Systematic Studies on the Antioxidant Capacity and Volatile Compound Profile of Yellow Mealworm Larvae (T. molitor L.) under Different Drying Regimes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Samples
2.2. Drying Procedures
2.3. Proximate Analysis
2.4. Colour Evaluation
2.5. Fatty Acid Composition and Fat Oxidation
2.6. Volatile Compound Measurements
2.7. Antioxidant Analysis
2.8. Statistical Analysis
3. Results
3.1. Energy Consumption and Efficiency of the Drying Methods
3.2. Colour Analysis
3.3. Fatty Acid Analysis
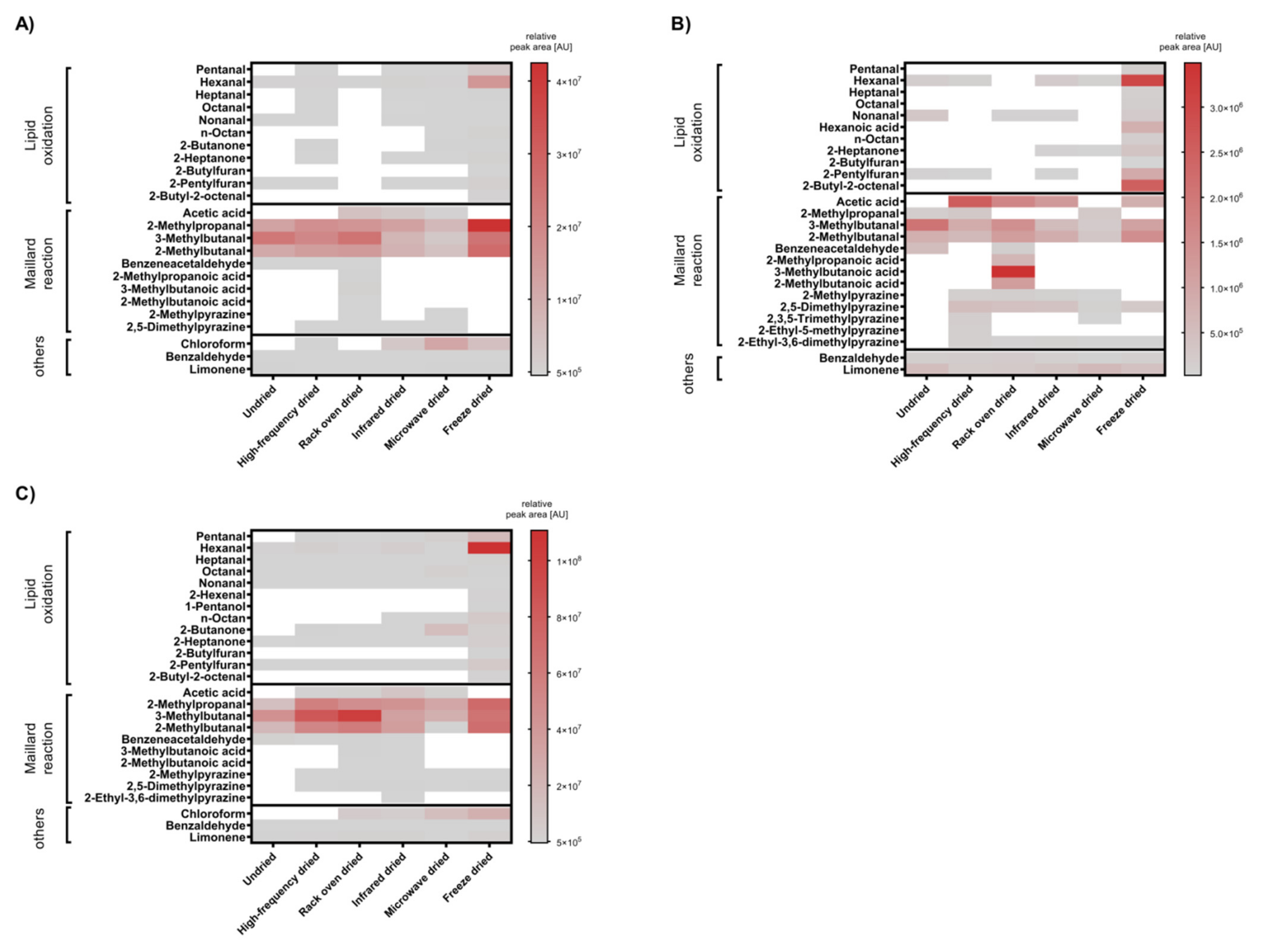
3.4. Volatile Compounds
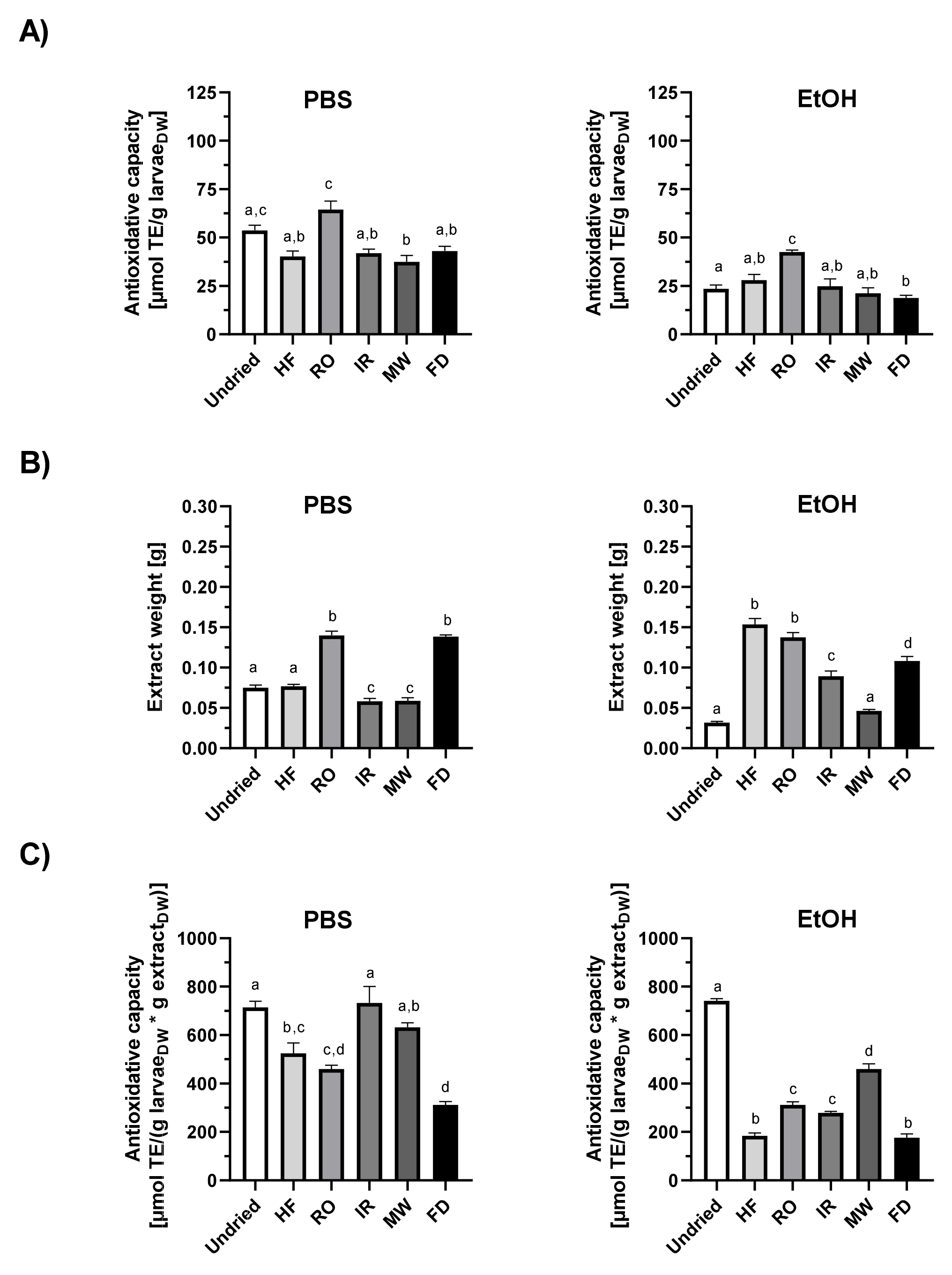
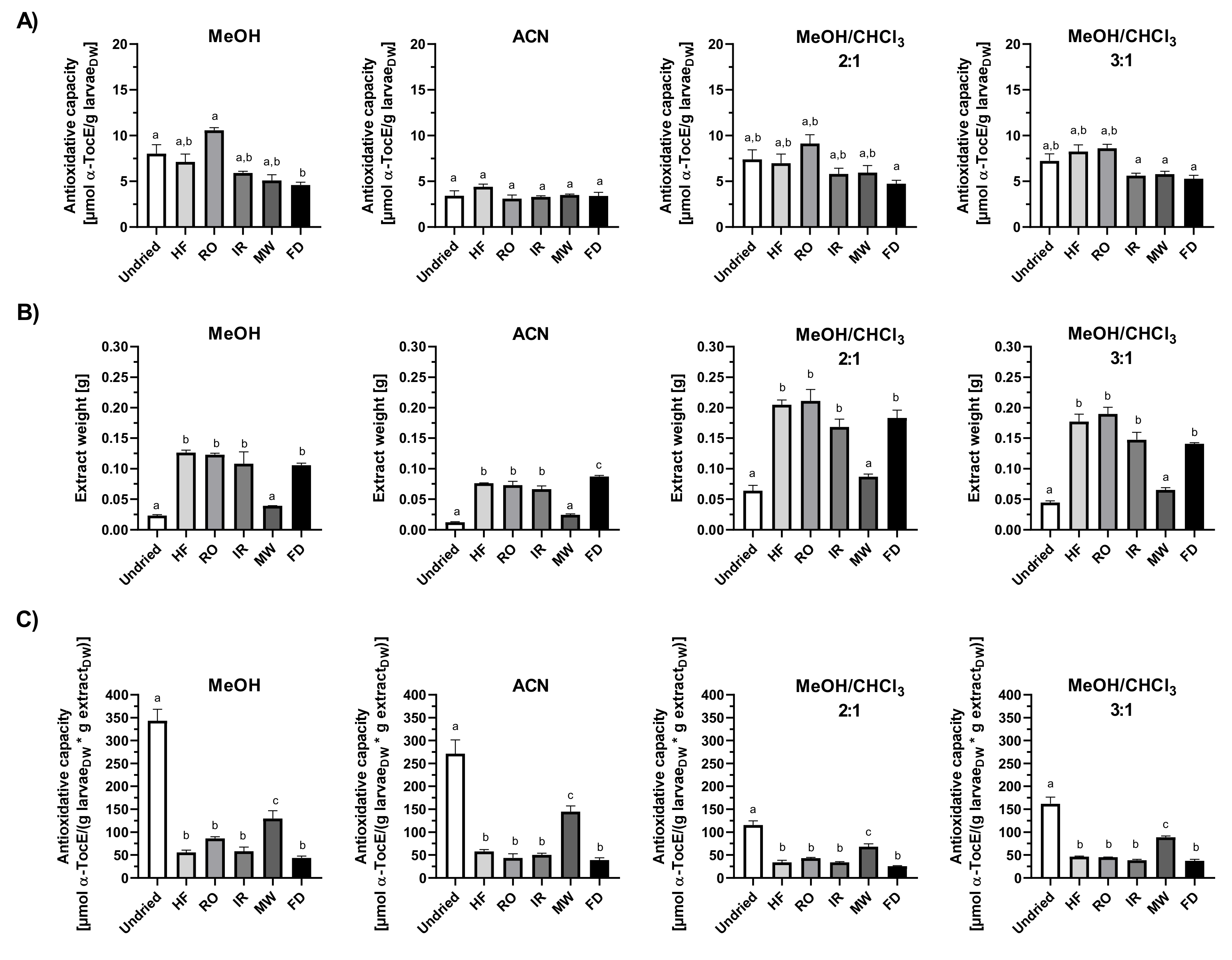
3.5. Total Antioxidant Capacity of Mealworm Larvae Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs, Population Dynamics. In World Population Prospects 2019; 2019; ISBN 9789211483161. [Google Scholar]
- White, R.R.; Hall, M.B. Nutritional and greenhouse gas impacts of removing animals from US agriculture. Proc. Natl. Acad. Sci. USA 2017, 114, E10301–E10308. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Vaskou, P.; Kountouris, Y. Insect Food Products in the Western World: Assessing the Potential of a New “Green” Market. Ann. Entomol. Soc. Am. 2019, 112, 518–528. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; Food and Agriculture Organization of the UN: Rome, Italy, 2012; Volume 12, ESA Working Paper. [Google Scholar]
