Pilot Testing of an Area-Wide Biological Control Strategy against the Coffee Berry Borer in Colombia Using African Parasitoids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
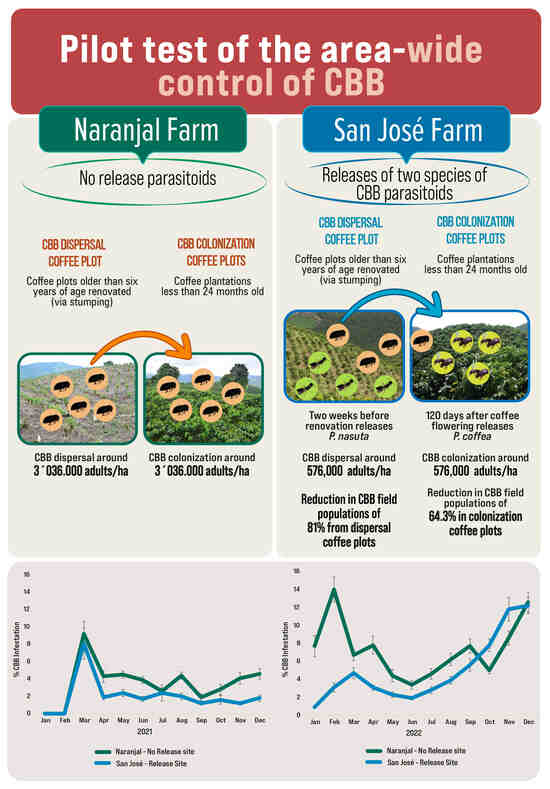
2.1. Study Area
2.1.1. Overview of Sites and Management
2.1.2. Designation of Release, Control No Release Locations, and Source of Parasitoids
2.1.3. Definitions Dispersal and Colonization Coffee Plots in Study Areas
2.1.4. Description of Parasitoid Releases
2.2. Estimation of Pest Density and Parasitism in the Dispersal Coffee Plots
2.3. Impacts of P. nasuta Releases in the Dispersal Coffee Plots
2.4. Estimation of Pest Density and Parasitism in the Colonization Coffee Plots
2.5. Estimation of Parasitism Rates for P. coffea and P. nasuta in the Colonization Coffee Plots
2.6. Impact of P. coffea and P. nasuta in the Colonization Coffee Plots
2.7. Statistical Analysis
3. Results
3.1. Size of Dispersal and Colonization Coffee Plots in Study Areas
3.2. Pest Density and Parasitism in the Dispersal Coffee Plots
3.3. Impact of P. nasuta Releases in the Dispersal Coffee Plots
3.4. Pest Density and Parasitism in the Colonization Coffee Plots
3.5. Parasitism Rates for P. coffea and P. nasuta in the Colonization Coffee Plots
3.6. Impact of P. coffea and P. nasuta in the Colonization Coffee Plots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Federación Nacional de Cafeteros de Colombia. Informe de Gestión 2021; Federación Nacional de Cafeteros de Colombia: Bogotá, Colombia, 2021. [Google Scholar]
- Federación Nacional de Cafeteros de Colombia. Informe de Gestión 2022; Federación Nacional de Cafeteros de Colombia: Bogotá, Colombia, 2022. [Google Scholar]
- Infante, F.; Jaramillo, J.; Castillo, A.; Vega, F. The coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthropod Rev. 2009, 2, 129–147. [Google Scholar] [CrossRef]
- Constantino, L.M.; Navarro, L.; Berrio, A.; Acevedo, F.E.; Rubio, D.; Benavides, P. Aspectos biológicos, morfológicos y genéticos de Hypothenemus obscurus e Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae). Rev. Colomb. Entomol. 2011, 37, 173–182. [Google Scholar] [CrossRef]
- Berthet, J.A. Caruncho do café Caruncho do café. Informação prestada pelo Sr. Dr. Director do Instituto Agronômico a respeito de amostras de café vindas do Congo Belga. Bol. Agric. São Paulo 1913, 14, 312–313. [Google Scholar]
- Bergamin, J. Contribuição para o conhecimento da biologia da broca do café “ Hypothenemus hampei (Ferrari, 1867)” (Col. Ipidae). Arq. Inst. Biológico 1943, 14, 31–72. [Google Scholar]
- Bustillo, A.E.; Cárdenas, R.; Villalba, D.A.; Benavides, P.; Orozco, J.; Posada, F.J. Manejo Integrado de la Broca del café: Hypothenemus hampei (Ferrari) en Colombia; Cenicafé: Manizales, Colombia, 1998; ISBN 978-958-96554-0-5. [Google Scholar]
