Integrated Biological Control Using a Mixture of Two Entomopathogenic Bacteria, Bacillus thuringiensis and Xenorhabdus hominickii, against Spodoptera exigua and Other Congeners
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Chemicals
2.3. Bacterial Culture
2.4. RNA Extraction, cDNA Preparation, and qPCR
2.5. Nodulation Assay
2.6. Antimicrobial Peptide (AMP) Gene Expression Analysis
2.7. Bt Bioassays
2.8. Bioassay with BtA+Bacterial Metabolites
2.9. Field Assay to Estimate Control Efficacy
2.10. Control Spectrum of BtA+XhE Mixture
2.11. Data Analysis
3. Results
3.1. Screening for the Optimal Bt Strain to Control S. exigua
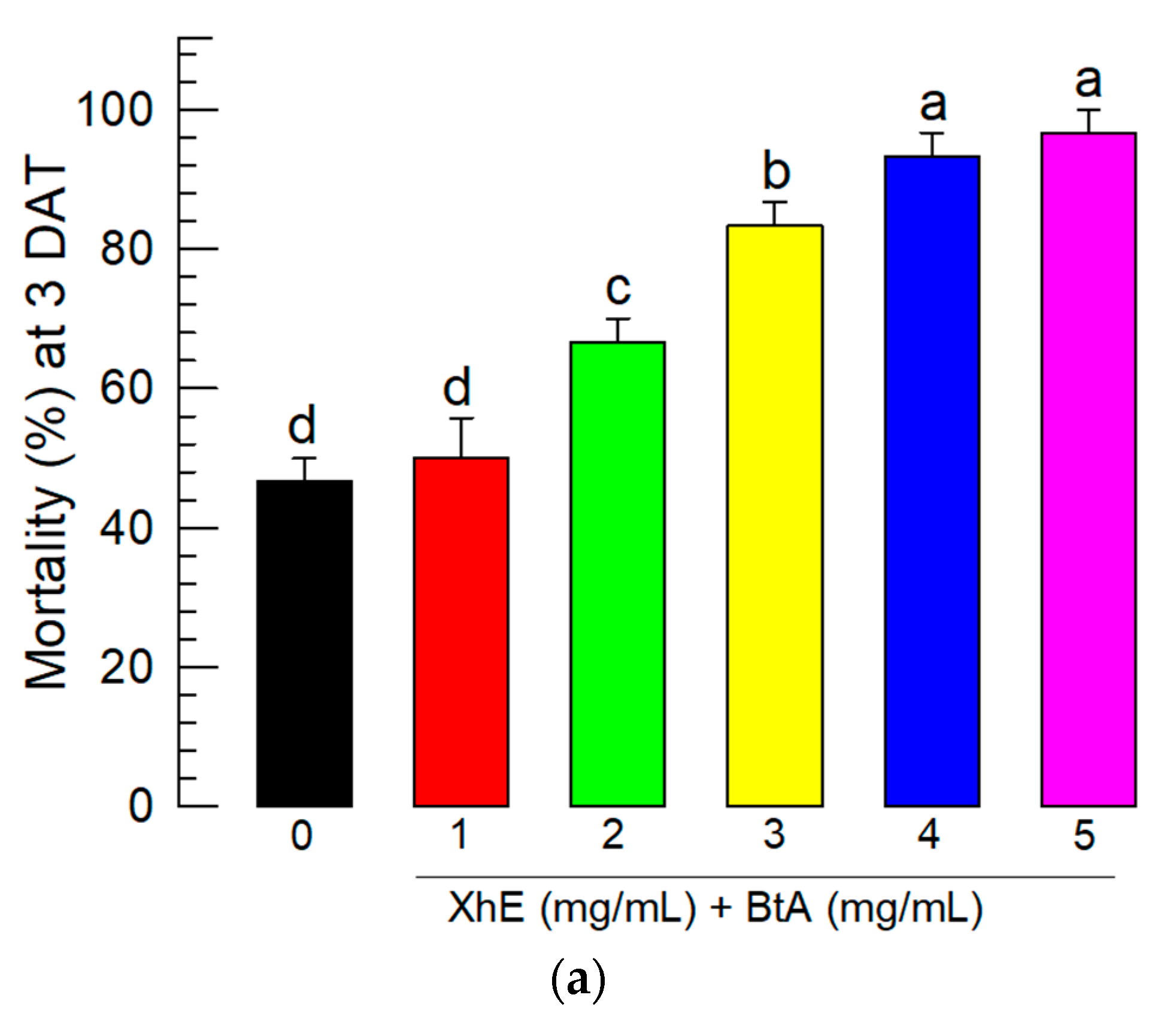
3.2. Culture Broth of X. hominickii ANU101 Enhances BtA Toxicity
3.3. BtA Induces Humoral and Cellular Immune Responses in S. exigua
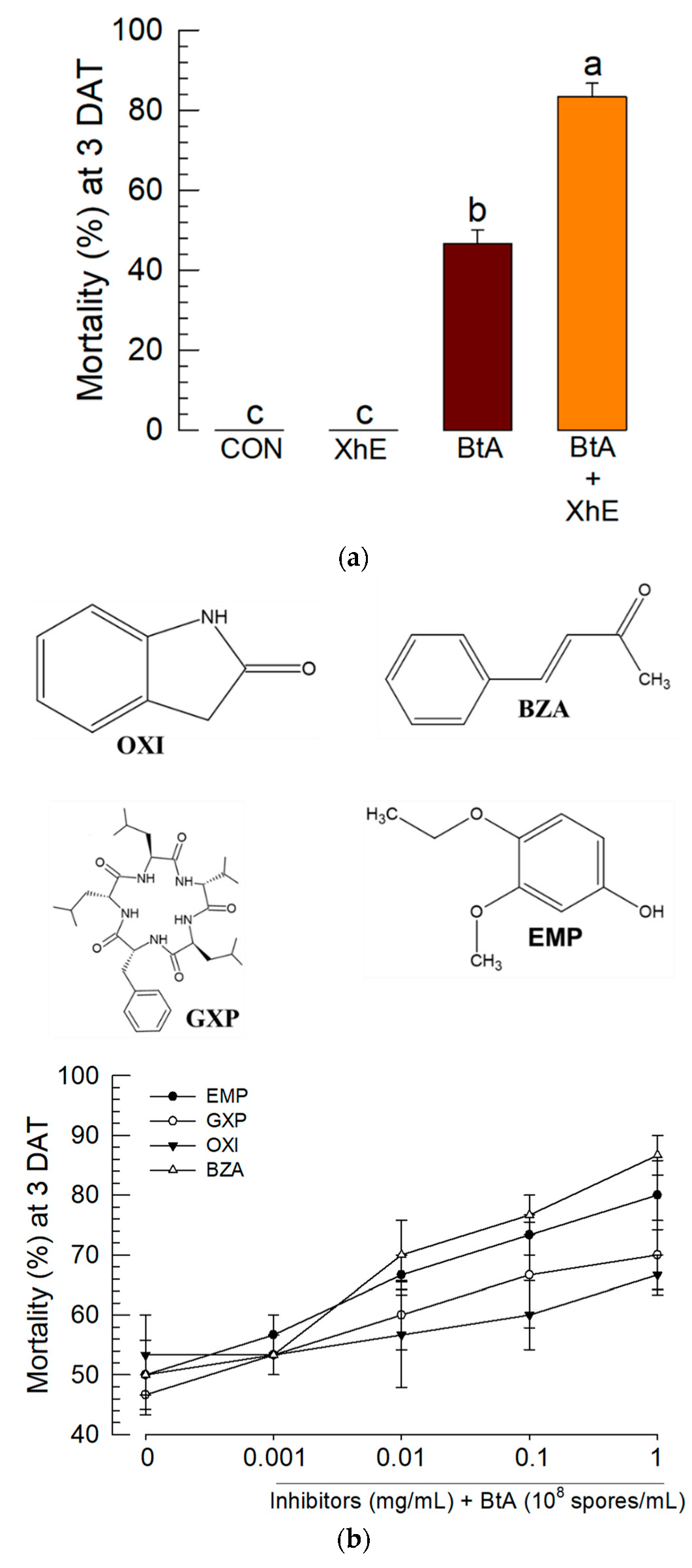
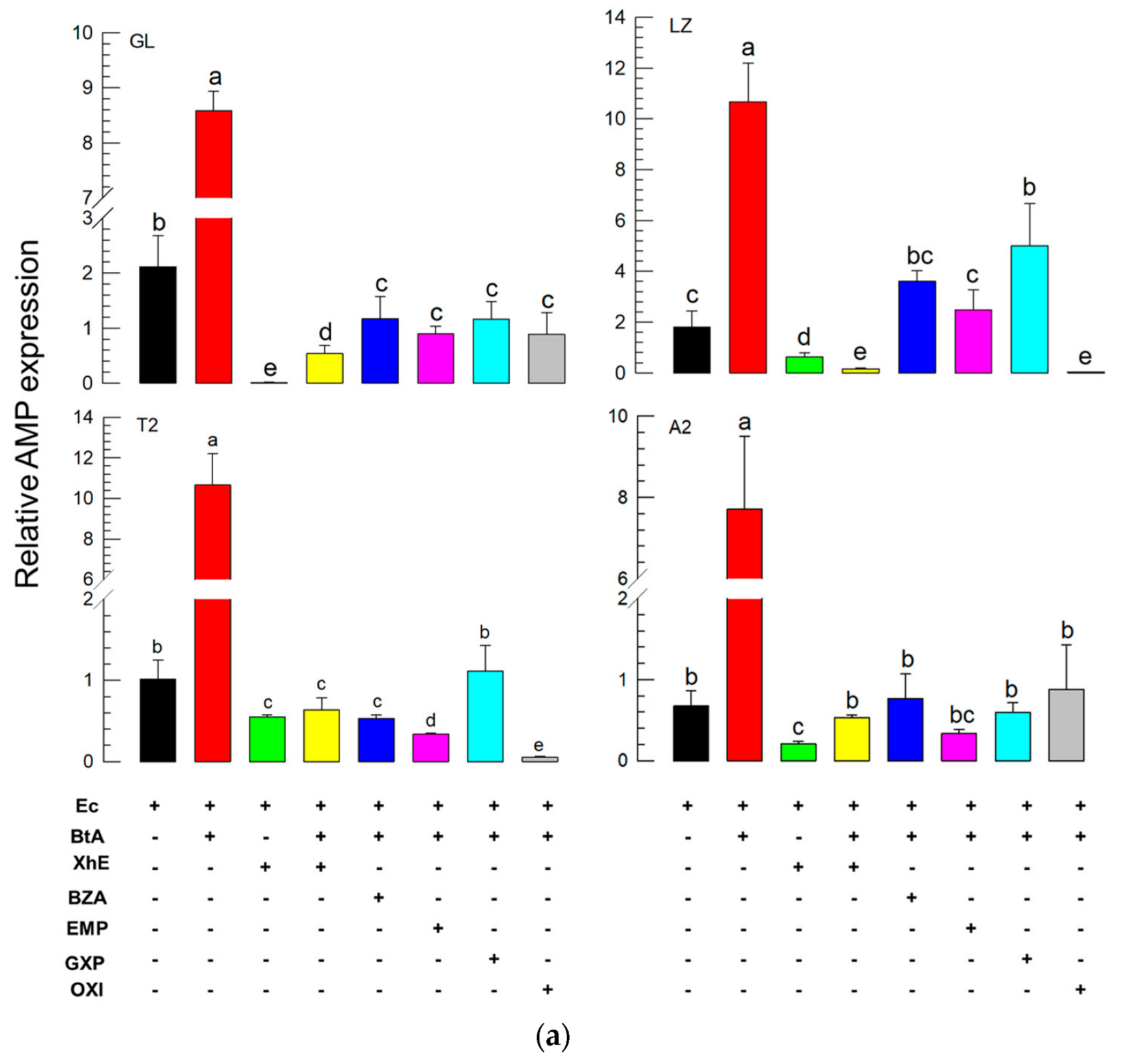
3.4. Suppression of the BtA-Induced Immune Responses by XhE and Four Secondary Metabolites
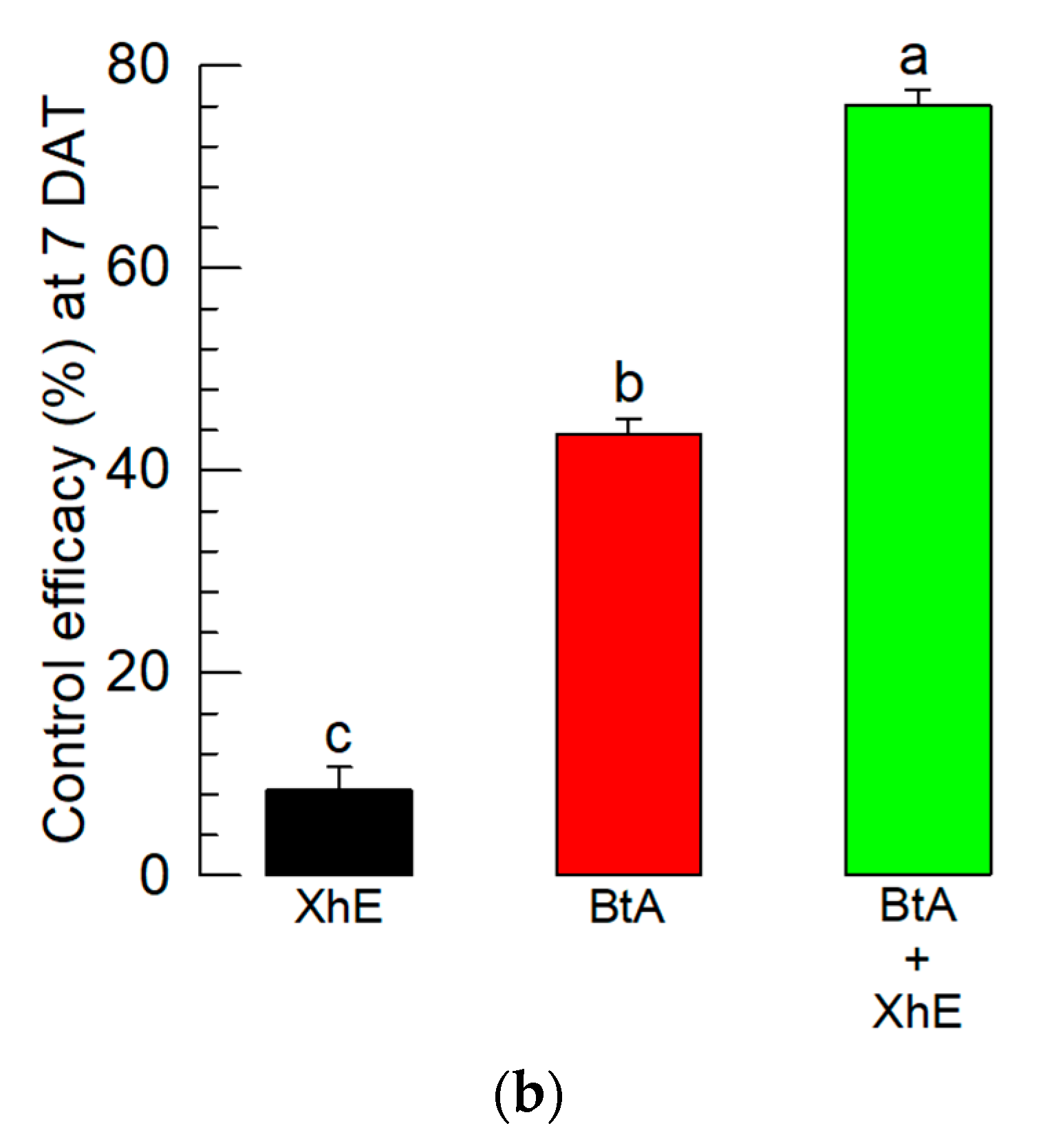
3.5. Field Assay of the BtA+XhE Mixture
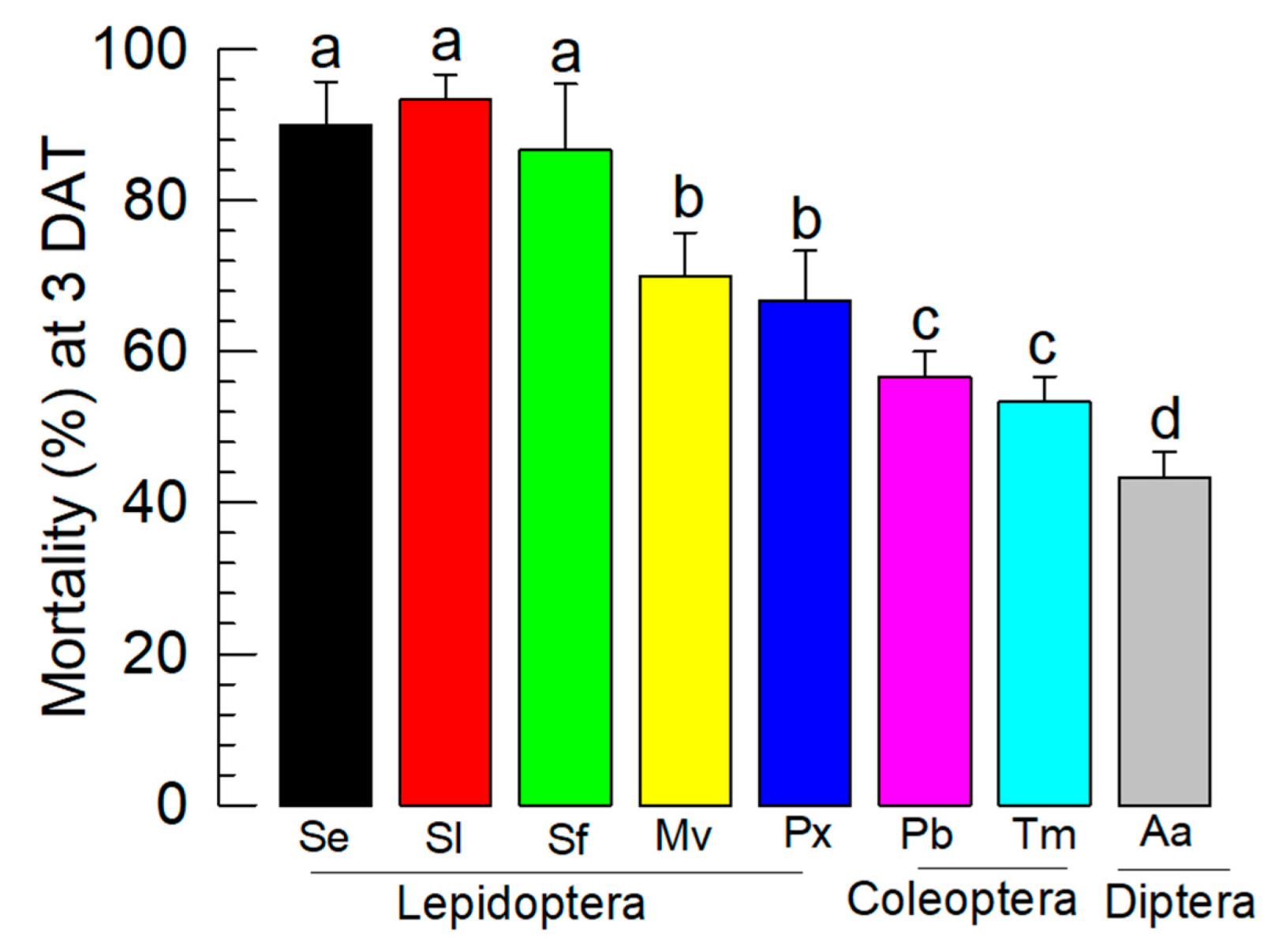
3.6. Control Spectrum of BtA+XhE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodrich-Blair, H.; Clarke, D.J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: Two roads to the same destination. Mol. Microbiol. 2007, 64, 260–268. [Google Scholar] [CrossRef]
