Physical Properties of Substrates as a Driver for Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Growth
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Soldier Fly Colony
2.2. Experiment 1: The Influence of Different Fibre Components and Nutrient Levels on BSFL Growth
Texture Measurements
2.3. Experiment 2: The Influence of Different Straw Particle Sizes on BSFL Growth
2.4. Determination of Growth and Biomass Parameters, and Chemical Analysis
2.5. Statistical Analyses
3. Results
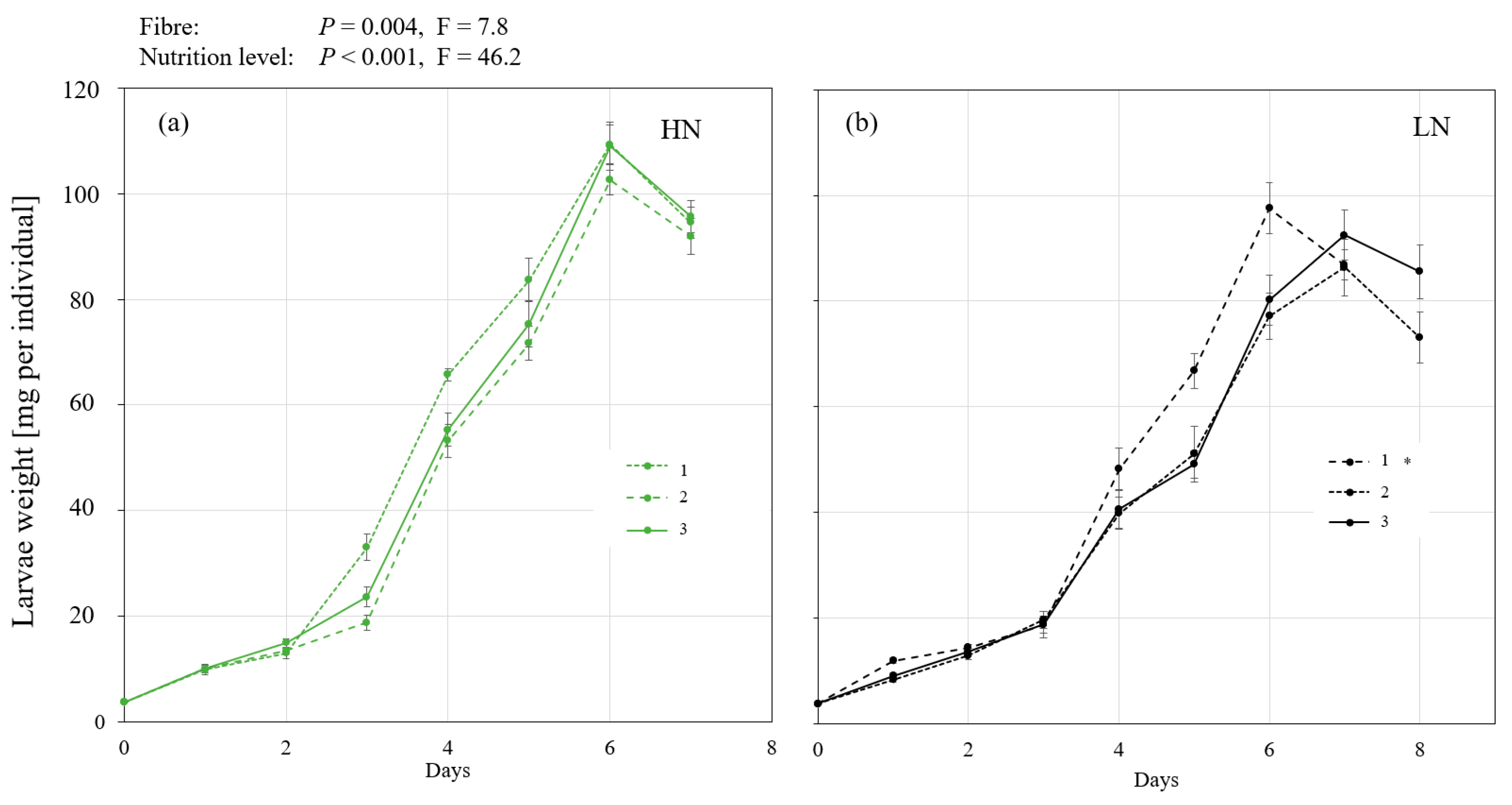
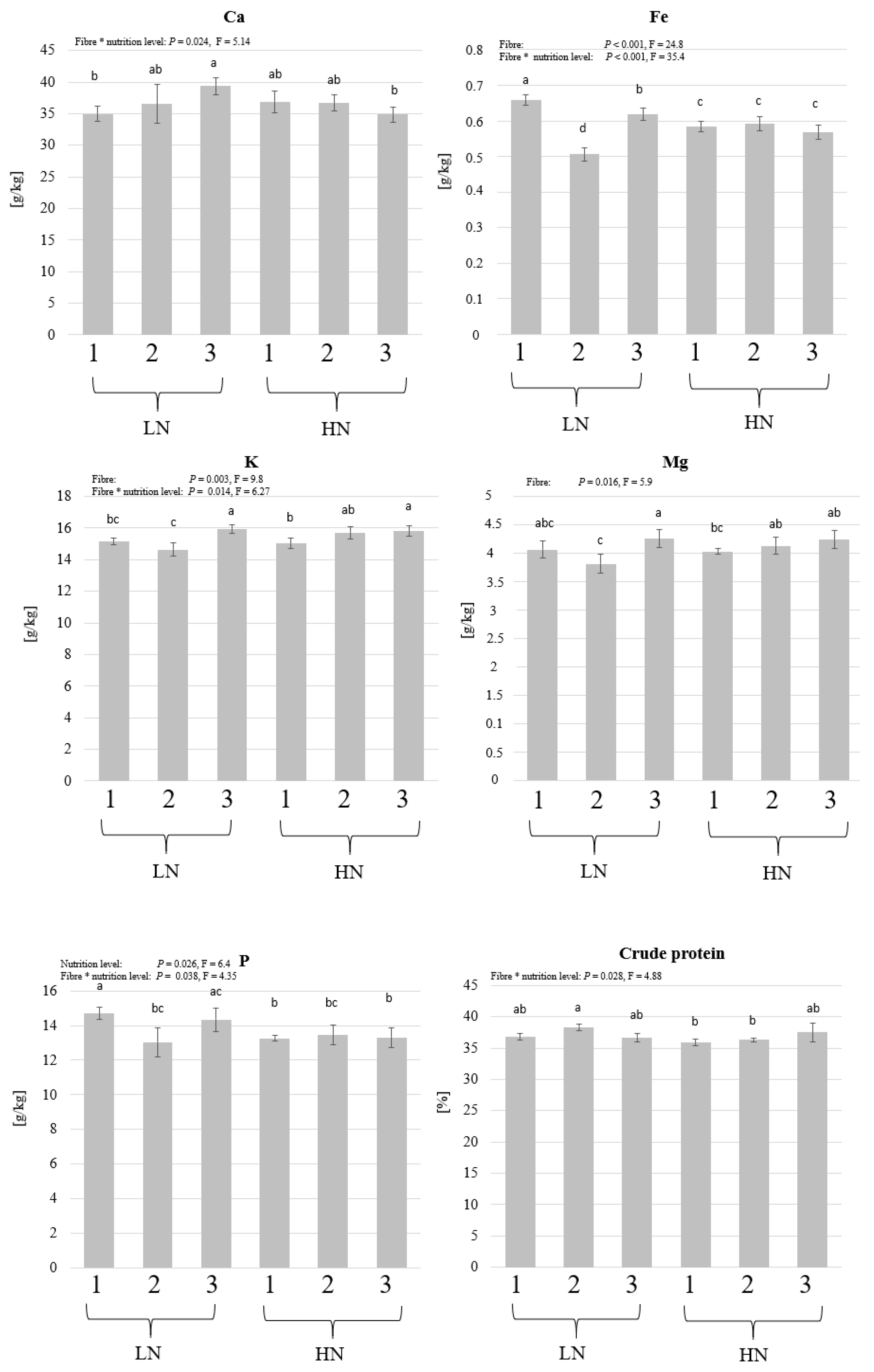
3.1. Experiment 1: The Influence of Different Fibre Components and Nutrient Levels on BSFL Growth
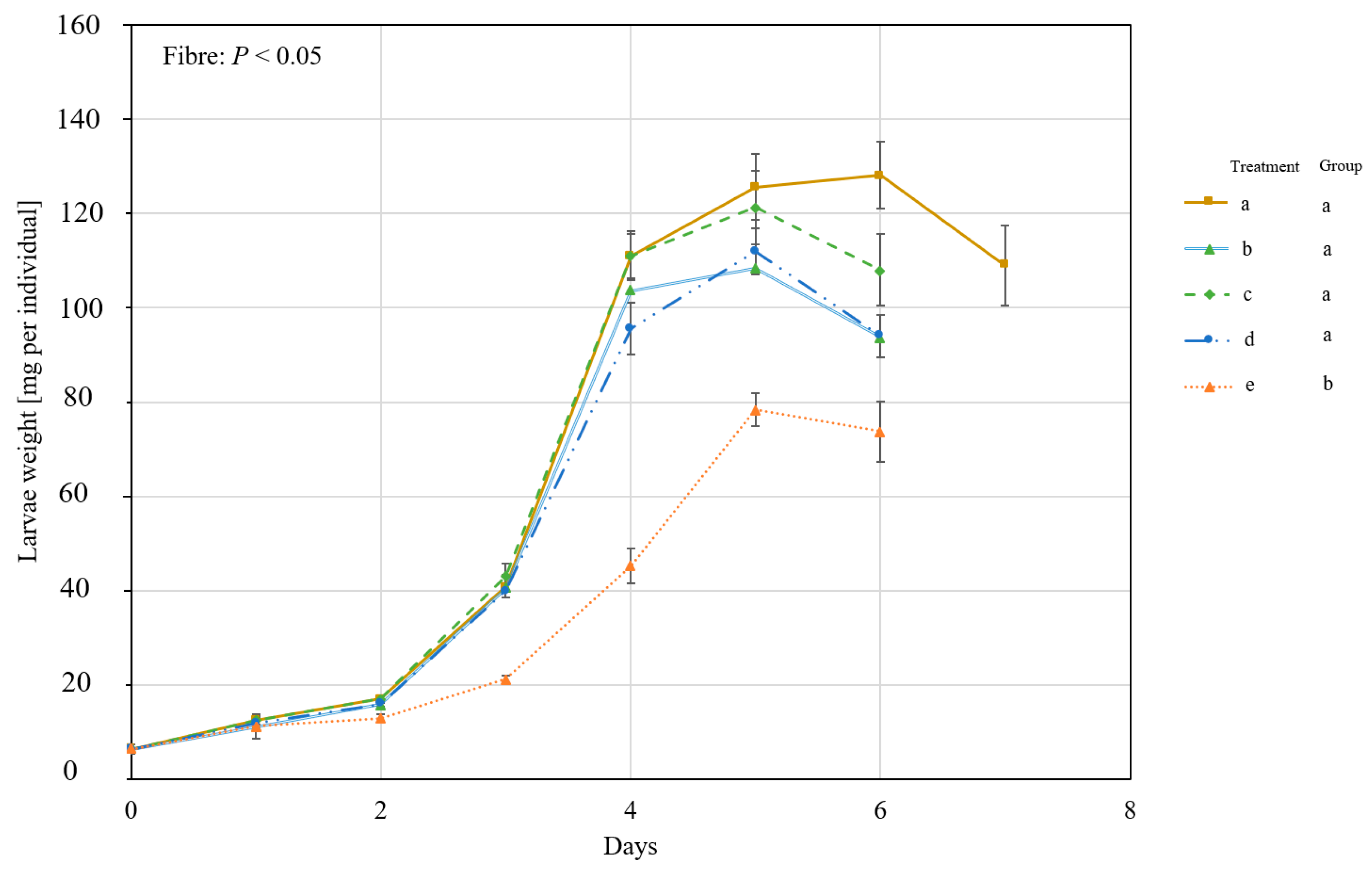
3.2. The Influence of Different Straw Particle Sizes
4. Discussion
5. Conclusions
6. Outlook and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 1 July 2022).
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting global feed protein demand: Challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci 2019, 7, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Oonincx, D.G. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Yu, J.; van Huis, A.; Yu, Z.; Fasulo, S. Efficient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L. (Diptera: Stratiomyidae) larvae and functional bacteria. J. Environ. Manag. 2018, 217, 668–676. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 110066. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from insects in cosmetics and for personal care products. Insects 2021, 13, 41. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Gärttling, D.; Schulz, H. Compilation of black soldier fly frass analyses. J. Soil Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.; Niassy, S.; van Loon, J.J.; Dicke, M. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Yakti, W.; Schulz, S.; Marten, V.; Mewis, I.; Padmanabha, M.; Hempel, A.-J.; Kobelski, A.; Streif, S.; Ulrichs, C. The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae. Sustainability 2022, 14, 1772. [Google Scholar] [CrossRef]
