Improved Tribocorrosion Resistance of a CoCrMo Implant Material by Carburising
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Low-Temperature Carburising Treatment
2.2. Electrolyte
2.3. Characterisation of Carburised Layer
2.4. Potentiodynamic Tests
2.5. Tribocorrosion Tests
- Cathodic (−700 mV vs. SCE): This potential suppresses metal dissolution and the formation of the passive film and it enables the measurement of material loss via mechanical wear in the absence of corrosion. The material loss is calculated from volume measurements of the resulting scars following tests under cathodic potential conditions;
- OCP: A passive film forms spontaneously on the sample surface. Sliding is expected to mechanically damage and/or remove this self-generating protective layer (Type I corrosion-wear) with an associated drop in potential at which equilibrium of the anodic and cathodic reactions is achieved. The OCP trace is recorded and provides a qualitative insight into the type and extent of electrochemical activity during tribocorrosion testing;
- Passive (100 mV vs. SCE): The passive film forms under this test condition but is damaged or removed by the mechanical action. During testing, the anodic current versus time is recorded. The measured current gives qualitative information on the type of electrochemical damage and is used for the quantitative determination of the material losses via corrosion and corrosion due to wear during tribocorrosion testing using Faraday’s equation.
3. Results
3.1. S-Phase Layer Characterisation
3.2. Potentiodynamic Curves
3.3. Wear Tests under Cathodic Potential Conditions
3.4. Tribocorrosion Tests under OCP Conditions
3.5. Tribocorrosion Tests under Passive Potential Conditions
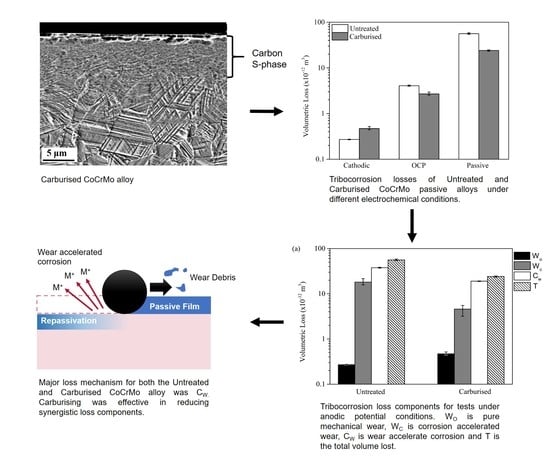
3.6. Tribocorrosion Losses
3.7. Characterisation of Resultant Scars
3.7.1. Cathodic Potential
3.7.2. OCP
3.7.3. Passive Potential
4. Discussion
4.1. Material Loss Dependence on the Electrochemical Potential
4.1.1. Volumetric Loss under Cathodic Potential Conditions
4.1.2. Volumetric Loss under OCP and Passive Potential Conditions
4.2. Material Loss Components
5. Conclusions
- The carburising of CoCrMo alloy was successful in lowering the tribocorrosion losses under both equilibrium and passive potential conditions. A similar volumetric loss was observed under cathodic potential conditions for both untreated and carburised CoCrMo samples.
- Under cathodic potential and OCP conditions, the morphology of the wear tracks produced on untreated CoCrMo was predominantly characterised by abrasion marks, as opposed to polishing wear experienced by the carburised CoCrMo alloy.
- Under anodic potential conditions, the morphology of wear tracks produced on the untreated and carburised samples was similar and comprised of fine micro-abrasion marks aligned with the direction of sliding. Tribocorrosion processes have resulted in the removal of almost the entire S-phase layer exposing a softer material with mechanical properties more similar to the untreated CoCrMo alloy.
- Synergism between corrosion and wear constituted 99.5% and 98.0% of the loss associated with the untreated and carburised CoCrMo samples, respectively.
- The improvement in corrosion-wear, CW (the dominant material loss component), of the carburised alloy was attributed to the better qualities of the passive film of the carburised sample coupled with an increased load support when compared to the untreated CoCrMo alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campbell, J.R.; Estey, M.P. Metal release from hip prostheses: Cobalt and chromium toxicity and the role of the clinical laboratory. Clin. Chem. Lab. Med. 2012, 51, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Dieppe, P.; Vernon, K.; Porter, M.; Blom, A.W. Failure rates of stemmed metal-on-metal hip replacements: Analysis of data from the National Joint Registry of England and Wales. Lancet 2012, 379, 1199–1204. [Google Scholar] [CrossRef]
- Gill, H.S.; Grammatopoulos, G.; Adshead, S.; Tsialogiannis, E.; Tsiridis, E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol. Med. 2012, 18, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mischler, S.; Muñoz, A.I. Wear of CoCrMo alloys used in metal-on-metal hip joints: A tribocorrosion appraisal. Wear 2013, 297, 1081–1094. [Google Scholar] [CrossRef] [Green Version]
- Dearnley, P.A.; Aldrich-Smith, G. Corrosion-wear mechanisms of hard coated austenitic 316L stainless steel. Wear 2004, 256, 491–499. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Mallia, B. The chemical wear (corrosion-wear) of novel Cr based hard coated 316L austenitic stainless steel in aqueous saline solution. Wear 2013, 306, 263–275. [Google Scholar] [CrossRef]
