Niche and Range Shifts of Aedes aegypti and Ae. albopictus Suggest That the Latecomer Shows a Greater Invasiveness
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Record Datasets
2.2. Niche Dynamic Analysis
2.3. Predictors Used in SDMs
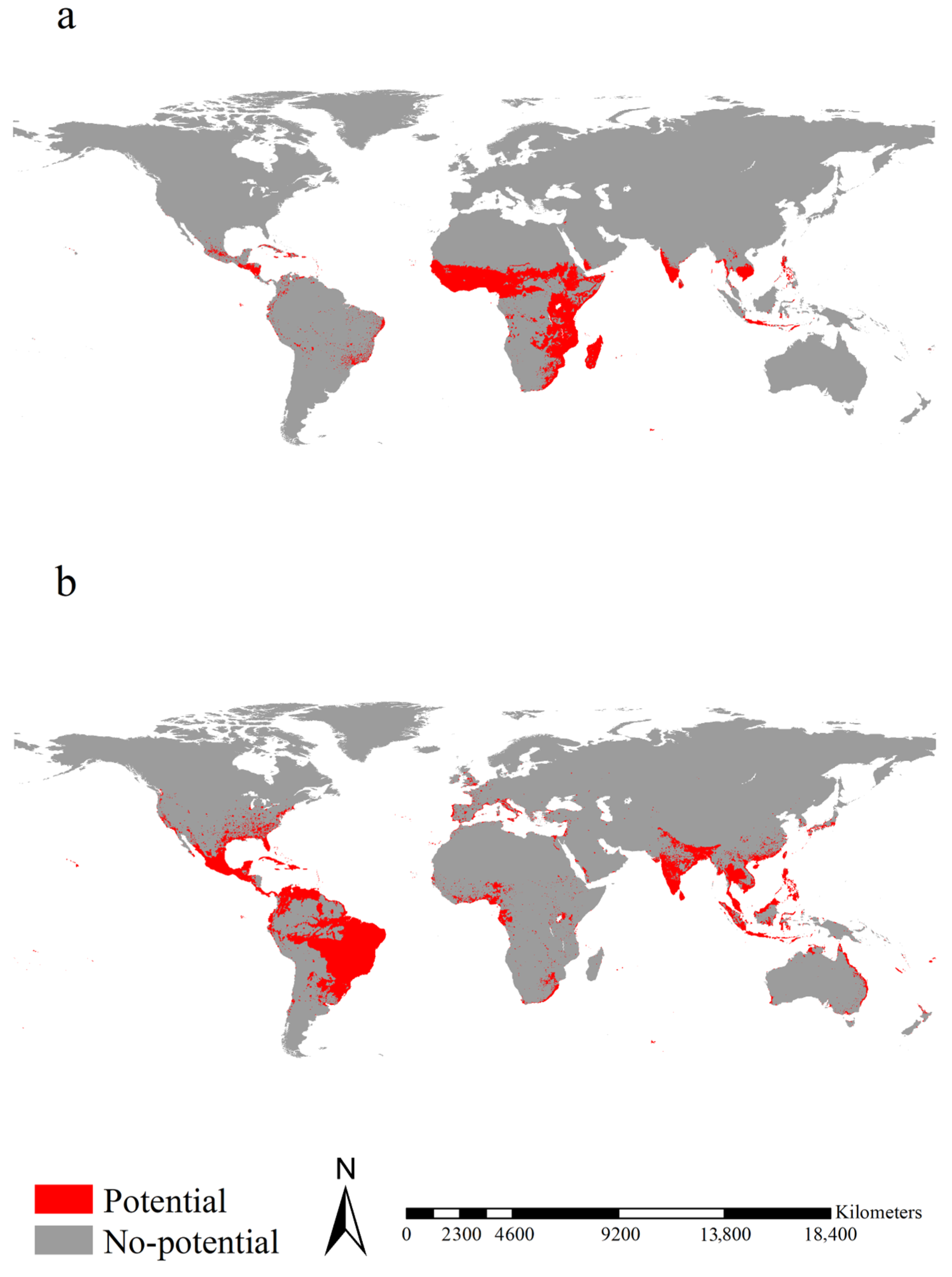
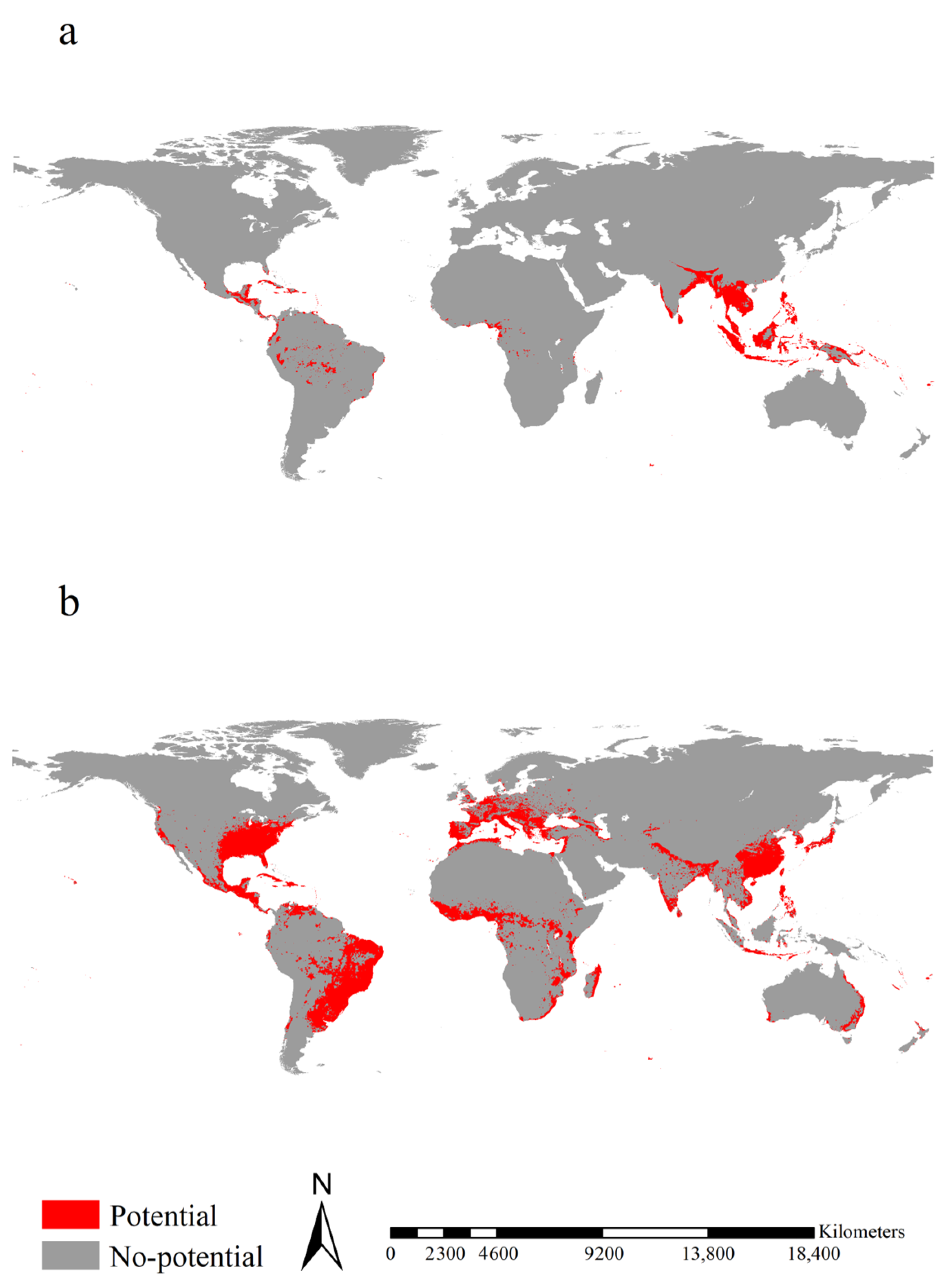
2.4. Potential Ranges of the Two Aedes Species
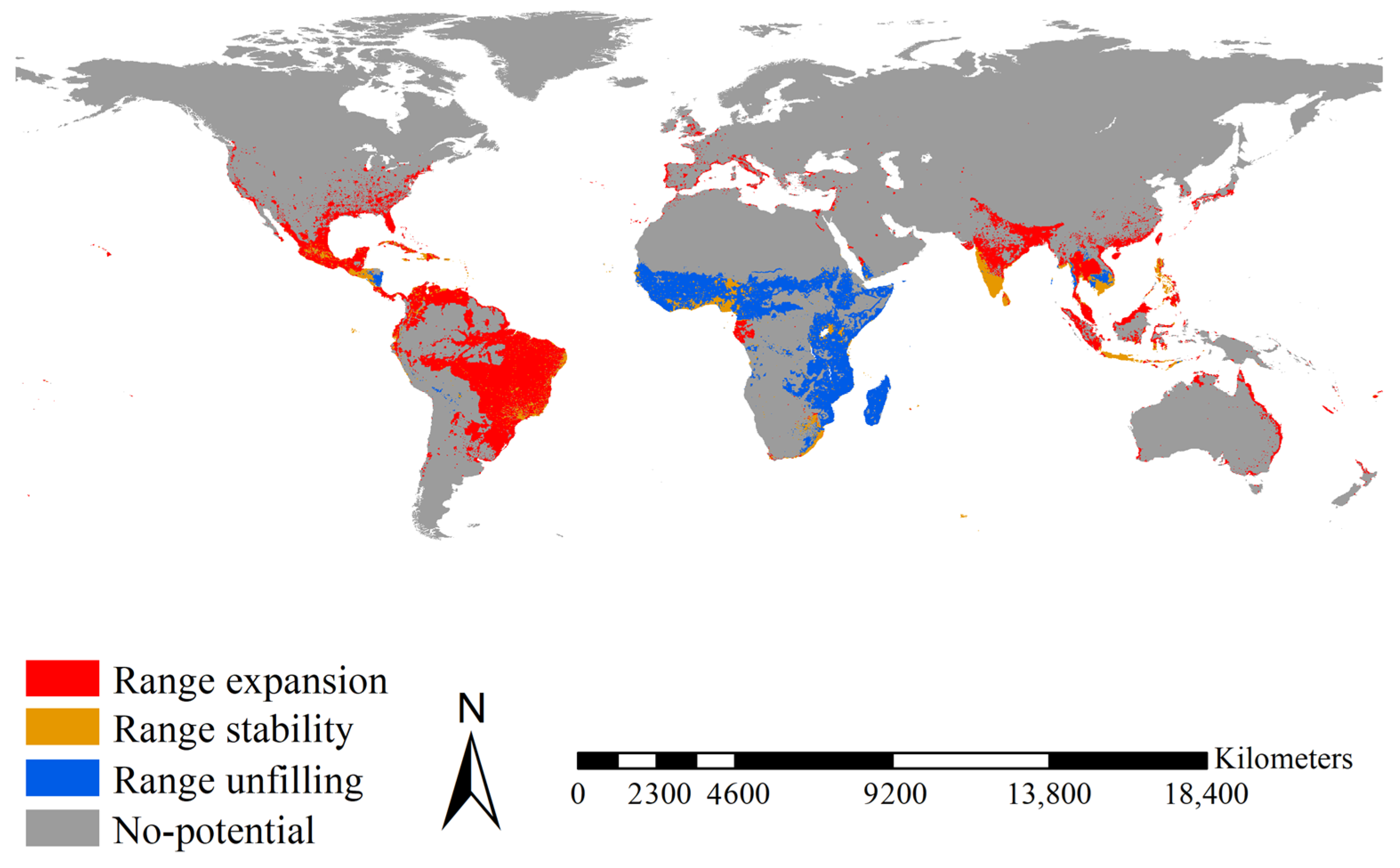
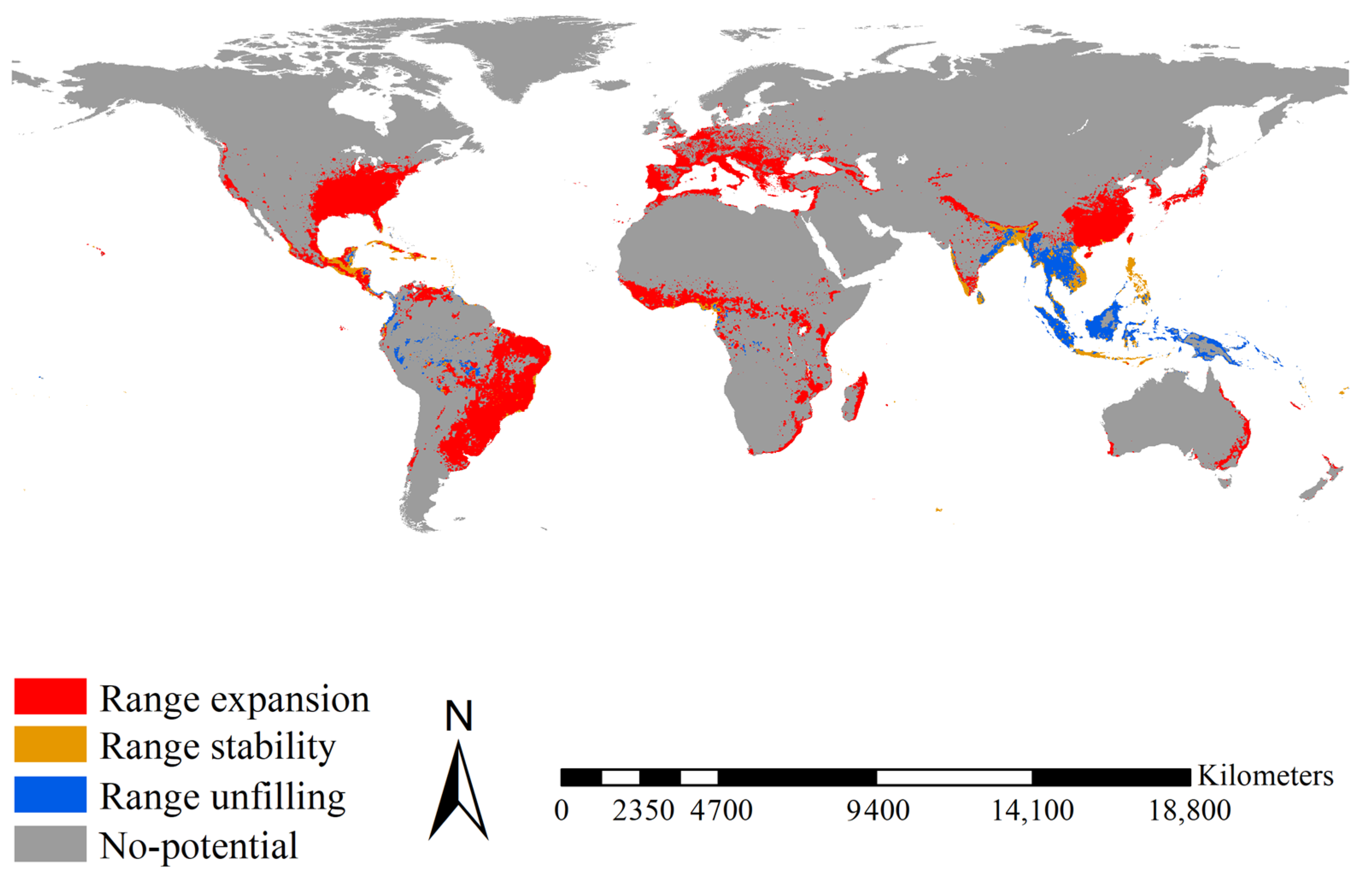
2.5. Range Shifts of the Two Aedes Species
3. Results
3.1. Major Predictors for the Potential Ranges
3.2. Niche Dynamics of the Two Aedes Species
3.3. Potential Ranges of the Two Aedes Species
3.4. Range Shifts of the Two Aedes Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jentes, E.S.; Poumerol, G.; Gershman, M.D.; Hill, D.R.; Lemarchand, J.; Lewis, R.F.; Staples, J.E.; Tomori, O.; Wilder-Smith, A.; Monath, T.P. The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 2011, 11, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Farrar, J.J.; Nguyen, V.V.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Leparc-Goffart, I.; Nougairede, A.; Cassadou, S.; Prat, C.; de Lamballerie, X. Chikungunya in the Americas. Lancet 2014, 383, 514. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B 2015, 370, 20130554. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.M.; Thomas, S.M.; Wahed, A.A.; Cohoon, K.P.; Peterson, A.T. Mapping the global geographic potential of Zika virus spread. Mem. Inst. Oswaldo Cruz 2016, 111, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Schaffner, F.; Medlock, J.M.; Van Bortel, W. Public health significance of invasive mosquitoes in Europe. Clin. Microbiol. Infec. 2013, 19, 685–692. [Google Scholar] [CrossRef]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Yellow Fever Expert Committee. Yellow Fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef]
- Powers, A.M. Chikungunya virus control: Is a vaccine on the horizon? Lancet 2014, 384, 2008–2009. [Google Scholar] [CrossRef]
- Sharp, T.M.; Roth, N.M.; Torres, J.; Ryff, K.R.; Rodriguez, N.M.P.; Mercado, C.; Diaz Padró, M.D.P.; Ramos, M.; Phillips, R.; Lozier, M. Chikungunya cases identified through passive surveillance and household investigations—Puerto Rico, May 5–August 12, 2014. MMWR-Morb. Mortal. Wkly. Rep. 2014, 63, 500–501. [Google Scholar]
- Staples, J.E.; Fischer, M. Chikungunya virus in the Americas—What a vectorborne pathogen can do. N. Engl. J. Med. 2014, 371, 887–889. [Google Scholar] [CrossRef]