- Dzepe, D.; Nana, P.; Fotso, A.; Tchuinkam, T.; Djouaka, R. Influence of larval density, substrate moisture content and feedstock ratio on life history traits of black soldier fly larvae. J. Insects Food Feed. 2020, 6, 133–140. [Google Scholar] [CrossRef]
- Raksasat, R.; Lim, J.W.; Kiatkittipong, W.; Kiatkittipong, K.; Ho, Y.C.; Lam, M.K.; Font-Palma, C.; Zaid, H.F.M.; Cheng, C.K. A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources. Environ. Pollut. 2020, 267, 115488. [Google Scholar] [CrossRef]
- Palma, L.; Fernandez-Bayo, J.; Niemeier, D.; Pitesky, M.; VanderGheynst, J.S. Managing high fiber food waste for the cultivation of black soldier fly larvae. NPJ Sci. Food 2019, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossule, V.; Lavagnolo, M.C. Lab tests on semi-aerobic landfilling of MSW under varying conditions of water availability and putrescible waste content. J. Environ. Manag. 2020, 256, 109995. [Google Scholar] [CrossRef]
- Grossule, V.; Vanin, S.; Lavagnolo, M.C. Potential treatment of leachate by Hermetia illucens (Diptera, Stratyomyidae) larvae: Performance under different feeding conditions. Waste Manag. Res. 2020, 38, 537–545. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef]
- Palma, L.; Ceballos, S.J.; Johnson, P.C.; Niemeier, D.; Pitesky, M.; VanderGheynst, J.S. Cultivation of black soldier fly larvae on almond byproducts: Impacts of aeration and moisture on larvae growth and composition. J. Sci. Food Agric. 2018, 98, 5893–5900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed. 