- Shi, Y.-M.; Hirschmann, M.; Shi, Y.-N.; Ahmed, S.; Abebew, D.; Tobias, N.J.; Grün, P.; Crames, J.J.; Pöschel, L.; Kuttenlochner, W.; et al. Global analysis of biosynthetic gene clusters reveals conserved and unique natural products in entomopathogenic nematode-symbiotic bacteria. Nat. Chem. 2022, 14, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Dziedziech, A.; Shivankar, S.; Theopold, U. High-Resolution Infection Kinetics of Entomopathogenic Nematodes Entering Drosophila melanogaster. Insects 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.; Park, Y.; Kim, Y. Sequential immunosuppressive activities of bacterial secondary metabolites from the entomopahogenic bacterium Xenorhabdus nematophila. J. Microbiol. 2014, 52, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ji, D.; Cho, S.; Park, Y. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J. Invertebr. Pathol. 2005, 89, 258–264. [Google Scholar] [CrossRef]
- Kim, Y.; Stanley, D. Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues. Genes 2021, 12, 211. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological Mediators of Insect Immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef]
- Sajjadian, S.M.; Ahmed, S.; Al Baki, M.A.; Kim, Y. Prostaglandin D2 synthase and its functional association with immune and reproductive processes in a lepidopteran insect, Spodoptera exigua. Gen. Comp. Endocrinol. 2020, 287, 113352. [Google Scholar] [CrossRef]
- Vatanparast, M.; Lee, D.-H.; Kim, Y. Biosynthesis and immunity of epoxyeicosatrienoic acids in a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2020, 107, 103643. [Google Scholar] [CrossRef]