- Bustillo Pardey, A.E. Aspectos sobre la broca del café, Hypothenemus hampei, en Colombia. In Los Insectos y su Manejo en la Caficultura Colombiana; Cenicafé: Chinchiná, Colombia, 2008; pp. 388–418. ISBN 978-958-98193-9-5. [Google Scholar]
- Benavides, P.; Góngora, C.E.; Bustillo, A.E. IPM Program to control coffee berry borer Hypothenemus hampei, with emphasis on highly pathogenic mixed strains of Beauveria bassiana, to overcome insecticide resistance in Colombia. In Insecticides-Advances in Integrated Pest Management; InTech: Rijeka, Croatia, 2012; pp. 511–540. ISBN 978-953-307-780-2. [Google Scholar]
- Bustillo, A.E. El Manejo de Cafetales y su Relación Con el Control de la Broca del Café en Colombia; Cenicafé: Manizales, Colombia, 2007; pp. 16–21. [Google Scholar]
- Duque, H.; Baker, P.S. Devouring Profit the Socio-Economics of Coffee Berry Borer IPM; Cenicafé: Cali, Colombia, 2003; pp. 62–63. ISBN 978-958-97218-4-1. [Google Scholar]
- Castaño, A.; Benavides, P.; Baker, P.S. Dispersión de Hypothenemus hampei en cafetales zoqueados. Rev. Cenicafé 2005, 56, 142–150. [Google Scholar]
- Alonzo, P. El Problema de la Broca (Hypothenemus hampei, Ferr.) (Coleoptera: Scolytidae) y la Caficultura: Aspectos Relacionados Con Importancia, Daño, Identificación, Biología, Ecología y Control; IICA: San José, Costa Rica, 1984. [Google Scholar]
- Benavides, P.; Bustillo, A.E.; Montoya, E.C.; Cárdenas, R.; Mejía, C.G. Participación del control cultural, químico y biológico en el manejo de la broca del café. Rev. Colomb. Entomol. 2002, 28, 161–165. [Google Scholar] [CrossRef]
- Benavides, P.; Gil, Z.N.; Constantino, L.M.; Villegas, C.; Jaramillo, M. Plagas del café: Broca, minador, cochinillas harinosas, arañita roja y monalonion. In Manual del Cafetero Colombiano: Investigación y Tecnología para la Sostenibilidad de la Caficultura; Cenicafé: Manizales, Colombia, 2013; Volume 2, pp. 215–260. [Google Scholar]
- Benavides, P. El uso de enemigos naturales en estrategias de manejo integrado de plagas a gran escala. In El Control Natural de Insectos en el Ecosistema Cafetero Colombiano; Benavides, P., Góngora, C.E., Eds.; Cenicafé: Chinchiná, Colombia, 2020; pp. 204–220. ISBN 978-958-8490-43-4. [Google Scholar]
- Vega, F.E.; Infante, F.; Johnson, A.J. The Genus Hypothenemus, with emphasis on H. hampei, the coffee berry borer. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 427–494. ISBN 978-0-12-417156-5. [Google Scholar]
- Góngora, C.E.; Marín, P.; Benavides, P. Claves para el Éxito del Hongo Beauveria bassiana Como Controlador Biológico de la Broca del Café; Technical Report; Federación Nacional de Cafeteros de Colombia: Bogotá, Colombia, 2009; Volume 384. [Google Scholar]
- Jaramillo, J.L.; Montoya, E.C.; Benavides, P.; Góngora, C.E. Beauveria bassiana y Metarhizium anisopliae para el control de broca del café en frutos del suelo. Rev. Colomb. Entomol. 2015, 41, 95–104. [Google Scholar]