- Van Meerbeek, K.; Svenning, J.C. Causing confusion in the debate about the transition toward amore plant-based diet. Proc. Natl. Acad. Sci. USA 2018, 115, E1701–E1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazac, R.; Renwick, K.; Seed, B.; Black, J.L. An Approach for Integrating and Analyzing Sustainability in Food-Based Dietary Guidelines. Front. Sustain. Food Syst. 2021, 5, 84. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional Qualities and Enhancement of Edible Insects. Annu. Rev. Nutr. 2021, 41, 551–576. [Google Scholar] [CrossRef]
- Ojha, S.; Bekhit, A.E.-D.; Grune, T.; Schlüter, O.K. Bioavailability of nutrients from edible insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Future Prospects for Food and Feed Security; Food and Agriculture Organization of the UN (FAO): Rome, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Wade, M.; Hoelle, J. A review of edible insect industrialization: Scales of production and implications for sustainability. Environ. Res. Lett. 2019, 15, 123013. [Google Scholar] [CrossRef]
- Pippinato, L.; Gasco, L.; Di Vita, G.; Mancuso, T. Current scenario in the European edible-insect industry: A preliminary study. J. Insects Food Feed 2020, 6, 371–381. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on insects as food and feed: A global comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Montanari, F.; Pinto de Moura, A.; Cunha, L.M. Production and Commercialization of Insects as Food and Feed, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- EFSA Panel on Nutrition, N.F. and F.A. (NDA). Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F. and F.A. (NDA). Safety of frozen and dried formulations from whole yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06778. Available online: https://onlinelibrary.wiley.com (accessed on 10 January 2022).
- EFSA. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Yi, L.; Han, R.; Giroud, L.; Jullien, R.L. Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Guadalupe Rojas, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar]
- Kröncke, N.; Baur, A.; Böschen, V.; Demtröder, S.; Benning, R.; Delgado, A. Automation of Insect Mass Rearing and Processing Technologies of Mealworms (Tenebrio molitor). In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Mariod, A.A., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 123–139. ISBN 978-3-030-32951-8. [Google Scholar]
- Nava, A.L.; Higareda, T.E.; Barreto, C.; Rodríguez, R.; Márquez, I.; Palacios, M.L. Circular economy approach for mealworm industrial production for human consumption. IOP Conf. Ser. Earth Environ. Sci. 2020, 463, 12087. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov Food Sci Emerg Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Schrögel, P.; Wätjen, W. Insects for food and feed-safety aspects related to mycotoxins and metals. Foods 2019, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.J.; Hwang, I.K.; Nho, C.W.; Kim, S.M.; Kim, S.H. Determination of carbohydrate composition in mealworm (Tenebrio molitor l.) larvae and characterization of mealworm chitin and chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of suitable drying processes for mealworms (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- Kanzler, C.; Haase, P.T.; Schestkowa, H.; Kroh, L.W. Antioxidant Properties of Heterocyclic Intermediates of the Maillard Reaction and Structurally Related Compounds. J. Agric. Food Chem. 2016, 64, 7829–7837. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, E.N. Lipid Oxidation: Second Edition, 2nd ed; Elsevier Inc: Amsterdam, The Netherlands, 2005; ISBN 9780953194988. [Google Scholar]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of secondary and tertiary volatile compounds resulting from the lipid oxidation of rapeseed oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate Contribution of Lipid Oxidation and Maillard Reaction to the Nonenzymatic Food Browning. Crit. Rev. Food Sci. Nutr. 2007, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bußler, S.; Psarianos, M.; Rossi, G.; Schlüter, O.K. Edible insect processing pathways and implementation of emerging technologies. J. Insects Food Feed 2021, 7, 877–900. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Platform of Insects for Food and Feed (IPIFF) IPIFF Guide on Good Hygiene Practices for European Union (EU) Producers of Insects as Food and Feed-Updated October 2021. Available online: https://ipiff.org/good-hygiene-practices/ (accessed on 10 January 2022).