- Tabachnick, W.J. Evolutionary genetics and arthropod-borne disease: The Yellow Fever Mosquito. Am. Entomol. 1991, 37, 14–26. [Google Scholar] [CrossRef]
- Kaplan, L.; Kendell, D.; Robertson, D.; Livdahl, T.; Khatchikian, C. Aedes aegypti and Aedes albopictus in Bermuda. Extinction, invasion, invasion and extinction. Biol. Invasions 2010, 12, 3277–3288. [Google Scholar] [CrossRef]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.E. Concluding remarks of Cold Spring Harbor Symposium. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Davies, S.J.; Hill, M.P.; McGeoch, M.A.; Clusella-Trullas, S. Niche Shift and Resource Supplementation Facilitate an Amphibian Range Expansion. Divers. Distrib. 2019, 25, 154–165. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Gilbert, B.; Levine, J.M. Plant Invasions and the Niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Fang, Y.Q.; Zhang, X.H.; Wei, H.Y.; Wang, D.J.; Chen, R.D.; Wang, L.K.; Gu, W. Predicting the Invasive Trend of Exotic Plants in China Based on the Ensemble Model under Climate Change: A Case for Three Invasive Plants of Asteraceae. Sci. Total Environ. 2021, 756, 143841. [Google Scholar] [CrossRef]
- Zhang, W.G.; Chen, X.Y.; Liu, R.L.; Song, X.J.; Liu, G.; Zou, J.B.; Qian, Z.Q.; Zhu, Z.; Cui, L. Realized Niche Shift Associated with Galinsoga quadriradiata (Asteraceae) Invasion in China. J. Plant Ecol. 2022, 15, 538–548. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring Ecological Niche Overlap from Occurrence and Spatial Environmental Data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic Niche Shifts Are Rare among Terrestrial Plant Invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
- Gutierrez-Ortega, J.S.; Salinas-Rodriguez, M.M.; Ito, T.; Perez-Farrera, M.A.; Vovides, A.P.; Martinez, J.F.; Molina-Freaner, F.; Hernández-López, A.; Kawaguchi, L.; Nagano, A.J.; et al. Niche Conservatism Promotes Speciation in Cycads: The Case of Dioon merolae (Zamiaceae) in Mexico. New Phytol. 2020, 227, 1872–1884. [Google Scholar] [CrossRef]
- Da Re, D.; Olivares, A.P.; Smith, W.; Vallejo-Marín, M. Global analysis of ecological niche conservation and niche shift in exotic populations of monkeyflowers (Mimulus guttatus, M. luteus) and their hybrid (M.× robertsii). Plant Ecol. Divers. 2020, 13, 133–146. [Google Scholar] [CrossRef]