- Laiton, L.A.; Constantino, L.M.; Benavides, P. Capacidad depredadora de Cathartus quadricollis y Ahasverus advena (Coleoptera: Silvanidae) sobre Hypothenemus hampei (Coleoptera: Curculionidae) en laboratorio. Rev. Colomb. Entomol. 2018, 44, 200–205. [Google Scholar] [CrossRef]
- Bustillo, A.E. Una revisión sobre la broca del café, Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae), en Colombia. Rev. Colomb. Entomol. 2006, 32, 101–116. [Google Scholar] [CrossRef]
- Bacca, R.T. Efecto del Parasitoide Prorops nasuta Waterston (Hymenoptera: Bethylidae) Sobre Poblaciones de Broca del Café Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae); Universidad Nacional de Colombia: Bogotá, Colombia, 1999; 186p. [Google Scholar]
- Maldonado, C.E.; Benavides, P. Evaluación del establecimiento de Cephalonomia stephanoderis y Prorops nasuta, controladores de Hypothenemus hampei, en Colombia. Rev. Cenicafé 2007, 58, 333–339. [Google Scholar]
- Jaramillo, J.; Bustillo, A.E.; Montoya, E.C. Parasitismo de Phymastichus coffea sobre poblaciones de Hypothenemus hampei en frutos de café de diferentes edades. Rev. Cenicafé 2002, 53, 317–326. [Google Scholar]
- Bustillo, A.E.; Orozco, J.; Benavides, P.; Portilla, M. Producción masiva y uso de parasitoides para el control de la broca del café en Colombia. Rev. Cenicafé 1996, 47, 215–230. [Google Scholar]
- Portilla, M.; Bustillo, A. Nuevas investigaciones en la cría masiva de Hypothenemus hampei y de sus parasitoides Cephalonomia stephanoderis y Prorops nasuta. Rev. Colomb. Entomol. 1995, 21, 25–33. [Google Scholar] [CrossRef]
- Orozco, J. Guía para la Producción del Parasitoide Phymastichus coffea para el Control de la Broca del Café, 1st ed.; Cenicafé: Manizales, Colombia, 2002. [Google Scholar]
- Gonzáles, A. Biocafé: Avispitas para el Control Biológico de la Broca del Café 2023. Available online: https://avispitas.blogspot.com/p/biocafe.html (accessed on 23 August 2023).
- Hanski, I. Metapopulation Ecology, 1st ed.; Oxford Series in Ecology and Evolution; Oxford University Press: Oxford, NY, USA, 1999; ISBN 978-0-19-854065-6. [Google Scholar]
- Vergara, J.D.; Orozco, J.; Bustillo, A.E.; Chaves, B. Biología de Phymastichus coffea en condiciones de campo. Rev. Cenicafé 2001, 52, 97–103. [Google Scholar]
- Borbón, M. Liberaciones, comportamiento y dispersión de los parasitoides de la broca del fruto del cafeto, Hypothenemus hampei (Ferrari, 1867), Prorops nasuta y Phymastichus coffea, procedentes de Colombia, En Costa Rica. In Manejo da Broca-do-Cafe: Workshop International, 28 de Novembro a 2 de Dezembro de 2004, Londrina-Paraná-Brasil; IAPAR: Pune, India, 2007; p. 282. [Google Scholar]
- James Barr, J. Statistical Analysis System. Available online: https://www.sas.com/es_co/home.html (accessed on 23 August 2023).