- SUSINCHAIN SUStainable INsect CHAIN (SUSINCHAIN) - Newsletter. 2020. Available online: https://susinchain.eu/newsletter/1/ (accessed on 10 January 2022).
- Selaledi, L.; Mabelebele, M. The influence of drying methods on the chemical composition and body color of yellow mealworm (Tenebrio molitor L.). Insects 2021, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Seho, R.E.Y.; Monteiro, R.L.; De Dea Lindner, J.; Miotto, M.; Carciofi, B.A.M.; Laurindo, J.B. Effects of vacuum and multiflash drying on the microbiota and colour of dried yellow mealworm ( Tenebrio molitor ). J. Insects Food Feed 2022, 8, 23–33. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.; Benning, R.; Haase, H.; Kröncke, N.; Grebenteuch, S.; et al. Effect of Different Drying Methods on Nutrient Quality of the Yellow Mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef]
- Purschke, B.; Brüggen, H.; Scheibelberger, R.; Jäger, H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2018, 244, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Álvarez, A.J.; Mondor, M.; Piña-Domínguez, I.A.; Sánchez-Velázquez, O.A.; Melgar Lalanne, G. Drying technologies for edible insects and their derived ingredients. Dry. Technol. 2021, 39, 1991–2009. [Google Scholar] [CrossRef]
- Sindermann, D.; Heidhues, J.; Kirchner, S.; Stadermann, N.; Kuhl, A. Industrial processing technologies for insect larvae. J. Insects Food Feed 2021, 7, 857–875. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten VDLUFA methodenbuch III. VDLUFA-Verlag (Vol. Ed.), Band III-Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Bonn, Germany, 2013; p. 2190. ISBN 9783941273146. [Google Scholar]
- de Oliveira, L.M.; da Silva Lucas, A.J.; Oliveira, F.G. Evaluation of Color Tenebrio molitor Larvae by Different Methods of Dehydration. J. Food Process. Technol. 2018, 9, 10–13. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fiebig, H.-J.; Godelmann, R. Bestimmung der Peroxidzahl (Methode nach Wheeler) - Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und verwandten Stoffen: Analyse von Fetten XXXVII. Lipid / Fett 1997, 99, 194–196. [Google Scholar] [CrossRef]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. Systematic comparison of static and dynamic headspace sampling techniques for gas chromatography. Anal. Bioanal. Chem. 2016, 408, 6567–6579. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanzler, C.; Schestkowa, H.; Haase, P.T.; Kroh, L.W. Formation of Reactive Intermediates, Color, and Antioxidant Activity in the Maillard Reaction of Maltose in Comparison to d -Glucose. J. Agric. Food Chem. 2017, 65, 8957–8965. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Esra Çapanoğlu Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- EU Report From The Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Energy prices and costs in Europe; COM/2020/951 final. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52016DC0805&from=EN (accessed on 10 January 2022).
- Jongema Y List of edible insects of the world. 2017, pp. 1–100. Available online: https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (accessed on 10 January 2022).
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor larva-based ingredients for the food industry: A review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; vander Gheynst, J.S. Critical moisture content for microbial growth in dried food-processing residues. J. Sci. Food Agric. 2010, 90, 2000–2005. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Severini, C. Understanding the drying kinetic and hygroscopic behaviour of larvae of yellow mealworm (Tenebrio molitor) and the effects on their quality. J. Insects as Food Feed. 2016, 2, 233–243. [Google Scholar] [CrossRef]
- Jiao, Y.; Tang, J.; Wang, Y.; Koral, T.L. Radio-Frequency Applications for Food Processing and Safety. Annu. Rev. Food Sci. Technol. 2018, 9, 105–127. [Google Scholar] [CrossRef] [Green Version]
- SUSINCHAIN Reporting Periodic Reporting for Period 1-SUSINCHAIN (SUStainable INsect CHAIN). 2021. Available online: https://cordis.europa.eu/project/id/861976/reporting (accessed on 10 January 2022).
