Tribocorrosion Response of Surface-Modified Ti in a 0.9% NaCl Solution
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation and Characterisation
2.2. Corrosion Testing
2.3. Tribocorrosion Testing
3. Results and Discussion
3.1. Corrosion Testing
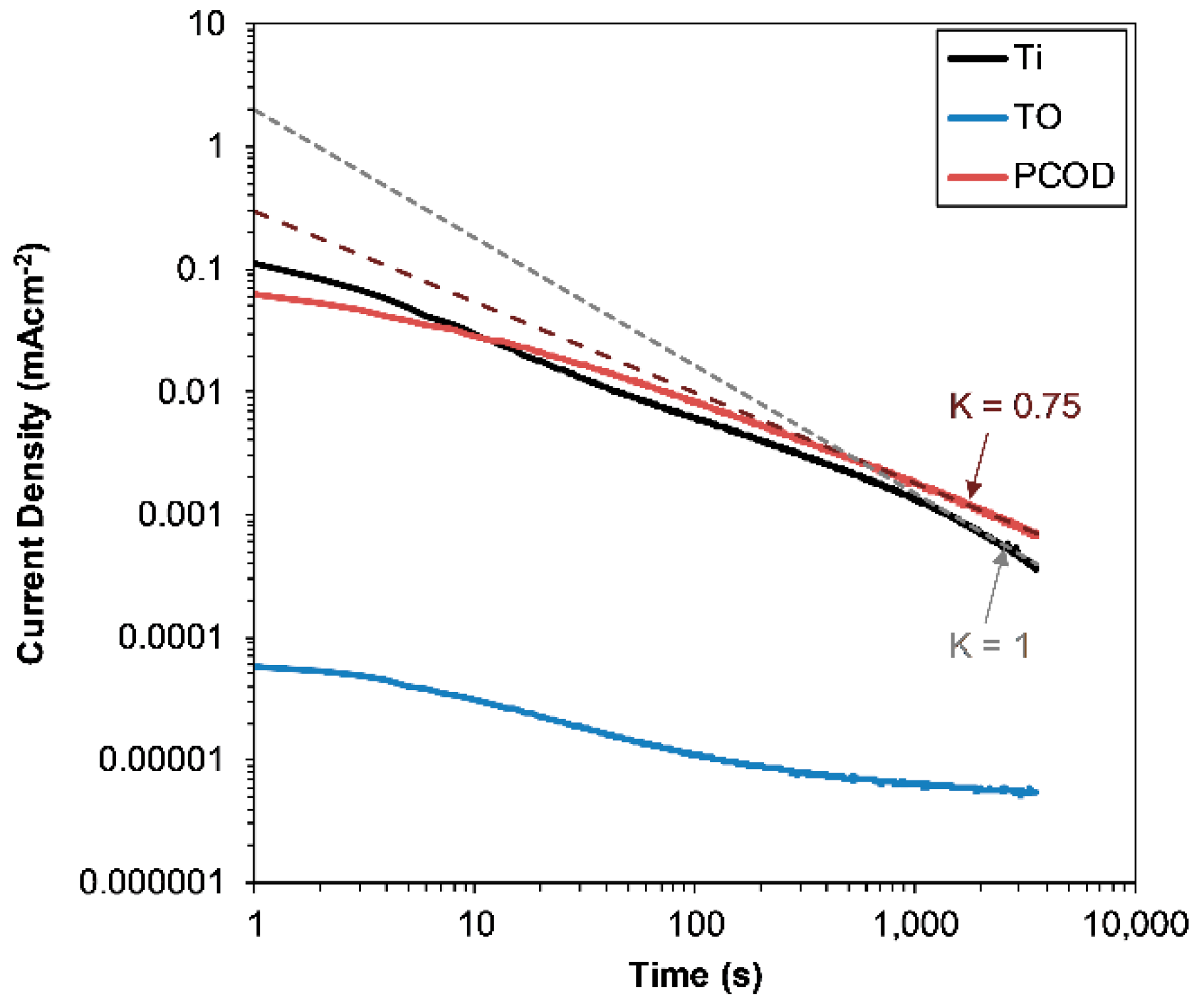
3.1.1. Potentiodynamic
3.1.2. Potentiostatic
3.2. OCP Tribocorrosion Testing
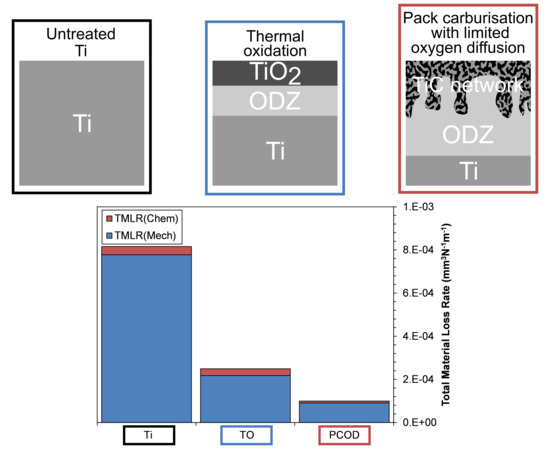
Wear Rates
4. Conclusions
- Under static corrosive conditions, TO-Ti offers the best improvement in corrosive resistance, due to the thick, non-porous rutile surface film.