- Moreno, D.; Bustillo, A.E.; Benavides, P.; Montoya, E.C. Escape y mortalidad de Hypothenemus hampei en los procesos de recolección y beneficio del café en Colombia. Rev. Cenicafé 2001, 52, 111–116. [Google Scholar]
- Martínez, A.; Gonzáles, R.; Hernández, S. Kairomona responsable de la atracción de la broca del café Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) al grano de café. In Memorias. 4. Congreso Nacional MIP; 3. Congreso Internacional “Humberto Tapia Barquero”; Universidad Nacional Agraria: Managua, Nicaragua, 1990; pp. 28–36. [Google Scholar]
- Gutiérrez, A.; Ondarza, R. Kairomone Effect of extracts from Coffea canephora over Hypothenemus hampei (Coleoptera: Scolytidae). Environ. Entomol. 1996, 25, 96–100. [Google Scholar] [CrossRef]
- Infante, F.; Mumford, J.; Baker, P. Life history studies of Prorops nasuta, a parasitoid of the coffee berry borer. Biol. Control 2005, 50, 259–270. [Google Scholar] [CrossRef]
- Liang, P.; Ladizinsky, N.C.; Asmus, G.; Hamilton, L.J.; Acebes, D.A.L.; Manoukis, N.C.; Follett, P.A. Artificial fruits and nuts for studying predation of cryptic prey: A case for 3d-printed coffee berries for studying predation of coffee berry borer by flat bark beetles. Entomol. Exp. Applic. 2023, 171, 716–720. [Google Scholar] [CrossRef]
- Follett, P.A.; Czokajlo, D.; Collignon, M.R.; Cha, D. A predator breeding station for augmentative biological control of Scolytine crop pests. Biol. Control 2023, 186, 105318. [Google Scholar] [CrossRef]
- Mejía, J.W.; Bustillo, A.E.; Orozco, J.; Cháves, B. Efecto de cuatro insecticidas y de Beauveria bassiana sobre Prorops nasuta (Hymenoptera: Bethylidae), parasitoide de la broca del café. Rev. Colomb. Entomol. 2000, 26, 117–123. [Google Scholar] [CrossRef]
- Centro Nacional de Investigaciones de Café. Informe Anual Cenicafé 2022; Cenicafé: Manizales, Colombia, 2022; p. 50. [Google Scholar]
- Giraldo, M.; Montoya, E.C.; Sarmiento, N.; Quiroga, A.; Espinosa, J.C.; García, J.C.; Duque, H.; Benavides, P. Vulnerabilidad de la caficultura de Caldas a la broca del café en diferentes eventos climáticos. AVT 2019, 505, 1–8. [Google Scholar] [CrossRef]








| Farm | Num. Trees | Berries/Branch | CBB-Infested Berries/Branch |
|---|---|---|---|
| Mean (±SE) | Mean (±SE) | ||
| Naranjal-No Release Site | 300 | 28.7 ± (1.3) A | 8.5 ± (0.5) A |
| San Jose-Release Site | 200 | 29.3 ± (1.4) A | 6.4 ± (0.4) A |
| Year | Farm | Number (Mean ± SE) of CBB Life Stages Per m2 |
|---|---|---|
| Mean & | ||
| 2021 | Naranjal—No Release Site | 303.6 ± (45.1) A |
| San Jose—Release Site | 57.6 ± (13.3) B | |
| 2022 | Naranjal—No Release Site | 8.7 ± (2.7) A |
| San Jose—Release Site | 10.2 ± (2.0) A |
| Year | Month | Coffee Seeds with CBB Parasitized | Released Wasps |
|---|---|---|---|
| 2021 | February | 35,400 | 123,600 |
| March | 37,200 | 130,000 | |
| April | 16,000 | 55,900 | |
| May | 12,000 | 41,900 | |
| June | 12,000 | 41,900 | |
| July | 4000 | 13,900 | |