- Ahmed, S.; Al Baki, M.A.; Lee, J.; Seo, D.Y.; Lee, D.; Kim, Y. The first report of prostacyclin and its physiological roles in insects. Gen. Comp. Endocrinol. 2021, 301, 113659. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raffa, K.F.; Handelsman, J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. USA 2006, 103, 15196–15199. [Google Scholar] [CrossRef]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B.; et al. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Heckel, D.G.; Ferré, J. Mechanisms of Resistance to Insecticidal Proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef]
- Kim, Y. Molecular action of prostaglandin to mediate insect immunity and its application to develop novel insect control techniques. Korean J. Appl. Entomol. 2022, 61, 173–195. [Google Scholar]
- Jung, S.; Kim, Y. Synergistic effect of Xenorhabdus nematophila K1 and Bacillus thuringiensis subsp. aizawai against Spodoptera exigua (Lepidoptera: Noctuidae). Biol. Control 2006, 39, 201–209. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Jung, S.; Kim, Y. An Entomopathogenic Bacterium, Xenorhabdus nematophila K1, Enhances Baculovirus Pathogenicity against Spodoptera exigua and Plutella xylostella. J. Asia Pac. Entomol. 2006, 9, 179–182. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y. Benzylideneacetone suppresses both cellular and humoral immune responses of Spodoptera exigua and enhances fungal pathogenicity. J. Asia Pac. Entomol. 2011, 14, 423–427. [Google Scholar] [CrossRef]