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Lalander, C.; Ermolaev, E.; Wiklicky, V.; Vinnerås, B. Process efficiency and ventilation requirement in black soldier fly larvae composting of substrates with high water content. Sci. Total Environ. 2020, 729, 138968. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Using Hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Peguero, D.A.; Gold, M.; Vandeweyer, D.; Zurbrügg, C.; Mathys, A. A review of pretreatment methods to improve agri-food waste bioconversion by black soldier fly larvae. Front. Sustain. Food Syst. 2021, 5, 549. [Google Scholar] [CrossRef]
- Yakti, W.; Förster, N.; Müller, M.; Mewis, I.; Ulrichs, C. Hemp Waste as a Substrate for Hermetia illucens (L.)(Diptera: Stratiomyidae) and Tenebrio molitor L. (Coleoptera: Tenebrionidae) Rearing. Insects 2023, 14, 183. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Chiu, S.L.; Lo, I.M. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakowski, Z.; Adamski, R.; Kokocińska, M.; Kwapisz, S. Generalized desorption equilibrium equation of lignite in a wide temperature and moisture content range. Fuel 2011, 90, 3330–3335. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Tang, J.; Villa-Rojas, R.; Sablani, S.; Carter, B.; Campbell, G. Influence of water activity on thermal resistance of microorganisms in low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 353–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Stewart, G.F.; Schweigert, B.S.; Hawthorn, J.; Bourne, M. Food Texture and Viscosity: Concept and Measurement; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Bruno, D.; Bonacci, T.; Reguzzoni, M.; Casartelli, M.; Grimaldi, A.; Tettamanti, G.; Brandmayr, P. An in-depth description of head morphology and mouthparts in larvae of the black soldier fly Hermetia illucens. Arthropod Struct. Dev. 2020, 58, 100969. [Google Scholar] [CrossRef]
