Characterization of Olive Fruit Damage Induced by Invasive Halyomorpha halys
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Field Surveys in an Olive Orchard Naturally Attacked by Stink Bugs
2.3. Field Assessment of H. halys Feeding Damage on Olive Fruits
2.4. Chemical and Molecular Investigation of H. halys Feeding Damage
2.5. Statistical Analysis
3. Results
3.1. Field Surveys in Olive Orchards Naturally Attacked by Stink Bugs
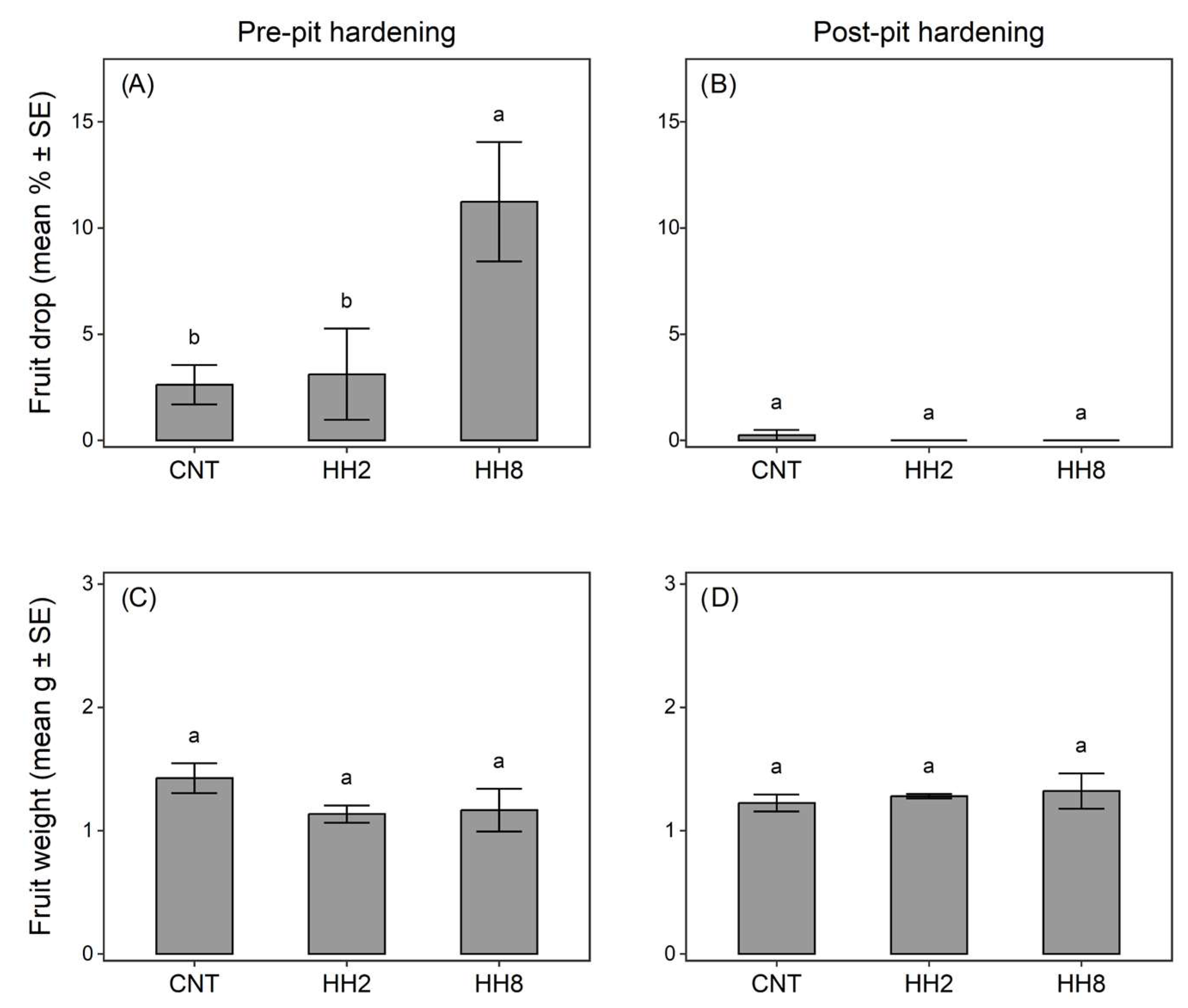
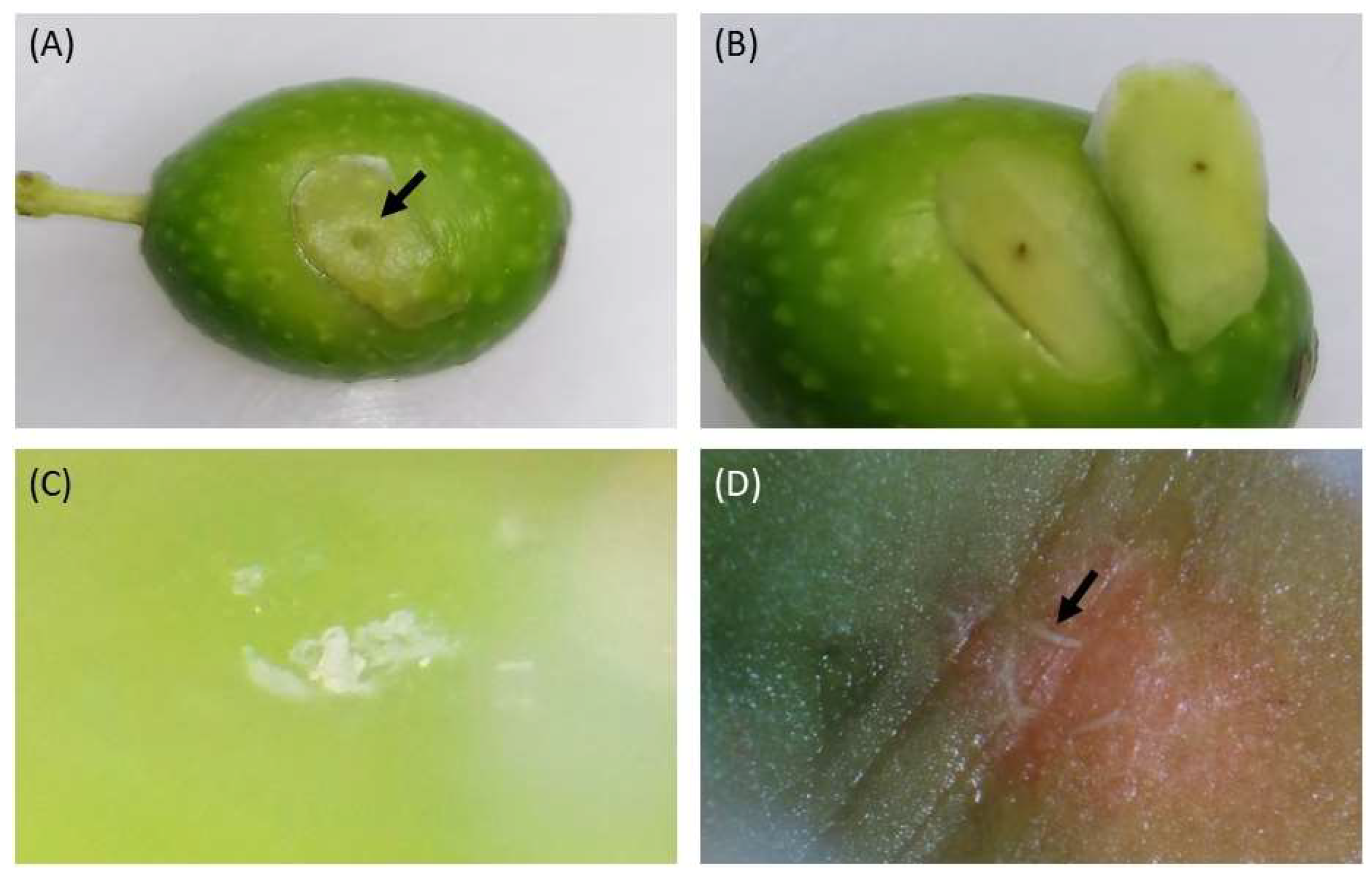
3.2. Field Assessment of H. halys Feeding Damage on Olive Fruits
3.3. Chemical and Molecular Investigation of H. halys Feeding Damage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. Effect of climate change on introduced and native agricultural invasive insect pests in Europe. Insects 2021, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Benvegnu, I.; Colombari, F.; Haack, R.A. Invasion by the chestnut gall wasp in Italy causes significant yield loss in Castanea sativa nut production. Agric. For. Entomol. 2014, 16, 75–79. [Google Scholar] [CrossRef]
- Reale, L.; Tedeschini, E.; Rondoni, G.; Ricci, C.; Bin, F.; Frenguelli, G.; Ferranti, F. Histological investigation on gall development induced by a worldwide invasive pest, Dryocosmus kuriphilus, on Castanea sativa. Plant Biosyst. 2016, 150, 35–42. [Google Scholar] [CrossRef]
- Snyder, W.E.; Evans, E.W. Ecological Effects of Invasive Arthropod Generalist Predators. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 95–122. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Economic impact of exotic insect pests in Brazilian agriculture. J. Appl. Entomol. 2013, 137, 1–15. [Google Scholar] [CrossRef]
- Rondoni, G.; Onofri, A.; Ricci, C. Differential susceptibility in a specialised aphidophagous ladybird, Platynaspis luteorubra (Coleoptera: Coccinellidae), facing intraguild predation by exotic and native generalist predators. Biocontrol Sci. Technol. 2012, 22, 1334–1350. [Google Scholar] [CrossRef]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitteilungen-Schweiz. Entomol. Ges. 2008, 81, 1–8. [Google Scholar]