- Liu, C.L.; Wolter, C.; Xian, W.W.; Jeschke, J.M. Most Invasive Species Largely Conserve Their Climatic Niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Yin, X.; Jarvie, S.; Guo, W.Y.; Deng, T.; Mao, L.F.; Zhang, M.H.; Chu, C.J.; Qian, H.; Svenning, J.; He, F. Niche Overlap and Divergence Times Support Niche Conservatism in Eastern Asia-Eastern North America Disjunct Plants. Glob. Ecol. Biogeogr. 2021, 30, 1990–2003. [Google Scholar] [CrossRef]
- Atwater, D.Z.; Ervine, C.; Barney, J.N. Climatic Niche Shifts Are Common in Introduced Plants. Nat. Ecol. Evol. 2018, 2, 34–43. [Google Scholar] [CrossRef]
- Loerch, M.; Mutke, J.; Weigend, M.; Luebert, F. Historical Biogeography and Climatic Differentiation of the Fulcaldea-Archidasyphyllum-Arnaldoa Clade of Barnadesioideae (Asteraceae) Suggest a Miocene, Aridity-Mediated Andean Disjunction Associated with Climatic Niche Shifts. Glob. Planet. Change 2021, 201, 103495. [Google Scholar] [CrossRef]
- Kelly, C.L.; Gordon, I.J.; Schwarzkopf, L.; Pintor, A.; Pople, A.; Hirsch, B.T. Invasive wild deer exhibit environmental niche shifts in Australia: Where to from here? Ecol. Evol. 2023, 13, e10251. [Google Scholar] [CrossRef]
- Bates, O.K.; Ollier, S.; Bertelsmeier, C. Smaller Climatic Niche Shifts in Invasive Than Non-Invasive Alien Ant Species. Nat. Commun. 2020, 11, 5213. [Google Scholar] [CrossRef] [PubMed]
- Escoriza, D.; Ben Hassine, J.; Boix, D. Factors Regulating the Invasive Success of an Alien Frog: A Comparison of the Ecology of the Native and Alien Populations. Hydrobiologia 2014, 730, 127–138. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J.; Tan, J.F.; Yu, F.H. Climatic Niche Divergence and Habitat Suitability of Eight Alien Invasive Weeds in China under Climate Change. Ecol. Evol. 2017, 7, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Piovezan-Borges, A.C.; Valente-Neto, F.; Urbieta, G.L.; Laurence, S.G.W.; Roque, F.D. Global trends in research on the effects of climate change on Aedes aegypti: International collaboration has increased, but some critical countries lag behind. Parasite Vector 2022, 15, 346. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, H.; Bolker, B.; Lord, C.C.; Rubio, C.; Lounibos, L.P. Food availability alters the effects of larval temperature on Aedes aegypti Growth. J. Med. Entomol. 2011, 48, 974–984. [Google Scholar] [CrossRef]
- Rueda, L.M.; Patel, K.J.; Axtell, R.C.; Stinner, R.E.; Carolina, N. Temperature dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Entomol. Soc. Am. 1990, 27, 892–898. [Google Scholar] [CrossRef]