- Gligorescu, A.; Fischer, C.H.; Larsen, P.F.; Nørgaard, J.V.; Heckman, L.-H.L. Production and optimization of Hermetia illucens (L.) larvae reared on food waste and utilized as feed ingredient. Sustainability 2020, 12, 9864. [Google Scholar] [CrossRef]
- Shumo, M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. Influence of temperature on selected life-history traits of black soldier fly (Hermetia illucens) reared on two common urban organic waste streams in Kenya. Animals 2019, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Villazana, J.; Alyokhin, A. Development of black soldier fly larvae (Diptera: Stratiomyidae) on seafood wastes. J. Insects Food Feed. 2019, 5, 313–319. [Google Scholar] [CrossRef]
- Gobbi, P.; Martinez-Sanchez, A.; Rojo, S. The effects of larval diet on adult life-history traits of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Eur. J. Entomol. 2013, 110, 461. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, L.; Sheppard, C.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005; Volume 17, pp. 1–17. [Google Scholar]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. The Contribution of Dietary Magnesium in Farm Animals and Human Nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef]
- Delezie, E.; Bierman, K.; Nollet, L.; Maertens, L. Impacts of calcium and phosphorus concentration, their ratio, and phytase supplementation level on growth performance, foot pad lesions, and hock burn of broiler chickens. J. Appl. Poult. Res. 2015, 24, 115–126. [Google Scholar] [CrossRef]
- Luquet, G. Biomineralizations: Insights and prospects from crustaceans. Zookeys 2012, 176, 103–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Treatments | Chicken Feed [%] | Water [%] | Powder Cellulose (%) | Concentrated Lignocellulose [%] | Blended Straw [%] | Bulk Densities of the Final Wet Substrate [g L−1] | |