- Chaowattanakul, T.; Khieu, V.M.; Rojviriya, C.; Siriwong, S.; Jittanit, W.; Chanput, W.P. Energy consumption, physical properties, protein structure and digestibility of edible insects dried using three methods. J. Insects Food Feed 2021, 1–12. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Enrichment in specific fatty acids profile of Tenebrio molitor and Hermetia illucens larvae through feeding. Future Foods 2021, 3, 100016. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1–107. [CrossRef] [Green Version]
- Grebenteuch, S.; Kanzler, C.; Klaußnitzer, S.; Kroh, L.W.; Rohn, S. The formation of methyl ketones during lipid oxidation at elevated temperatures. Molecules 2021, 26, 1104. [Google Scholar] [CrossRef]
- Ramos, P.R.; Characterisation of Volatile Compounds Found in Tenebrio Molitor l. Reared with Different Dietary Regimes Using Headspace Solid-Phase Microextraction. Northumbria University Newcastle. 2020. Available online: https://www.researchgate.net/publication/341769184 (accessed on 10 January 2022).
- Halarnkar, P.P.; Schooley, D.A. A comparative catabolism study of isoleucine by insect and mammalian tissues. Comp. Biochem. Physiol. Part B Biochem. 1995, 110, 357–365. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, F.J.; León, M.M.; Zamora, R. Amino acid decarboxylations produced by lipid-derived reactive carbonyls in amino acid mixtures. Food Chem. 2016, 209, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, T.; Münch, P.; Schieberle, P. Quantitative model studies on the formation of aroma-active aldehydes and acids by Strecker-type reactions. J Agric Food Chem. 2000, 48, 434–440. [Google Scholar] [CrossRef]
- Flament, I. Coffee Flavor Chemistry; Wiley: Hoboken, NJ, USA, 2002; p. 424. [Google Scholar]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, M.; Wendin, K.; Olsson, V.; Langton, M.; Elhassan, M.; Wendin, K.; Olsson, V.; Langton, M. Quality Aspects of Insects as Food—Nutritional, Sensory, and Related Concepts. Foods 2019, 8, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.; Kim, H.R.; Cho, I.H. Aroma characteristics of raw and cooked tenebrio molitor larvae (mealworms). Food Sci. Anim. Resour. 2020, 40, 649–658. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Szumny, A. Sensory and chemical characteristic of two insect species: Tenebrio molitor and zophobas morio larvae affected by roasting processes. Molecules 2021, 26, 2697. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, V.; Serafini, M.; Battista, N. Dietary Modulation of Oxidative Stress From Edible Insects: A Mini-Review. Front. Nutr. 2021, 8, 38. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, M.; Kim, M.A.; Kwon, Y.S.; Hwang, J.S.; Goo, T.W.; Jun, M.; Yun, E.Y. Effects of processing methods on nutritional composition and antioxidant activity of mealworm (Tenebrio molitor) larvae. Entomol. Res. 2019, 49, 284–293. [Google Scholar] [CrossRef]