- Kim, E.; Jeoung, S.; Park, Y.; Kim, K.; Kim, Y. A novel formulation of Bacillus thuringiensis for the control of brassica leaf beetle, Phaedon brassicae (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2015, 108, 2556–2565. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jung, J.K.; Kim, Y. A mixture of Bacillus thuringiensis subsp. israelensis with Xenorhabdus nematophila-cultured broth enhances toxicity against mosquitoes Aedes albopictus and Culex pipiens pallens (Diptera: Culicidae). J. Econ. Entomol. 2016, 109, 1086–1093. [Google Scholar]
- Kim, J.; Kim, Y. Benzylideneacetone, an eicosanoid biosynthesis inhibitor enhances baculovirus pathogenicity in the diamondback moth, Plutella xylostella. J. Invertebr. Pathol. 2011, 106, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Caramella, S.; Quadroni, S.; Brivio, M.F. Drosophila suzukii susceptibility to the oral administration of Bacillus thuringiensis, Xenorhabdus nematophila and its secondary metabolites. Insects 2021, 12, 635. [Google Scholar] [CrossRef]
- Goh, H.G.; Park, J.D.; Choi, Y.M.; Choi, K.M.; Park, I.S. The host plants of beet armyworm, Spodoptera exigua (Hübner), (Lepidoptera: Noctuidae) and its occurrence. Korean J. Appl. Entomol. 1991, 30, 111–116. [Google Scholar]
- Kim, Y.; Lee, J.; Kang, S.; Han, S. Age Variation in Insecticide Susceptibility and Biochemical Changes of Beet Armyworm, Spodoptera exigua (Hüubner). J. Asia Pac. Entomol. 1998, 1, 109–113. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.R.; Lee, J.; Kang, S.; Han, S.C.; Hong, K.J.; Kim, H.S.; Yoo, J.K.; Lee, J.O. Insecticide resistance in the tobacco cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). J. Asia Pac. Entomol. 1998, 1, 115–122. [Google Scholar]