- Carrington, L.B.; Armijos, M.V.; Lambrechts, L.; Barker, C.M.; Scott, T.W. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS ONE 2013, 8, e58824. [Google Scholar] [CrossRef]
- Costa, E.; Santos, E.; Correia, J.; Albuquerque, C. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Rev. Bras. Entomol. 2010, 54, 488–493. [Google Scholar] [CrossRef]
- Armbruster, P.; Conn, J.E. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2006, 99, 1234–1243. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Blanford, S.; Bell, A.S.; Blanford, J.I.; Read, A.F.; Thomas, M.B. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 2010, 107, 15135–15139. [Google Scholar] [CrossRef]
- Leonel, B.F.; Koroiva, R.; Hamada, N.; Ferreira-Keppler, R.L.; Roque, F.O. Potential effects of climate change on ecological interaction outcomes between two disease-vector mosquitoes: A mesocosm experimental study. J. Med. Entomol. 2015, 52, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Cohen, J.M.; Evans, M.V.; Gudapati, P.; Johnson, L.R.; Lippi, C.A.; Miazgowicz, K.; Murdock, C.C.; Rohr, J.R.; Ryan, S.J.; et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl. Trop. Dis. 2017, 11, 1–18. [Google Scholar] [CrossRef]
- Piovezan-Borges, A.C.; Valente-Neto, F.; Tadei, W.P.; Hamada, N.; Roque, F.O. Simulated climate change, but not predation risk, accelerates Aedes aegypti emergence in a microcosm experiment in western Amazonia. PLoS ONE 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Winokur, O.C.; Main, B.J.; Nicholson, J.; Barker, C.M. Impact of temperature on the extrinsic incubation period of Zika virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Gao, X.; Ma, J.; Jiao, Z.H.; Xiao, J.H.; Hayat, M.A.; Wang, H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Laporta, G.Z.; Potter, A.M.; Oliveiram, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.M. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Cunze, S.; Koch, L.K.; Kochmann, J.; Klimpel, S. Aedes albopictus and Aedes japonicus—Two invasive mosquito species with different temperature niches in Europe. Parasite Vector 2016, 9, 573. [Google Scholar] [CrossRef]
- Gratz, N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 215–227. [Google Scholar] [CrossRef]
- Simard, F.; Nchoutpouen, E.; Toto, J.C.; Fontenille, D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005, 42, 726–731. [Google Scholar] [CrossRef]