- Bariselli, M.; Bugiani, R.; Maistrello, L. Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 2016, 46, 332–334. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2020/465 of 30 March 2020. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0465&from=EN (accessed on 30 September 2023).
- Kriticos, D.J.; Kean, J.M.; Phillips, C.B.; Senay, S.D.; Acosta, H.; Haye, T. The Potential Global Distribution of the Brown Marmorated Stink Bug, Halyomorpha halys, a Critical Threat to Plant Biosecurity. J. Pest Sci. 2017, 90, 1033–1043. [Google Scholar] [CrossRef]
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.P.; Streito, J.C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Damos, P.; Soulopoulou, P.; Thomidis, T. First record and current status of the brown marmorated sting bug Halyomorpha halys damaging peaches and olives in northern Greece. J. Plant Prot. Res. 2020, 60, 323–326. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Gogolashvili, N.E.; Fifis, G.T.; Navrozidis, E.I.; Thomidis, T. First report of native parasitoids of Halyomorpha halys (Hemiptera: Pentatomidae) in Greece. Insects 2021, 12, 984. [Google Scholar] [CrossRef]
- Zapponi, L.; Morten, M.; Chiesa, S.G.; Angeli, G.; Borri, G.; Mazzoni, V.; Sofia, M.; Anfora, G. Brown marmorated stink bug (Halyomorpha halys) feeding damage determines early drop in olive crops. J. Appl. Entomol. 2022, 146, 791–795. [Google Scholar] [CrossRef]
- Daher, E.; Cinosi, N.; Chierici, E.; Rondoni, G.; Famiani, F.; Conti, E. Field and Laboratory Efficacy of Low-Impact Commercial Products in Preventing Olive Fruit Fly, Bactrocera oleae, Infestation. Insects 2022, 13, 213. [Google Scholar] [CrossRef]
- Daher, E.; Rondoni, G.; Cinosi, N.; Conti, E.; Famiani, F. Particle Films Combined with Propolis Have Positive Effects in Reducing Bactrocera oleae Attacks on Olive Fruits. Horticulturae 2023, 9, 397. [Google Scholar] [CrossRef]