- Lee, G.S.; Yoon, S.B.; Lee, J.H.; Kim, H.; Song, J.H.; Lee, W. First report of the fall armyworm, Spodoptera frugiperda (Smith, 1797) (Lepidoptera, Noctuidae), a new migratory pest in Korea. Korean J. Appl. Entomol. 2020, 59, 73–78. [Google Scholar]
- Mollah, M.I.; Choi, H.W.; Yeam, I.; Lee, J.M.; Kim, Y. Salicylic Acid, a Plant Hormone, Suppresses Phytophagous Insect Immune Response by Interrupting HMG-Like DSP1. Front. Physiol. 2021, 12, 744272. [Google Scholar] [CrossRef]
- Goh, H.G.; Lee, S.G.; Lee, B.P.; Choi, K.M.; Kim, J.H. Simple mass-rearing of beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), on an artificial diet. Korean J. Appl. Entomol. 1990, 29, 180–183. [Google Scholar]
- Jung, J.-K.; Seo, B.-Y.; Cho, J.-R.; Kwon, Y.-H.; Kim, G.-H. Occurrence of Lepidopteran Insect Pests and Injury Aspects in Adzuki Bean Fields. Korean J. Appl. Entomol. 2009, 48, 29–35. [Google Scholar] [CrossRef]
- Vatanparast, M.; Park, Y. Differential Transcriptome Analysis Reveals Genes Related to Low- and High-Temperature Stress in the Fall Armyworm, Spodoptera frugiperda. Front. Physiol. 2022, 12, 827077. [Google Scholar] [CrossRef]
- Park, Y.; Kang, S.; Sadekuzzaman, M.; Kim, H.; Jung, J.-K.; Kim, Y. Identification and bacterial characteristics of Xenorhabdus hominickii ANU101 from an entomopathogenic nematode, Steinernema monticolum. J. Invertebr. Pathol. 2017, 144, 74–87. [Google Scholar] [CrossRef]