- Dickens, B.L.; Sun, H.; Jit, M.; Cook, A.R.; Carrasco, L.R. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus. BMJ Glob. Health 2018, 3, e000801. [Google Scholar] [CrossRef]
- Abílio, A.P.; Abudasse, G.; Kampango, A.; Candrinho, B.; Sitoi, S.; Luciano, J.; Tembisse, D.; Sibindy, S.; de Almeida, A.P.G.; Garcia, G.A.; et al. Distribution and breeding sites of Aedes aegypti and Aedes albopictus in 32 urban/peri-urban districts of Mozambique: Implication for assessing the risk of arbovirus outbreaks. PLoS Negl. Trop. Dis. 2018, 12, e0006692. [Google Scholar] [CrossRef] [PubMed]
- Holeva-Eklund, W.M.; Young, S.J.; Will, J.; Busser, N.; Townsend, J.; Hepp, C.M. Species distribution modeling of Aedes aegypti in Maricopa County, Arizona from 2014 to 2020. Front. Environ. Sci. 2022, 10, 1001190. [Google Scholar] [CrossRef]
- Chardon, N.I.; Cornwell, W.K.; Flint, L.E.; Flint, A.; Ackerly, D. Topographic, latitudinal and climatic distribution of Pinus coulteri: Geographic range limits are not at the edge of the climate envelope. Ecography 2015, 38, 590–601. [Google Scholar] [CrossRef]
- Michalak, J.L.; Lawler, J.J.; Roberts, D.R.; Carroll, C. Distribution and protection of climatic refugia in North America. Conserv. Biol. 2018, 32, 1414–1425. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Sandel, B.; Blank, D.; Liu, Z.T.; Liu, X. Climate and topography explain range sizes of terrestrial vertebrates. Nat. Clim. Chang. 2016, 6, 498–502. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Stanley, G.; Misinzo, G.; Mboera, L.E.G. Climate Change Influences Potential Distribution of Infected Aedes aegypti Co-Occurrence with Dengue Epidemics Risk Areas in Tanzania. PLoS ONE 2016, 11, e0162649. [Google Scholar] [CrossRef]
- Estallo, E.L.; Sangermano, F.; Grech, M.; Luduena-Almeida, F.; Frias-Cespedes, M.; Ainete, M.; Almirón, W.; Livdahl, T. Modelling the distribution of the vector Aedes aegypti in a central Argentine city. Med. Vet. Entomol. 2018, 32, 451–461. [Google Scholar] [CrossRef]
- Echeverry-Cárdenas, E.; LÓpez-Castañeda, C.; Carvajal-Castro, J.D.; Aguirre-Obando, O.A. Potential geographic distribution of the tiger mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) in current and future conditions for Colombia. PLoS Negl. Trop. Dis. 2021, 15, e0008212. [Google Scholar] [CrossRef]
- Nurjanah, S.; Atmowidi, T.; Hadi, U.K.; Solihin, D.D.; Priawandiputra, W.; Santoso, B.; Asmarani, D.; Setiawan, T.; Meidaliyantisyah. Distribution modelling of Aedes aegypti in three dengue-endemic areas in Sumatera, Indonesia. Trop. Biomed. 2022, 39, 373–383. [Google Scholar] [CrossRef]