| 2022 | March | 45,500 | 127,400 |
| April | 34,500 | 96,600 | |
| May | 18,500 | 48,100 | |
| June | 37,000 | 116,180 | |
| July | 6500 | 20,410 |
| Year | Farm | Number of CBB-Infested Berries Per Branch |
|---|---|---|
| Mean ± (SE) | ||
| 2021 | Naranjal-No Release Site | 78.6 ± (11.2) A |
| San Jose-Release Site | 17.7 ± (3.2) B | |
| 2022 | Naranjal-No Release Site | 90.4 ± (11.2) A |
| San Jose-Release Site | 44.2 ± (6.9) B |
| Farm | ||||||
|---|---|---|---|---|---|---|
| Year | Naranjal-No Release Site | San Jose-Release Site | ||||
| Month | Mean CBB Life Stages/Branch (±SE) | Month | Mean CBB Life Stages/Branch (±SE) | |||
| 2021 | January | 0.0 ± (0.0) A | January | 0.0 ± (0.0) A | ||
| February | 0.0 ± (0.0) A | February | 0.0 ± (0.0) A | |||
| March | 11.7 ± (2.4) A | March | 0.9 ± (0.2) B | |||
| April | 7.9 ± (1.9) A | April | 1.2 ± (0.3) B | |||
| May | 11.7 ± (1.5) A | May | 4.8 ± (1.1) B | |||
| June | 11.9 ± (1.7) A | June | 3.7 ± (1.0) B | |||
| July | 13.4 ± (1.9) A | July | 6.4 ± (1.7) B | |||
| August | 29.0 ± (3.3) A | August | 7.8 ± (1.7) B | |||
| September | 6.7 ± (1.1) A | September | 4.1 ± (0.9) A | |||
| October | 5.9 ± (1.3) A | October | 2.3 ± (0.6) B | |||
| November | 5.9 ± (1.1) A | November | 1.3 ± (0.4) B | |||
| December | 3.4 ± (0.7) A | December | 1.9 ± (0.5) A | |||
| 2022 | January | 4.7 ± (0.8) A | January | 0.8 ± (0.2) B | ||
| February | 17.2 ± (1.9) A | February | 3.3 ± (0.6) B | |||
| March | 11.6 ± (1.2) A | March | 2.1 ± (0.4) B | |||
| April | 3.7 ± (0.7) A | April | 5.5 ± (1.0) A | |||
| May | 5.3 ± (1.0) A | May | 7.4 ± (1.3) A | |||
| June | 6.3 ± (1.1) A | June | 2.3 ± (0.5) B | |||
| July | 11.5 ± (1.8) A | July | 7.2 ± (1.2) B | |||
| August | 7.7 ± (1.1) A | August | 5.8 ± (0.9) A | |||
| September | 18.7 ± (2.2) A | September | 15.2 ± (2.0) A | |||
| October | 16.8 ± (2.3) A | October | 9.8 ± (1.3) B | |||
| November | 11.0 ± (1.6) A | November | 5.4 ± (0.9) B | |||
| December | 14.9 ± (1.9) A | December | 11.8 ± (2.0) B | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavides, P.; Gil, Z.N.; Escobar, L.E.; Navarro-Escalante, L.; Follett, P.; Diaz-Soltero, H. Pilot Testing of an Area-Wide Biological Control Strategy against the Coffee Berry Borer in Colombia Using African Parasitoids. Insects 2023, 14, 865. https://doi.org/10.3390/insects14110865
Benavides P, Gil ZN, Escobar LE, Navarro-Escalante L, Follett P, Diaz-Soltero H. Pilot Testing of an Area-Wide Biological Control Strategy against the Coffee Berry Borer in Colombia Using African Parasitoids. Insects. 2023; 14(11):865. https://doi.org/10.3390/insects14110865
Chicago/Turabian StyleBenavides, Pablo, Zulma Nancy Gil, Luis Eduardo Escobar, Lucio Navarro-Escalante, Peter Follett, and Hilda Diaz-Soltero. 2023. "Pilot Testing of an Area-Wide Biological Control Strategy against the Coffee Berry Borer in Colombia Using African Parasitoids" Insects 14, no. 11: 865. https://doi.org/10.3390/insects14110865