- Leskey, T.C.; Lee, D.H.; Short, B.D.; Wright, S.E. Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. J. Econ. Entomol. 2012, 105, 1726–1735. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Kamminga, K. Review of the chemical control research on Halyomorpha halys in the USA. J. Pest Sci. 2017, 90, 1021–1031. [Google Scholar] [CrossRef]
- Cianferoni, F.; Graziani, F.; Dioli, P.; Ceccolini, F. Review of the occurrence of Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) in Italy, with an update of its European and World distribution. Biologia 2018, 73, 599–607. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Marchetti, E.; Nasi, S.; Ferrari, R.; Conti, E. Improved Captures of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys, Using a Novel Multimodal Trap. Insects 2022, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, M.; Felton, G.W. Insights into the saliva of the brown marmorated stink bug Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2014, 9, e88483. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.W. The Saliva of Hemiptera. Adv. Insect Phys. 1972, 9, 183–255. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J. Econ. Entomol. 2009, 102, 1133–1140. [Google Scholar] [CrossRef]
- Chen, J.H.; Avila, G.A.; Zhang, F.; Guo, L.F.; Sandanayaka, M.; Mi, Q.Q.; Shi, S.S.; Zhang, J.P. Field cage assessment of feeding damage by Halyomorpha halys on kiwifruit orchards in China. J. Pest Sci. 2020, 93, 953–963. [Google Scholar] [CrossRef]
- Schumm, Z.R.; Alston, D.G.; Spears, L.R.; Manlove, K. Impact of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Feeding on Tart Cherry (Rosales: Rosaceae) Quality and Yield in Utah. J. Econ. Entomol. 2020, 113, 2328–2334. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Injury to apples and peaches at harvest from feeding by Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) nymphs early and late in the season. Crop Prot. 2016, 89, 58–65. [Google Scholar] [CrossRef]