- Eom, S.; Park, Y.; Kim, H.; Kim, Y. Development of a High Efficient “Dual Bt-Plus” insecticide using a primary form of an entomopathogenic bacterium, Xenorhabdus nematophila. J. Microbiol. Biotechnol. 2014, 24, 507–521. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data analysis using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT User’s Guide, Release 6.03; SAS Institute Inc.: Cary, NC, USA, 1989. [Google Scholar]
- Seo, S.; Lee, S.; Hong, Y.; Kim, Y. Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata spp. temperata. Appl. Environ. Microbiol. 2012, 78, 3816–3823. [Google Scholar] [CrossRef]
- Nollmann, F.I.; Dauth, C.; Mulley, G.; Kegler, C.; Kaiser, M.; Waterfield, N.R.; Bode, H.B. Insect-specific production of new GameXPeptides in Photorhabdus luminescens TTO1, widespread natural products in entomopathogenic bacteria. Chembiochem 2015, 16, 205–208. [Google Scholar] [CrossRef]
- Endo, H. Molecular and Kinetic Models for Pore Formation of Bacillus thuringiensis Cry Toxin. Toxins 2022, 14, 433. [Google Scholar] [CrossRef]
- Knowles, B.H.; Ellar, D.J. Colloid-osmotic lysis is a general feature of the mechanism of action on Bacillus thuringiensis delta-endotoxins with different insect specificity. Biochim. Biophys. Acta 1987, 924, 509–518. [Google Scholar] [CrossRef]
