Evaluation of a Push–Pull Strategy for Spotted-Wing Drosophila Management in Highbush Blueberry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Insects
2.3. Oviposition Deterrence Laboratory Assays
2.4. Field Tests
3. Statistical Analysis
3.1. General
3.2. Oviposition Deterrence Laboratory Assays
3.3. Field Tests
4. Results
4.1. Oviposition Deterrence Laboratory Tests
4.2. Field Tests
5. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying host potentials: Indexing postharvest fresh fruits for spotted wing Drosophila, Drosophila suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Adrion, J.R.; Kousathanas, A.; Pascual, M.; Burrack, H.J.; Haddad, N.M.; Bergland, A.O.; Machado, H.; Sackton, T.B.; Schlenke, T.A.; Watada, M.; et al. Drosophila suzukii: The genetic footprint of a recent, worldwide invasion. Mol. Biol. Evol. 2014, 31, 3148–3163. [Google Scholar]
- Asplen, M.; Anfora, G.; Biondi, A.; Choi, D.; Chu, D.; Daane, K.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchinson, W.D.; et al. Invasion biology of spotted wing drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest. Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Blaxter, M.; Anfora, G. Drosophila suzukii. Curr. Biol. 2013, 23, R8–R9. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing Drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; de Toni, D.C.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Ometto, L.; Tait, G.; Ghirotto, S.; Kaur, R.; Drago, F.; González, J.; Walton, V.M.; Anfora, G.; Rossi-Stacconi, M.V. Distinct genotypes and phenotypes in European and American strains of Drosophila suzukii: Implications for biology and management of an invasive organism. J. Pest Sci. 2020, 93, 77–89. [Google Scholar] [CrossRef]
- Boughdad, A.; Haddi, K.; El Bouazzati, A.; Nassiri, A.; Tahiri, A.; El Anbri, C.; Eddaya, T.; Zaid, A.; Biondi, A. First record of the invasive spotted wing Drosophila infesting berry crops in Africa. J. Pest Sci. 2020, 94, 261–271. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Okwaro, L.A.; Kleman, I.; Rehermann, G.; Revadi, S.; Ndlela, S.; Khamis, F.M.; Nderitu, P.W.; Kasina, M.; George, M.K.; et al. Detection of the spotted wing drosophila, Drosophila suzukii, in continental sub-Saharan Africa. J. Pest Sci. 2021, 94, 251–259. [Google Scholar] [CrossRef]
- Knapp, L.; Mazzi, D.; Finger, R. The economic impact of Drosophila suzukii: Perceived costs and revenue losses of Swiss cherry, plum and grape growers. Pest Manag. Sci. 2021, 77, 978–1000. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Rosensteel, D.O.; Hardin, J.A.; Sial, A.A.; Burrack, H.J. Season-long programs for control of Drosophila suzukii in southeastern U.S. blueberries. Crop Prot. 2016, 81, 76–84. [Google Scholar] [CrossRef]
- Steck, G.J.; Dixon, W.; Dean, D. Spotted Wing Drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), a Fruit Pest New to North America. Pest Alerts 2009 DACS-P-01674. Available online: https://ccmedia.fdacs.gov/content/download/66350/file/pest_alert_-_spotted_wing_drosophila_-_drosophila_suzukii.pdf (accessed on 14 September 2023).
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A decade of research towards a sustainable integrated pest management program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Bruck, D.J.; Bolda, M.; Tanigoshi, L.; Klick, J.; Kleiber, J.; DeFrancesco, J.; Gerdeman, B.; Spitler, H. Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag. Sci. 2011, 67, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, S.; Isaacs, R. Control of spotted wing Drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
- Deans, C.; Hutchinson, W.D. Propensity for resistance development in the invasive berry pest, spotted-wing drosophila (Drosophila suzukii), under laboratory selection. Pest Manag. Sci. 2022, 78, 5203–5212. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Gress, B.E.; Demkovich, M.R.; Nicola, N.L.; Chiu, J.C.; Zalom, F.G. Spatio-temporal variation of spinosad susceptibility in Drosophila suzukii (Diptera: Drosophilidae), a three-year study in California’s Monterey Bay region. J. Econ. Entomol. 2022, 115, 972–980. [Google Scholar] [CrossRef]
- Feng, Y.; Bruton, R.; Park, A.; Zhang, A. Identification of attractive blend for spotted wing drosophila, Drosophila suzukii (Matsumura) from apple juice. J. Pest Sci. 2018, 91, 1251–1267. [Google Scholar] [CrossRef]
- Kirkpatrick, D.M.; Gut, L.J.; Miller, J.R. Estimating monitoring trap plume reach and trapping area for Drosophila suzukii (Diptera: Drosophilidae) in Michigan tart cherry. J. Econ. Entomol. 2018, 111, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gut, L.; Grieshop, M. Evaluation of food-based attractants for Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2017, 46, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing Drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Adams, T.; Rogg, H. Trapping spotted wing Drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2012, 136, 148–154. [Google Scholar] [CrossRef]