- Both treated samples show significant material loss reductions when compared with untreated Ti. When compared with untreated Ti, the TO-Ti reduced material loss by 3.2 times and the PCOD-Ti reduced wear by 7.6 times.
- Under tribo-electrochemical conditions, the PCOD treatment outperformed the TO treatment and was able to maintain limited protection when subjected to a contact load of 20 N. Low friction coefficients observed can be attributed to an oxygen deficient film formed during sliding contact.
- The TiC network structure formed during the PCOD process shows excellent adhesion and an ability to sustain high loads without critical failure.
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Mohammed, M.T. Development of a new metastable beta titanium alloy for biomedical applications. Karbala Int. J. Mod. Sci. 2017, 3, 224–230. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, Y.M.; Biao, M.N.; Zhang, X.D.; Yang, B.C. Bio-functionalization of biomedical metals. Mater. Sci. Eng. C 2017, 70, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Bloyce, A. Surface engineering of titanium alloys for wear protection. Proc. Inst. Mech. Eng. 1998, 212, 467–476. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Prashanth, K.G.; Attar, H.; Chaubey, A.K.; Cao, G.H.; Zhang, L.C. Evaluation of mechanical and wear properties of TixNb7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Haghighi, S.E.; Lu, H.B.; Jian, G.Y.; Cao, G.H.; Habibi, D.; Zhang, L.C. Effect of α martensite on the microstructure and mechanical properties of beta-type Ti–Fe–Ta alloys. Mater. Des. 2015, 76, 47–54. [Google Scholar] [CrossRef]
- Sivakumar, B.; Pathak, L.C.; Singh, R. Fretting corrosion response of boride coated titanium in ringer’s solution for bio-implant use: Elucidation of degradation mechanism. Tribol. Int. 2018, 127, 219–230. [Google Scholar] [CrossRef]
- Brama, M.; Rhodes, N.; Hunt, J.; Ricci, A.; Teghil, R.; Migliaccio, S.; Rocca, C.D.; Leccisotti, S.; Lioi, A.; Scandurra, M.; et al. Effect of titanium carbide coating on the osseointegration response in vitro and in vivo. Biomaterials 2007, 28, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, S.; Li, F.; Li, D.; Zhang, J.; Tian, M.; Zhang, Q.; Tong, Y.; Zhang, Z.; Wang, G.; et al. Effect of plasma nitriding and titanium nitride coating on the corrosion resistance of titanium. J. Prosthet. Dent. 2016, 116, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Aniołek, K. The influence of thermal oxidation parameters on the growth of oxide layers on titanium. Vacuum 2017, 144, 94–100. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Designer surfaces for titanium components. Anti-Corros. Methods. Mater. 1999, 46, 338–346. [Google Scholar] [CrossRef]
- Güleryüz, H.; Çimenoğlu, H. Effect of thermal oxidation on corrosion and corrosion-wear behaviour of a Ti–6Al–4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sankara Narayanan, T.S.N.; Ganesh Sundara Raman, S.; Seshadri, S.K. Fretting corrosion behaviour of thermally oxidized CP-ti in ringer’s solution. Corros. Sci. 2010, 52, 711–721. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Barylski, A. Sliding wear resistance of oxide layers formed on a titanium surface during thermal oxidation. Wear 2016, 356–357, 23–29. [Google Scholar] [CrossRef]
- Alansari, A.; Sun, Y. A comparative study of the mechanical behaviour of thermally oxidised commercially pure titanium and zirconium. J. Mech. Behav. Biomed. Mater. 2017, 74, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dearnley, P.A.; Dahm, K.L.; Çimenoǧlu, H. The corrosion-wear behaviour of thermally oxidised CP-ti and Ti–6Al–4V. Wear 2004, 256, 469–479. [Google Scholar] [CrossRef]
- Bailey, R.; Sun, Y. Pack carburisation of commercially pure titanium with limited oxygen diffusion for improved tribological properties. Surf. Coat. Technol. 2015, 261, 28–34. [Google Scholar] [CrossRef]