- Wright, D.J.; Iqbal, M.; Granero, F.; Ferre, J. A Change in a Single Midgut Receptor in the Diamondback Moth (Plutella xylostella) Is Only in Part Responsible for Field Resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl. Environ. Microbiol. 1997, 63, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Banks, D.; Adang, M.J. Toxicity, binding, and permeability analyses of four Bacillus thuringiensis Cry1 δ-endotoxins using brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl. Environ. Microbiol. 1999, 65, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Jin, F.L.; Wang, Y.S.; Freed, S.; Hu, Q.B.; Ren, S.X. Molecular cloning and characterization of gloverin from the diamondback moth, Plutella xylostella L. and its interaction with bacterial membrane. World J. Microbiol. Biotechnol. 2015, 31, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Manachini, B.; Arizza, V.; Parrinello, D.; Parrinello, N. Hemocytes of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) and their response to Saccharomyces cerevisiae and Bacillus thuringiensis. J. Invertebr. Pathol. 2011, 106, 360–365. [Google Scholar] [CrossRef]
- Ahmed, S.; Sajjadian, S.M.; Kim, Y. HMGB1-Like Dorsal Switch Protein 1 Triggers a Damage Signal in Mosquito Gut to Activate Dual Oxidase via Eicosanoids. J. Innate Immun. 2022, 5, 1–16. [Google Scholar] [CrossRef]
- Mollah, M.M.I.; Kim, Y. Virulence secondary metabolites of the entomopathogenic bacteria, Xenorhabdus and Photorhabdus, against insect immune defences through inhibiting PLA2. BMC Microbiol. 2020, 20, 359. [Google Scholar] [CrossRef]