- Mancini, S.; Mattioli, S.; Paolucci, S.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Paci, G. Effect of Cooking Techniques on the in vitro Protein Digestibility, Fatty Acid Profile, and Oxidative Status of Mealworms (Tenebrio molitor). Front. Vet. Sci. 2021, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.K.; Yeo, J.D.; Jang, E.Y.; Kim, M.J.; Lee, J.H. Aldehydes from oxidized lipids can react with 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals in isooctane systems. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1831–1838. [Google Scholar] [CrossRef]
- Cämmerer, B.; Chodakowski, K.; Gienapp, C.; Wohak, L.; Hartwig, A.; Kroh, L.W. Pro-oxidative effects of melanoidin-copper complexes on isolated and cellular DNA. Eur. Food Res. Technol. 2012, 234, 663–670. [Google Scholar] [CrossRef]
- Fiol, M.; Weckmüller, A.; Neugart, S.; Schreiner, M.; Rohn, S.; Krumbein, A.; Kroh, L.W. Thermal-induced changes of kale’s antioxidant activity analyzed by HPLC-UV/Vis-online-TEAC detection. Food Chem. 2013, 138, 857–865. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]

| Drying Method | DTemp (°C) | DTemp (°C) | MO (kW) | t (h) | EC (kWh) | Larvae Material (kg) | EC/kg Larvae Material (kWh/kg) * | Energy Cost (EUR /kg Larvae Material) # |
|---|---|---|---|---|---|---|---|---|
| Infrared drying | 130 | 130 | 22 | 0.25 | 5.5 | 1 | 5.5 | 0.99 |
| Rack oven drying | 60 | 250 | 0.43 | 19 | 1.96 | 0.4 | 4.9 | 0.88 |
| P (kW) | t (h) | EC (kWh) | larvae material (kg) | EC/kg larvae material (kWh/kg) * | Energy cost (EUR /kg larvae material) # | |||
| Freeze-drying | 0.51 | 27 | 13.8 | 0.5 | 27.5 | 4.96 | ||
| Microwave drying | 7.2 | 0.25 | 1.8 | 0.15 | 12.0 | 2.16 | ||
| High frequency drying | Real-time online measurement of energy consumption | 1 | 0.27 | 0.54 | 0.5 | 0.09 | ||
| Drying Method | Undried | High-Frequency Dried | Rack Oven Dried | Infrared Dried | Microwave Dried | Freeze- Dried |
|---|---|---|---|---|---|---|
| Water activity | 0.69 ± 0.00 a | 0.30 ± 0.00 b | 0.13 ± 0.01 c | 0.18 ± 0.01 d | 0.13 ± 0.01 c | 0.11 ± 0.00 e |
| Moisture (g/100g) | 59.53 ± 0.24 a | 1.38 ± 0.01 b | 7.87 ± 0.03 c | 2.23 ± 0.03 d | 2.67 ± 0.03 d | 5.47 ± 0.03 e |
| Protein (g/100 g DM) | 57.57 ± 1.09 a | 53.00 ± 1.80 b | 55.77 ± 0.07 a.b | 57.07 ± 0.03 a.b | 56.77 ± 0.07 a.b | 55.50 ± 0.15 a.b |
| Fat (g/100 g DM) | 26.80 ± 0.45 a | 23.07 ± 1.06 b | 28.83 ± 0.09 a | 27.47 ± 0.03 a | 27.47 ± 0.09 a | 28.43 ± 0.03 a |
| Ash (g/100 g DM) | 3.90 ± 0.00 a | 3.67 ± 0.09 a.b | 3.73 ± 0.07 a.c | 3.47 ± 0.03 b | 3.67 ± 0.03 a.b | 3.63 ± 0.03 b,c |
| Colour Parameters | High-Frequency Dried | Rack Oven Dried | Infrared Dried | Microwave Dried | Freeze Dried |
|---|---|---|---|---|---|
| L* | 33.00 ± 0.43 a | 41.27 ± 1.44 b | 50.27 ± 0.99 c | 60.60 ± 0.47 d | 66.13 ± 0.71 e |
| a* | 9.73 ± 0.05 a | 11.33 ± 0.11 a | 17.47 ± 0.52 b | 16.20 ± 0.16 b | 12.07 ± 0.20 c |
| b* | 19.00 ± 0.19 a | 20.13 ± 0.24 a | 36.00 ± 0.16 b | 46.93 ± 0.29 c | 44.47 ± 0.05 d |