- Bailey, R.; Sun, Y. An investigation into the effects of process conditions on the tribological performance of pack carburized titanium with limited oxygen diffusion. J. Mater. Eng. Perform. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Bailey, R.; Sun, Y. Corrosion and tribocorrosion performance of pack-carburized commercially pure titanium with limited oxygen diffusion in a 0.9% NaCl solution. J. Bio-Tribo-Corros. 2018, 4, 6. [Google Scholar] [CrossRef]
- Siva Rama Krishna, D.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Int. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- Bailey, R.; Sun, Y. Corrosion and tribocorrosion performance of thermally oxidized commercially pure titanium in a 0.9% NaCl solution. J. Mater. Eng. Perform. 2015, 24, 1669–1678. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.Y. Oxygen boost diffusion for the deep-case hardening of titanium alloys. Mater. Sci. Eng. A 2000, 280, 303–310. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, H.; Cheng, G.; Liu, H. Effect of carburization on the mechanical properties of biomedical grade titanium alloys. J. Bionic Eng. 2011, 8, 86–89. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- Sun, Y.; Haruman, E. Effect of electrochemical potential on tribocorrosion behavior of low temperature plasma carburized 316L stainless steel in 1 M H2SO4 solution. Surf. Coat. Technol. 2011, 205, 4280–4290. [Google Scholar] [CrossRef]
- Sun, Y.; Rana, V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5 M NaCl solution. Mater. Chem. Phys. 2011, 129, 138–147. [Google Scholar] [CrossRef]
- Ashrafizadeh, A.; Ashrafizadeh, F. Structural features and corrosion analysis of thermally oxidized titanium. J. Alloys Compd. 2009, 480, 849–852. [Google Scholar] [CrossRef]
- Kumar, S.; Sankara Narayanan, T.S.N.; Ganesh Sundara Raman, S.; Seshadri, S.K. Thermal oxidation of CP Ti—An electrochemical and structural characterization. Mater. Charact. 2010, 61, 589–597. [Google Scholar] [CrossRef]
- Oláh, N.; Fogarassy, Z.; Furkó, M.; Balázsi, C.; Balázsi, K. Sputtered nanocrystalline ceramic TiC/amorphous C thin films as potential materials for medical applications. Ceram. Int. 2015, 41, 5863–5871. [Google Scholar] [CrossRef] [Green Version]
- Avgustinik, A.I.; Drozdetskaya, G.V.; Ordan’yan, S.S. Reaction of titanium carbide with water. Soviet Powder Metall. Met. Ceram. 1967, 6, 470–473. [Google Scholar]
- Raja, K.S.; Jones, D.A. Effects of dissolved oxygen on passive behavior of stainless alloys. Corros. Sci. 2006, 48, 1623–1638. [Google Scholar] [CrossRef]
- Zhang, Y.; Macdonald, D.D.; Urquidi-Macdonald, M.; Engelhardt, G.R.; Dooley, R.B. Passivity breakdown on AISI type 403 stainless steel in chloride-containing borate buffer solution. Corros. Sci. 2006, 48, 3812–3823. [Google Scholar] [CrossRef]
- Sun, Y. Depth-profiling electrochemical measurements of low temperature plasma carburised 316L stainless steel in 1 M H2SO4 solution. Surf. Coat. Technol. 2010, 204, 2789–2796. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K.; Lämmel, C.; Sempf, K.; Herrmann, M. Galvanic corrosion of metal/ceramic coupling. Corros. Sci. 2014, 80, 191–196. [Google Scholar] [CrossRef]
- Gardos, M.N.; Hong, H.; Winer, W.O. The effect of anion vacancies on the tribological properties of rutile (TiO2−x), part II: Experimental evidence. Tribol. Trans. 1990, 33, 209–220. [Google Scholar] [CrossRef]








© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, R. Tribocorrosion Response of Surface-Modified Ti in a 0.9% NaCl Solution. Lubricants 2018, 6, 86. https://doi.org/10.3390/lubricants6040086
Bailey R. Tribocorrosion Response of Surface-Modified Ti in a 0.9% NaCl Solution. Lubricants. 2018; 6(4):86. https://doi.org/10.3390/lubricants6040086
Chicago/Turabian StyleBailey, Richard. 2018. "Tribocorrosion Response of Surface-Modified Ti in a 0.9% NaCl Solution" Lubricants 6, no. 4: 86. https://doi.org/10.3390/lubricants6040086