- Yang, B.; Borgert, B.A.; Alto, B.W.; Boohene, C.K.; Brew, J.; Deutsch, K.; DeValerio, J.T.; Dinglasan, R.R.; Dixon, D.; Faella, J.M.; et al. Modelling distributions of Aedes aegypti and Aedes albopictus using climate, host density and interspecies competition. PLoS Negl. Trop. Dis. 2021, 15, e0009063. [Google Scholar] [CrossRef]
- Wint, W.; Jones, P.; Kraemer, M.; Alexander, N.; Schaffner, F. Past, present and future distribution of the yellow fever mosquito Aedes aegypti: The European paradox. Sci. Total Environ. 2022, 847, 157566. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation python–based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, R.C.; Linton, Y.M.; Strickman, D. Mosquitoes of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2020. [Google Scholar]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying Niche Shift Studies: Insights from Biological Invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.J.; Wang, T.; Jiang, X.F.; Hu, X.K.; Feng, J.M. Double—Edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araujo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 7–46. [Google Scholar] [CrossRef]
- Gallien, L.; Douzet, R.; Pratte, S.; Zimmermann, N.E.; Thuiller, W. Invasive species distribution models—How violating the equilibrium assumption can create new insights. Glob. Ecol. Biogeogr. 2012, 21, 1126–1136. [Google Scholar] [CrossRef]
- Cao, R.Y.; Gong, X.; Feng, J.M.; Yang, R.J. Niche and range dynamics of Tasmanian blue gum (Eucalyptus globulus Labill.), a globally cultivated invasive tree. Ecol. Evol. 2022, 12, e9305. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence–only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ulrich, W. Statistical challenges in null model analysis. Oikos 2012, 121, 171–180. [Google Scholar] [CrossRef]
- Bohl, C.L.; Kass, J.M.; Anderson, R.P. A new null model approach to quantify performance and significance for ecological niche models of species distributions. J. Biogeogr. 2019, 46, 1101–1111. [Google Scholar] [CrossRef]
- Yang, R.J.; Yu, X.L.; Nie, P.X.; Cao, R.Y.; Feng, J.M. Climatic niche and range shifts of grey squirrels (Sciurus carolinensis Gmelin) in Europe: An invasive pest displacing native squirrels. Pest Manag. Sci. 2023, 79, 3731–3739. [Google Scholar] [CrossRef]