- Igual Muñoz, A.; Casabán Julián, L. Influence of electrochemical potential on the tribocorrosion behaviour of high carbon CoCrMo biomedical alloy in simulated body fluids by electrochemical impedance spectroscopy. Electrochim. Acta 2010, 55, 5428–5439. [Google Scholar] [CrossRef]
- Casabán Julián, L.; Igual Muñoz, A. Influence of microstructure of HC CoCrMo biomedical alloys on the corrosion and wear behaviour in simulated body fluids. Tribol. Int. 2011, 44, 318–329. [Google Scholar] [CrossRef]
- Igual Muñoz, A.; Mischler, S. Effect of the Environment on Wear ranking and Corrosion of Biomedical CoCrMo Alloys. J. Mater. Sci. Mater. Med. 2011, 22, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Neville, A.; Dowson, D. Tribo-corrosion properties of cobalt-based medical implant alloys in simulated biological environments. Wear 2007, 263, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S. Tribocorrosion in implants—Assessing high carbon and low carbon Co–Cr–Mo alloys by in situ electrochemical measurements. Tribol. Int. 2006, 39, 1509–1517. [Google Scholar] [CrossRef]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S.; Fisher, J. Effect of metallic nanoparticles on the biotribocorrosion behaviour of Metal-on-Metal hip prostheses. Wear 2009, 267, 683–688. [Google Scholar] [CrossRef]
- Yan, Y.; Neville, A.; Dowson, D. Biotribocorrosion of CoCrMo orthopaedic implant materials—Assessing the formation and effect of the biofilm. Tribol. Int. 2007, 40, 1492–1499. [Google Scholar] [CrossRef]
- Yan, Y.; Dowson, D.; Neville, A. In-situ electrochemical study of interaction of tribology and corrosion in artificial hip prosthesis simulators. J. Mech. Behav. Biomed. Mater. 2013, 18, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Caligari Conti, M.; Karl, A.; Schembri Wismayer, P.; Buhagiar, J. Biocompatibility and characterization of a Kolsterised® medical grade cobalt-chromium-molybdenum alloy. Biomatter 2014, 4, 1–10. [Google Scholar]
- Dong, H. S-phase surface engineering of Fe-Cr, Co-Cr and Ni-Cr alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Li, X.Y.; Habibi, N.; Bell, T.; Dong, H. Microstructural characterisation of a plasma carburised low carbon Co–Cr alloy. Surf. Eng. 2007, 23, 45–51. [Google Scholar] [CrossRef]
- Luo, X.; Li, X.; Sun, Y.; Dong, H. Tribocorrosion behavior of S-phase surface engineered medical grade Co–Cr alloy. Wear 2013, 302, 1615–1623. [Google Scholar] [CrossRef]
- Cassar, J.; Mallia, B.; Karl, A.; Buhagiar, J. EIS of carburised CoCrMo: Evolution of parameters characterising the metal-electrolyte interface. Surf. Coat. Technol. 2016, 292, 90–98. [Google Scholar] [CrossRef]
- Bidiville, A.; Favero, M.; Sadelmann, P.; Mischler, S. Effect of surface chemistry on the mechanical response of metals in sliding tribocorrosion systems. Wear 2007, 263, 207–217. [Google Scholar] [CrossRef]
- Bazzoni, A.; Mischler, S.; Espallargas, N. Tribocorrosion of pulsed plasma-nitrided CoCrMo implany alloy. Tribol. Lett. 2013, 49, 157–167. [Google Scholar] [CrossRef]
- Cassar, J.; Mallia, B.; Karl, A.; Buhagiar, J. The effect of sliding onto the metal-electrolyte interface: Studying model parameter modifications by means of EIS. Mater. Sci. Eng. C 2017, 75, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.O.A.; Mischler, S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M.; Barril, S. Third body effects and material fluxes in tribocorrosion systems involving a sliding contact. Wear 2004, 256, 517–524. [Google Scholar] [CrossRef]
- Jemmely, P.; Mischler, S.; Landolt, D. Electrochemical modelling of passivation phenomena in tribocorrosion. Wear 2000, 237, 63–76. [Google Scholar] [CrossRef]
- Watson, S.W.; Friedersdorf, F.J.; Madsen, B.W.; Cramer, S.D. Methods of measuring wear-corrosion synergism. Wear 1995, 181–183, 476–484. [Google Scholar] [CrossRef]












| Composition (wt %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | P | S | Cr | Ni | Mo | Cu | N | W | Fe | Co |
| 0.05 | 0.80 | 0.62 | 0.003 | 0.0005 | 27.64 | 0.07 | 5.46 | 0.01 | 0.169 | 0.02 | 0.2 | Bal. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassar, J.; Mallia, B.; Mazzonello, A.; Karl, A.; Buhagiar, J. Improved Tribocorrosion Resistance of a CoCrMo Implant Material by Carburising. Lubricants 2018, 6, 76. https://doi.org/10.3390/lubricants6030076
Cassar J, Mallia B, Mazzonello A, Karl A, Buhagiar J. Improved Tribocorrosion Resistance of a CoCrMo Implant Material by Carburising. Lubricants. 2018; 6(3):76. https://doi.org/10.3390/lubricants6030076
Chicago/Turabian StyleCassar, Josianne, Bertram Mallia, Antonino Mazzonello, Andreas Karl, and Joseph Buhagiar. 2018. "Improved Tribocorrosion Resistance of a CoCrMo Implant Material by Carburising" Lubricants 6, no. 3: 76. https://doi.org/10.3390/lubricants6030076