| Fatty acid | High-Frequency Dried | Rack Oven Dried | Infrared Dried | Microwave Dried | Freeze- Dried |
|---|---|---|---|---|---|
| Lauric acid (C12:0) | 0.31 ± 0.01 a | 0.34 ± 0.04 a | 0.30 ± 0.01 a | 0.26 ± 0.00 a | 0.20 ± 0.01 b |
| Myristic acid (C14:0) | 3.48 ± 0.14 a | 4.42 ± 0.01 b | 3.19 ± 0.02 a | 3.49 ± 0.02 a | 2.88 ± 0.05 c |
| Palmitic acid (C16:0) | 20.60 ± 0.14 a | 19.78 ± 0.15 b | 10.89 ± 0.09 c | 17.25 ± 0.02 d | 19.94 ± 0.16 b,e |
| Palmitoleic acid (C16:1 ω-7) | 1.34 ± 0.11 a | 1.46 ± 0.01 b | 1.77 ± 0.01 a | 1.57 ± 0.01 a | 1.47 ± 0.02 a |
| Stearic acid (C18:0) | 8.00 ± 0.38 a | 3.45 ± 0.01 b | 3.39 ± 0.01 b | 2.18 ± 0.06 c | 4.48 ± 0.01 d |
| Oleic acid (C18:1 ω-9) | 40.73 ± 0.13 a | 36.78 ± 0.21 b | 36.82 ± 0.12 b | 43.76 ± 0.03 c | 32.82 ± 0.04 d |
| Linoleic acid (C18:2 ω-6) | 25.54 ± 1.10 a | 31.91 ± 0.12 b | 41.27 ± 0.16 c | 30.18 ± 0.02 b | 35.89 ± 0.14 d |
| Linolenic acid (C18:3 ω-3) | n.d. | 1.12 ± 0.01 a | 1.18 ± 0.03 b | 1.01 ± 0.01 c | 1.48 ± 0.02 d |
| Arachidic acid (C20:0) | n.d. | 0.34 ± 0.01 a | 0.22 ± 0.01 b | 0.17 ± 0.01 c | 0.23 ± 0.02 b |
| Behenic acid (C22:0) | n.d. | 0.33 ± 0.01 a | 0.22 ± 0.01 a | 0.08 ± 0.01 b | 0.35 ± 0.04 c |
| Lignoceric acid (C24:0) | n.d. | 0.21 ± 0.01 a,b | 0.19 ± 0.01 a | 0.06 ± 0.01 c | 0.27 ± 0.02 b |
| ∑SFA | 32.38 ± 0.27 a | 28.74 ± 0.10 b | 18.34 ± 0.07 c | 23.48 ± 0.07 d | 28.35 ± 0.17 b |
| ∑MUFA | 42.07 ± 1.34 a | 38.24 ± 0.20 b | 38.59 ± 0.14 b | 45.33 ± 0.04 c | 34.29 ± 0.03 d |
| ∑PUFA | 25.54 ± 1.10 a | 33.02 ± 0.13 b | 43.07 ± 0.17 c | 31.19 ± 0.04 b | 37.36 ± 0.15 d |
| ∑UFA | 67.62 ± 0.28 a | 71.26 ± 0.09 b | 81.66 ± 0.07 c | 76.52 ± 0.04 d | 71.65 ± 0.17 b |
| PUFA/SFA | 0.78 ± 0.03a | 1.15 ± 0.01b | 2.35 ± 0.02c | 1.33 ± 0.01d | 1.2 ± 0.01d |
| Peroxide value | 0.69 ± 0.05 a | 1.31 ± 0.07 a | 1.28 ± 0.22 a | 1.92 ± 0.35 a | 7.22 ± 0.78 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keil, C.; Grebenteuch, S.; Kröncke, N.; Kulow, F.; Pfeif, S.; Kanzler, C.; Rohn, S.; Boeck, G.; Benning, R.; Haase, H. Systematic Studies on the Antioxidant Capacity and Volatile Compound Profile of Yellow Mealworm Larvae (T. molitor L.) under Different Drying Regimes. Insects 2022, 13, 166. https://doi.org/10.3390/insects13020166
Keil C, Grebenteuch S, Kröncke N, Kulow F, Pfeif S, Kanzler C, Rohn S, Boeck G, Benning R, Haase H. Systematic Studies on the Antioxidant Capacity and Volatile Compound Profile of Yellow Mealworm Larvae (T. molitor L.) under Different Drying Regimes. Insects. 2022; 13(2):166. https://doi.org/10.3390/insects13020166
Chicago/Turabian StyleKeil, Claudia, Sandra Grebenteuch, Nina Kröncke, Fenja Kulow, Sebastian Pfeif, Clemens Kanzler, Sascha Rohn, Georg Boeck, Rainer Benning, and Hajo Haase. 2022. "Systematic Studies on the Antioxidant Capacity and Volatile Compound Profile of Yellow Mealworm Larvae (T. molitor L.) under Different Drying Regimes" Insects 13, no. 2: 166. https://doi.org/10.3390/insects13020166