- Gömez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of fly attack (Bactrocera oleae) on the phenolic profile and selected chemical parameters of olive oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Mertoǵlu, G.; Kumrala, N.A. Economic evaluation of different insecticide applications for control of the olive moth, Prays oleae (Bern.), in “Gemlik” olive trees. Acta Hortic. 2018, 1199, 177–182. [Google Scholar] [CrossRef]
- Giovannini, L.; Sabbatini-Peverieri, G.; Marianelli, L.; Rondoni, G.; Conti, E.; Roversi, P.F. Physiological host range of Trissolcus mitsukurii, a candidate biological control agent of Halyomorpha halys in Europe. J. Pest Sci. 2022, 95, 605–618. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Giovannini, L.; Sabbatini-Peverieri, G.; Roversi, P.F.; Conti, E. Olfactory responses of Trissolcus mitsukurii to plants attacked by target and non-target stink bugs suggest low risk for biological control. Sci. Rep. 2022, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, M.; Caruso, G.; Gennai, C.; Urbani, S.; Frioni, E.; Ruzzi, M.; Servili, M.; Gucci, R.; Poerio, E.; Muleo, R. The role of polyphenoloxidase, peroxidase, and β-glucosidase in phenolics accumulation in Olea europaea L. Fruits under different water regimes. Front. Plant Sci. 2017, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Rondoni, G.; Fenjan, S.; Bertoldi, V.; Ielo, F.; Djelouah, K.; Moretti, C.; Buonaurio, R.; Ricci, C.; Conti, E. Molecular detection of field predation among larvae of two ladybird beetles is partially predicted from laboratory experiments. Sci. Rep. 2018, 8, 2594. [Google Scholar] [CrossRef]
- Valentin, R.E.; Maslo, B.; Lockwood, J.L.; Pote, J.; Fonseca, D.M. Real-time PCR assay to detect brown marmorated stink bug, Halyomorpha halys (Stål), in environmental DNA. Pest Manag. Sci. 2016, 72, 1854–1861. [Google Scholar] [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.8.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 27 June 2023).