- Tobias, N.J.; Shi, Y.-M.; Bode, H.B. Refining the Natural Product Repertoire in Entomopathogenic Bacteria. Trends Microbiol. 2018, 26, 833–840. [Google Scholar] [CrossRef]
- Ji, D.; Yi, Y.; Kang, G.H.; Choi, Y.H.; Kim, P.; Baek, N.I.; Kim, Y. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett. 2004, 239, 241–248. [Google Scholar] [CrossRef]
- Vasko, R.C.; Rodriguez, R.A.; Cunningham, C.N.; Ardi, V.C.; Agard, D.A.; McAlpine, S.R. Mechanistic Studies of Sansalvamide A-Amide: An Allosteric Modulator of Hsp90. ACS Med. Chem. Lett. 2010, 1, 4–8. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrithik, M.T.H.; Park, Y.; Park, H.; Kim, Y. Integrated Biological Control Using a Mixture of Two Entomopathogenic Bacteria, Bacillus thuringiensis and Xenorhabdus hominickii, against Spodoptera exigua and Other Congeners. Insects 2022, 13, 860. https://doi.org/10.3390/insects13100860
Hrithik MTH, Park Y, Park H, Kim Y. Integrated Biological Control Using a Mixture of Two Entomopathogenic Bacteria, Bacillus thuringiensis and Xenorhabdus hominickii, against Spodoptera exigua and Other Congeners. Insects. 2022; 13(10):860. https://doi.org/10.3390/insects13100860
Chicago/Turabian StyleHrithik, Md Tafim Hossain, Youngjin Park, Hyemi Park, and Yonggyun Kim. 2022. "Integrated Biological Control Using a Mixture of Two Entomopathogenic Bacteria, Bacillus thuringiensis and Xenorhabdus hominickii, against Spodoptera exigua and Other Congeners" Insects 13, no. 10: 860. https://doi.org/10.3390/insects13100860




