reduction via an Ni–B electroless bath coating for metal alloys. JOM 2005, 57, 40–45. [Google Scholar] [CrossRef]
- Bonin, L.; Vitry, V. Mechanical and wear characterization of electroless nickel mono and bilayers and high boron-mid phosphorus electroless nickel duplex coatings. Surf. Coat. Technol. 2016, 307, 957–962. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Formation and characterization of multilayers borohydride and hypophosphite reduced electroless nickel deposits. Electrochim. Acta 2017, 243, 7–17. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Tribological behavior of sodium borohydride reduced electroless nickel alloy coatings at room and elevated temperatures. Surf. Coat. Technol. 2017, 321, 464–476. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Effects of heat treatment on tribological behavior of electroless Ni–B coating at elevated temperatures. Surf. Rev. Lett. 2017, 24 (Suppl. 1), 1850014. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Tribological behavior of electroless Ni–B–W coating at room and elevated temperatures. Proc. IMechE Part J J. Eng. Tribol. 2018. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Effect of heat treatment on tribological behavior of electroless Ni–B–Mo coatings at different operating temperatures. Silicon 2018, 10, 1203–1215. [Google Scholar] [CrossRef]
- Pal, S.; Sarkar, R.; Jayaram, V. Characterization of thermal stability and high-temperature tribological behavior of electroless Ni–B coating. Metall. Mater. Trans. A 2018. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Friction and wear performance of electroless Ni–B coatings at different operating temperatures. Silicon 2018. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Wear and friction characteristics of electroless Ni–B–W coatings at different operating temperatures. Mater. Res. Express 2018, 5, 026526. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Tribological characteristics of electroless Ni–B–Mo coatings at different operating temperatures. Surf. Rev. Lett. 2018, 1850175. [Google Scholar] [CrossRef]
- Mattox, D.M. Film Characterization and Some Basic Film Properties. In Handbook of Physical Vapor Deposition (PVD) Processing: Film Formation, Adhesion, Surface Preparation and Contamination Contro; Mattox, D.M., Ed.; Noyes Publications: Westwood, NJ, USA, 1998; pp. 569–615. [Google Scholar]
- Cai, F.; Huang, X.; Yang, Q. Mechanical properties, sliding wear and solid particle erosion behaviors of plasma enhanced magnetron sputtering CrSiCN coating systems. Wear 2015, 324, 27–35. [Google Scholar] [CrossRef]
- Berg, G.; Friedrich, C.; Broszeit, E.; Berger, C. Development of chromium nitride coatings substituting titanium nitride. Surf. Coat. Technol. 1996, 86, 184–191. [Google Scholar] [CrossRef]
- Zhen, J.; Zhu, S.; Cheng, J.; Qiao, Z.; Liu, W.; Yang, J. Effects of sliding speed and testing temperature on the tribological behavior of a nickel-alloy based solid-lubricating composite. Wear 2016, 368, 45–52. [Google Scholar] [CrossRef]


















| Parameters | Levels | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Load (N) | 10 | 30 | 50 | × | × |
| Sliding speed (m/s) | 0.25 | 0.29 | 0.33 | 0.38 | 0.42 |
| Temperature (°C) | 25 | 100 | 300 | 500 | × |
| Test duration = 300 s | |||||
| Elements | Weight Percentage in Different Coatings | ||
|---|---|---|---|
| Ni–B | Ni–B–Mo | Ni–B–W | |
| Ni | 94.5 | 90.7 | 91.4 |
| B | 5.5 | 5.4 | 5.2 |
| W | × | × | 3.4 |
| Mo | × | 3.9 | × |
| Coating | Peak Position (2θ) | Absolute Integrated Intensity (au) | Crystallite Size (nm) | |||
|---|---|---|---|---|---|---|
| Ni | Ni3B | Ni | Ni3B | Ni | Ni3B | |
| Ni–B (350 °C) | 46 | 47 | 2455 | 2014 | 18.1 | 18.15 |
| Ni–B–W (350 °C) | 44.9 | 45.95 | 715 | 639 | 38.2 | 26.64 |
| Ni–B–Mo (350 °C) | 44.7 | 46 | 1892 | 1209 | 51 | 24.96 |
| Ni–B–Mo (450 °C) | 44.7 | 45.85 | 2268 | 708 | 51 | 23.81 |
| Coating | Surface Roughness, Ra (μm) | |
|---|---|---|
| Heat Treated at 350 °C | Heat Treated at 450 °C | |
| Ni–B | 0.66 | - |
| Ni–B–Mo | 0.76 | 0.87 |
| Ni–B–W | 0.46 | - |
| Coating | Microhardness (HV100) | |
|---|---|---|
| Heat Treated at 350 °C | Heat Treated at 450 °C | |
| Ni–B | 1060 ± 20 | × |
| Ni–B–Mo | 738 ± 20 | 690 ± 20 |
| Ni–B–W | 1181 ± 20 | × |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhopadhyay, A.; Barman, T.K.; Sahoo, P.; Davim, J.P. Comparative Study of Tribological Behavior of Electroless Ni–B, Ni–B–Mo, and Ni–B–W Coatings at Room and High Temperatures. Lubricants 2018, 6, 67. https://doi.org/10.3390/lubricants6030067
Mukhopadhyay A, Barman TK, Sahoo P, Davim JP. Comparative Study of Tribological Behavior of Electroless Ni–B, Ni–B–Mo, and Ni–B–W Coatings at Room and High Temperatures. Lubricants. 2018; 6(3):67. https://doi.org/10.3390/lubricants6030067
Chicago/Turabian StyleMukhopadhyay, Arkadeb, Tapan Kumar Barman, Prasanta Sahoo, and J. Paulo Davim. 2018. "Comparative Study of Tribological Behavior of Electroless Ni–B, Ni–B–Mo, and Ni–B–W Coatings at Room and High Temperatures" Lubricants 6, no. 3: 67. https://doi.org/10.3390/lubricants6030067