- Nie, P.X.; Yang, R.J.; Cao, R.Y.; Hu, X.K.; Feng, J.M. Niche and range shifts of the fall webworm (Hyphantria cunea Dury) in Europe imply its huge invasion potential in the future. Insects 2023, 14, 316. [Google Scholar] [CrossRef]
- Lacour, G.; Chanaud, L.; L’Ambert, G.; Hance, T. Seasonal synchronization of diapause phases in Aedes albopictus (Diptera: Culicidae). PLoS ONE 2015, 10, e0145311. [Google Scholar] [CrossRef]
- Marini, G.; Manica, M.; Arnoldi, D.; Inama, E.; Rosà, R.; Rizzoli, A. Influence of temperature on the life-cycle dynamics of Aedes albopictus population established at temperate latitudes: A laboratory experiment. Insects 2020, 11, 808. [Google Scholar] [CrossRef]
- Medley, K.A. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010, 19, 122–133. [Google Scholar] [CrossRef]
- Diaz-Nieto, L.M.; Macia, A.; Perotti, M.A.; Beron, C.M. Geographical limits of the Southeastern distribution of Aedes aegypti (Diptera, Culicidae) in Argentina. PLoS Negl. Trop. Dis. 2013, 7, e1963. [Google Scholar] [CrossRef] [PubMed]
- Seixas, G.; Salgueiro, P.; Bronzato-Badial, A.; Goncalves, Y.; Reyes-Lugo, M.; Gordicho, V. Origin and expansion of the mosquito Aedes aegypti in Madeira Island (Portugal). Sci. Rep. 2019, 9, 2241. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Brännström, Å.; Sewe, M.; Semenza, J.C.; Rocklöv, J. Estimating past, present and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front. Public Health 2019, 7, 148. [Google Scholar] [CrossRef]
- Bargielowski, I.E.; Lounibos, L.P.; Carrasquilla, M.C. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl. Acad. Sci. USA 2013, 110, 2888–2892. [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109, 141–161. [Google Scholar] [CrossRef]
- Juliano, S.A.; Philip, L.L. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol. Lett. 2005, 8, 558–574. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.-G. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.-L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Ding, F.Y.; Fu, J.Y.; Jiang, D.; Hao, M.M.; Lin, G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018, 178, 155–162. [Google Scholar] [CrossRef]
- Tsuda, Y.; Suwonkerd, W.; Chawprom, S.; Prajakwong, S.; Takagi, M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Control. 2006, 22, 222–228. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Invasive Species Specialist Group. 100 of the World’s Worst Invasive Alien Species. 2023. Available online: http://www.iucngisd.org/gisd/species.php?sc=109 (accessed on 4 March 2023).