- Rapoport, H.F.; Pérez-López, D.; Hammami, S.B.M.; Agüera, J.; Moriana, A. Fruit pit hardening: Physical measurement during olive fruit growth. Ann. Appl. Biol. 2013, 163, 200–208. [Google Scholar] [CrossRef]
- Futch, S.H.; Childers, C.C.; Mccoy, C.W. Identification of Insect Pests: HS-893/HS142, 11/2002. EDIS 2002, 9, 1–4. [Google Scholar]
- Spooner-Hart, R.; Tesoriero, L.; Hall, B.H. Field Guide to Olive Pests, Diseases and Disorders in Australia; Rural Industries Research and Development Corporation: Brisbane, QLD, Australia, 2007. [Google Scholar]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S. Performance and oil quality of “Arbequina” and four Italian olive cultivars under super high-density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- Wiman, N.G.; Parker, J.E.; Rodriguez-Saona, C.; Walton, V.M. Characterizing Damage of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) in Blueberries. J. Econ. Entomol. 2015, 108, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Rather, B.A.; Wani, A.R.; Ganie, M.A. Resistance against insect pests by plant phenolics and their derivative dompounds. Chem. Sci. Rev. Lett. 2017, 6, 1073–1081. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry 2015, 661, 23–67. [Google Scholar]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Djelouah, K.; Moretti, C.; Buonaurio, R.; Conti, E. Vicia faba plants respond to oviposition by invasive Halyomorpha halys activating direct defences against offspring. J. Pest Sci. 2018, 91, 671–679. [Google Scholar] [CrossRef]
- Serteyn, L.; Quaghebeur, C.; Ongena, M.; Cabrera, N.; Barrera, A.; Molina-Montenegro, M.A.; Francis, F.; Ramírez, C.C. Induced systemic resistance by a plant growth-promoting rhizobacterium impacts development and feeding behavior of aphids. Insects 2020, 11, 234. [Google Scholar] [CrossRef]
- Peterson, H.M.; Ray, S.; Ali, J.G.; Krawczyk, G. Feeding and oviposition by the brown marmorated stink bug, Halyomorpha halys (Stål) induce direct and systemic changes in volatile compound emissions from potted peach and tree of heaven. Arthropod-Plant Interact. 2022, 6, 227–247. [Google Scholar] [CrossRef]
- Chierici, E.; Sabbatini-Peverieri, G.; Roversi, P.F.; Rondoni, G.; Conti, E. Phenotypic plasticity in an egg parasitoid affects olfactory response to odors from the plant–host complex. Front. Ecol. Evol. 2023, 11, 1233655. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Ivancic, T.; Grohar, M.C.; Jakopic, J.; Veberic, R.; Hudina, M. Effect of Brown Marmorated Stink Bug (Halyomorpha Halys Stål.) Infestation on the Phenolic Response and Quality of Olive Fruits (Olea europaea L.). Agronomy 2022, 12, 2200. [Google Scholar] [CrossRef]
- Amiot, M.J.; Fleuriet, A.; Macheix, J.J. Importance and Evolution of Phenolic Compounds in Olive during Growth and Maturation. J. Agric. Food Chem. 1986, 34, 823–826. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. Phenolic compounds in olive oil and olives. Curr. Top. Nutraceutical Res. 2005, 3, 125–136. [Google Scholar]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Effect of ripening on the phenolic composition and mineral content of three varieties of olive fruits. Foods 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Seçmeler, Ö.; Galanakis, C.M. Chapter 8—Olive Fruit and Olive Oil. In Innovations in Traditional Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 193–220. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.F.; Morozzi, G. Health and Sensory Properties of Virgin Olive Oil Hydrophilic Phenols: Agronomic and Technological Aspects of Production That Affect Their Occurrence in the Oil. J. Chromatogr. 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espı, J.C.; Wichers, H.J. Review Oleuropein and Related Compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Whitehill, J.G.A.; Rigsby, C.; Cipollini, D.; Herms, D.A.; Bonello, P. Decreased emergence of emerald ash borer from ash treated with methyl jasmonate is associated with induction of general defense traits and the toxic phenolic compound Verbascoside. Oecologia 2014, 176, 1047–1059. [Google Scholar] [CrossRef]
- Muñoz, E.; Lamilla, C.; Marin, J.C.; Alarcon, J.; Cespedes, C.L. Antifeedant, insect growth regulatory and insecticidal effects of Calceolaria talcana (Calceolariaceae) on Drosophila melanogaster and Spodoptera frugiperda. Ind. Crops Prod. 2013, 42, 137–144. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
| CNT | HH2 | HH8 | |
|---|---|---|---|
| 3,4-DHPEA | 0.11 ± 0.019 b | 0.08 ± 0.010 b | 0.19 ± 0.020 a |
| p-HPEA | 0.06 ± 0.011 | 0.06 ± 0.017 | 0.05 ± 0.013 |
| Demethyloleuropein | n.d. | n.d. | n.d. |
| Verbascoside | 1.70 ± 0.099 b | 3.55 ± 0.555 a | 4.54 ± 0.615 a |
| 3,4-DHPEA-EDA | 5.32 ± 0.478 | 6.53 ± 0.802 | 4.42 ± 0.767 |
| Oleuropein | 8.09 ± 0.355 b | 7.2 ± 0.510 b | 12.95 ± 2.188 a |
| P-HPEA-EDA | n.d. | n.d. | n.d. |
| Ligustroside | 1.86 ± 0.177 | 2.35 ± 0.147 | 1.93 ± 0.145 |
| Rutin | 1.02 ± 0.045 | 1.38 ± 0.161 | 1.25 ± 0.055 |
| (+)-1-Acetoxypinoresinol | n.d. | n.d. | n.d. |
| (+)-Pinoresinol | n.d. | n.d. | n.d. |
| Sum of the phenolic fractions | 18.16 ± 0.123 c | 21.14 ± 0.631 b | 25.34 ± 1.55 a |
| CNT | HH2 | HH8 | |
|---|---|---|---|
| 3,4-DHPEA | 0.13 ± 0.010 | 0.11 ± 0.005 | 0.14 ± 0.006 |
| p-HPEA | 0.07 ± 0.010 | 0.05 ± 0.016 | 0.04 ± 0.003 |
| Demethyloleuropein | n.d. | n.d. | n.d. |
| Verbascoside | 0.95 ± 0.036 b | 2.37 ± 0.166 a | 2.17 ± 0.198 a |
| 3,4-DHPEA-EDA | 4.67 ± 0.789 | 5.64 ± 0.366 | 5.15 ± 0.383 |
| Oleuropein | 5.99 ± 0.509 b | 6.44 ± 0.378 b | 10.10 ± 1.275 a |
| P-HPEA-EDA | n.d. | n.d. | n.d. |
| Ligustroside | 1.43 ± 0.197 | 1.91 ± 0.170 | 1.63 ± 0.087 |
| Rutin | 0.98 ± 0.115 | 1.15 ± 0.126 | 0.99 ± 0.052 |
| (+)-1-Acetoxypinoresinol | n.d. | n.d. | n.d. |
| (+)-Pinoresinol | n.d. | n.d. | n.d. |
| Sum of the phenolic fractions | 14.21 ± 0.796 b | 17.68 ± 0.713 a | 20.22 ± 1.090 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daher, E.; Chierici, E.; Urbani, S.; Cinosi, N.; Rondoni, G.; Servili, M.; Famiani, F.; Conti, E. Characterization of Olive Fruit Damage Induced by Invasive Halyomorpha halys. Insects 2023, 14, 848. https://doi.org/10.3390/insects14110848
Daher E, Chierici E, Urbani S, Cinosi N, Rondoni G, Servili M, Famiani F, Conti E. Characterization of Olive Fruit Damage Induced by Invasive Halyomorpha halys. Insects. 2023; 14(11):848. https://doi.org/10.3390/insects14110848
Chicago/Turabian StyleDaher, Elissa, Elena Chierici, Stefania Urbani, Nicola Cinosi, Gabriele Rondoni, Maurizio Servili, Franco Famiani, and Eric Conti. 2023. "Characterization of Olive Fruit Damage Induced by Invasive Halyomorpha halys" Insects 14, no. 11: 848. https://doi.org/10.3390/insects14110848