|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | ||||||
| 1 | High nutrition (HN) | 20.33 | 73.17 | 6.50 | - | - | 635 |
| 2 | 20.33 | 73.17 | - | 6.50 | - | 720 | |
| 3 | 20.33 | 73.17 | - | - | 6.50 | 836 | |
| 1 | Low nutrition (LN) | 14.63 | 73.17 | 6.50 | - | 5.70 | 814 |
| 2 | 14.63 | 73.17 | - | 6.50 | 5.70 | 1032 | |
| 3 | 14.63 | 73.17 | - | - | 12.20 | 1072 | |
| Treatment | F15mm [N] | Slope [N/mm] | R2slope | |
|---|---|---|---|---|
| Low nutrition (LN) | 1 | 2.44 a ± 0.44 | 0.162 | 0.9911 |
| 2 | 1.24 a,b ± 0.39 | 0.084 | 0.9997 | |
| 3 | 0.29 b ± 0.02 | 0.019 | 0.9996 | |
| High nutrition (HN) | 1 | 6.63 c ± 0.82 | 0.457 | 0.9993 |
| 2 | 4.68 d ± 0.58 | 0.342 | 0.9952 | |
| 3 | 2.07 a ± 0.33 | 0.145 | 0.9975 | |
| Treatment | Particle Size [mm] | WatHC [mL g−1] | DryBDens [g L−1] | WetBDens [g L−1] |
|---|---|---|---|---|
| a | 3–15 | 6.46 | 83.2 | 762.8 |
| b | 2–3 | 6.38 | 110.5 | 1031 |
| c | 1.25–2 | 3.7 | 165.7 | 1179.9 |
| d | 1–1.25 | 3.68 | 312.1 | 1223.7 |
| e | ≤1 | 3.16 | 442.1 | 1494.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakti, W.; Müller, M.; Klost, M.; Mewis, I.; Dannehl, D.; Ulrichs, C. Physical Properties of Substrates as a Driver for Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Growth. Insects 2023, 14, 266. https://doi.org/10.3390/insects14030266
Yakti W, Müller M, Klost M, Mewis I, Dannehl D, Ulrichs C. Physical Properties of Substrates as a Driver for Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Growth. Insects. 2023; 14(3):266. https://doi.org/10.3390/insects14030266
Chicago/Turabian StyleYakti, Wael, Marcus Müller, Martina Klost, Inga Mewis, Dennis Dannehl, and Christian Ulrichs. 2023. "Physical Properties of Substrates as a Driver for Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae Growth" Insects 14, no. 3: 266. https://doi.org/10.3390/insects14030266