- Reiter, P.; Sprenger, D. The used tire trade: A mechanism for the worldwide dispersal of container breeding mosquitoes. J. Am. Mosq. Control. Assoc. 1987, 3, 494–501. [Google Scholar] [PubMed]
- Kramer, I.M.; Pfeiffer, M.; Steffens, O.; Schneider, F.; Gerger, V.; Phuyal, P. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci. Total Environ. 2021, 778, 146128. [Google Scholar] [CrossRef]

| Aedes aegypti | Aedes albopictus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Native Ae. aegypti | Introduced Ae. aegypti | Native Ae. albopictus | Introduced Ae. albopictus | ||||||||
| Category | Predictors | Importance Values | Category | Predictors | Importance Values | Category | Predictors | Importance Values | Category | Predictors | Importance Values |
| Climate | Bio3 | 0.351 | Climate | Bio4 | 0.369 | Climate | Bio1 | 0.230 | Climate | Bio10 | 0.150 |
| GDP | GDP | 0.196 | GDP | GDP | 0.194 | Climate | Bio4 | 0.197 | Climate | Bio4 | 0.149 |
| POP | POP | 0.113 | POP | POP | 0.112 | Climate | Bio12 | 0.195 | Climate | Bio13 | 0.108 |
| Climate | Bio7 | 0.052 | Climate | Bio10 | 0.099 | Climate | Bio18 | 0.148 | Land-use | Pastr | 0.082 |
| Land-use | Primf | 0.030 | Climate | Bio9 | 0.096 | POP | POP | 0.136 | Land-use | Urban | 0.056 |
| Land-use | Range | 0.028 | Land-use | Crop | 0.045 | Land-use | Range | 0.059 | POP | POP | 0.056 |
| Climate | Bio13 | 0.024 | Climate | Bio13 | 0.033 | Climate | Bio5 | 0.050 | Climate | Bio17 | 0.050 |
| Land-use | Secdn | 0.024 | Land-use | Primf | 0.015 | GDP | GDP | 0.049 | Climate | Bio18 | 0.03 |
| Land-use | Primn | 0.020 | Land-use | Urban | 0.011 | Land-use | Pastr | 0.032 | Land-use | Crop | 0.032 |
| Climate | Bio1 | 0.019 | Climate | Bio19 | 0.011 | Land-use | Crop | 0.021 | Climate | Bio8 | 0.030 |
| Climate | Bio14 | 0.017 | Topography | Ele | 0.010 | Land-use | Primf | 0.018 | Climate | Bio19 | 0.028 |
| Land-use | Pastr | 0.015 | Climate | Bio18 | 0.010 | Topography | Ele | 0.018 | Land-use | Primf | 0.025 |
| Climate | Bio18 | 0.012 | Climate | Bio17 | 0.008 | Climate | Bio14 | 0.018 | Topography | Ele | 0.022 |
| Land-use | Crop | 0.011 | Land-use | Pastr | 0.007 | Topography | Slop | 0.011 | Land-use | Range | 0.020 |
| Climate | Bio15 | 0.007 | Land-use | Range | 0.007 | Climate | Bio2 | 0.007 | Land-use | Primn | 0.016 |
| Topography | Slop | 0.007 | Land-use | Primn | 0.007 | Land-use | Secdn | 0.003 | Climate | Bio2 | 0.010 |
| Land-use | Secdf | 0.004 | Topography | Slop | 0.005 | Land-use | Secdf | 0.003 | Land-use | Secdn | 0.010 |
| Topography | Asp | 0.003 | Land-use | Secdf | 0.004 | Land-use | Urban | 0.003 | Topography | Slop | 0.007 |
| Land-use | Urban | 0.002 | Land-use | Secdn | 0.003 | Land-use | Primn | 0.002 | Land-use | Secdf | 0.005 |
| Topography | Asp | 0.003 | Topography | Asp | 0.002 | Topography | Asp | 0.001 | |||
| Climate | Bio2 | 0.002 | |||||||||
| Species (Native Range) | Introduced Population | Expan | Stable | Unfill | Breadth | EquaT | Similar | SimiT |
|---|---|---|---|---|---|---|---|---|
| Aedes aegypti (Africa) | Global | 0.045 | 0.955 | 0.027 | 1.017 | ns | 0.964 | ns |
| Asia | 0.087 | 0.913 | 0.005 | 1.090 | ns | 0.952 | ns | |
| North America | 0.137 | 0.863 | 0.058 | 1.085 | ns | 0.899 | ns | |
| South America | 0.008 | 0.992 | 0.148 | 0.877 | ns | 0.927 | ns | |
| Ae. albopictus (Asia) | Global | 0.382 | 0.618 | 0.101 | 1.391 | ns | 0.719 | ns |
| Africa | 0.055 | 0.945 | 0.208 | 0.867 | ns | 0.878 | ns | |
| Europe | 0.499 | 0.501 | 0.806 | 0.765 | ns | 0.434 | ns | |
| North America | 0.332 | 0.668 | 0.359 | 0.974 | ns | 0.659 | ns | |
| South America | 0.022 | 0.978 | 0.286 | 0.791 | ns | 0.864 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, P.; Feng, J. Niche and Range Shifts of Aedes aegypti and Ae. albopictus Suggest That the Latecomer Shows a Greater Invasiveness. Insects 2023, 14, 810. https://doi.org/10.3390/insects14100810
Nie P, Feng J. Niche and Range Shifts of Aedes aegypti and Ae. albopictus Suggest That the Latecomer Shows a Greater Invasiveness. Insects. 2023; 14(10):810. https://doi.org/10.3390/insects14100810
Chicago/Turabian StyleNie, Peixiao, and Jianmeng Feng. 2023. "Niche and Range Shifts of Aedes aegypti and Ae. albopictus Suggest That the Latecomer Shows a Greater Invasiveness" Insects 14, no. 10: 810. https://doi.org/10.3390/insects14100810