- Burrack, H.J.; Asplen, M.; Bahder, L.; Collins, J.; Drummond, F.A.; Guédot, C.; Isaacs, R.; Johnson, D.; Blanton, A.; Lee, J.C.; et al. Multistate comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries and caneberries. Environ. Entomol. 2015, 44, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, F.; Gonella, E.; Crotti, E.; Vacchini, V.; Syrpas, M.; Pontini, M.; Mangelinckx, S.; Daffonchio, D.; Alma, A. Olfactory attraction of Drosophila suzukii by symbiotic acetic acid bacteria. J. Pest Sci. 2016, 89, 783–792. [Google Scholar] [CrossRef]
- Ðurović, G.; Alawamleh, A.; Carlin, S.; Maddalena, G.; Guzzon, R.; Mazzoni, V.; Dalton, D.T.; Walton, V.M.; Suckling, D.M.; Butler, R.C.; et al. Liquid baits with Oenococcus oeni increase captures of Drosophila suzukii (Part II; characterization and development of baits and traps). Insects 2021, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, L.E.; Nyoike, T.W.; Liburd, O.E. Effect of trap design, bait type, and age on captures of Drosophila suzukii (Diptera: Drosophilidae) in berry crops. J. Econ. Entomol. 2014, 107, 1508–1518. [Google Scholar] [CrossRef]
- Hamby, K.A.; Bolda, M.P.; Sheehan, M.E.; Zalom, F.G. Seasonal monitoring for Drosophila suzukii (Diptera: Drosophilidae) in California commercial raspberries. Environ. Entomol. 2014, 43, 1008–1018. [Google Scholar] [CrossRef]
- Wollmann, J.; Schlesener, D.C.H.; Vieira, J.G.A.; Bernardi, D.; Garcia, M.S.; Garcia, F.R.M. Evaluation of food baits to capture Drosophila suzukii in the southern of Brazil. An. Acad. Bras. Ciênc. 2019, 91, e20180375. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.R.; Strickland, J.; Shields, V.D.; Rodriguez-Saona, C.; Cloonan, K.; Short, D.B.; Leskey, T.C.; Zhang, A. Field evaluation of different attractants for detecting and monitoring Drosophila suzukii. Front. Ecol. Evol. 2021, 9, 620445. [Google Scholar] [CrossRef]
- Larson, N.R.; Strickland, J.; Shields, V.D.; Zhang, A. Controlled-release dispenser and dry trap developments for Drosophila suzukii detection. Front. Ecol. Evol. 2020, 8, 45. [Google Scholar] [CrossRef]
- Zhang, A.; Feng, Y. Methods of Attracting Drosophila suzukii Using Acetoin Blend. U.S. Patent 10638754, 5 May 2020. [Google Scholar]
- Feng, Y.; Zhang, A. A floral fragrance, methyl benzoate, is an efficient green pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef]
- Zhang, A.; Feng, Y. Methods for Killing Insects Using Methyl Benzoate. U.S. Patent 9629362 B1, 25 April 2017. [Google Scholar]
- Hardy, P.J.; Michael, B.J. Volatile components of feijoa fruits. Phytochemistry 1970, 9, 1355–1357. [Google Scholar] [CrossRef]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental Regulation of Methyl Benzoate Biosynthesis and Emission in Snapdragon Flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef]
- Kolosova, N.; Gorenstein, N.; Kish, C.M.; Dudareva, N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 2001, 13, 2333–2347. [Google Scholar] [CrossRef]
- Negre, F.; Kish, C.M.; Boatright, J.; Underwood, B.; Shibuya, K.; Wagner, C.; Clark, D.G.; Dudareva, N. Regulation of methyl benzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 2003, 15, 2992–3006. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, e7902. [Google Scholar] [CrossRef]
- Morrison, W.R.; Larson, N.; Brabec, D.; Zhang, A. Methyl benzoate as a putative alternative, environmentally-friendly fumigant for the control of stored product insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef]
- Chen, J.; Rashid, T.; Feng, G.; Feng, Y.; Zhang, A.; Grodowitz, M.J. Insecticidal activity of methyl benzoate analogs against red imported fire ants, Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 2019, 112, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Feng, Y.; Zhang, A. Methyl benzoate fumigation for post-harvest pests and its effects on apple quality. J. Appl. Entomol. 2020, 144, 191–200. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Wang, Y.; Portilla, M.; Parys, K.; Li, W. Risk and toxicity assessment of a potential natural insecticide, methyl benzoate, in honey bees (Apis mellifera L.). Insects 2019, 10, 382. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef]
- van Timmerman, S.; O’Donnell, K.; Isaacs, R. Spotted Wing Drosophila Identification Guide; Department of Entomology, Michigan State University: East Lansing, MI, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 31 October 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multilevel/Mixed) Regression Models, R Package Version 0.4.6; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion Guide to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, AKA Least-Squared Means, R Package Version 1.8.8; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Mating technology with mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Stelinski, L.L. Behavior-modifying strategies in IPM: Theory and practice. In Integrated Pest Management: Innovation—Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 263–315. [Google Scholar]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate exhibits insecticidal and repellent activities against Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Shim, J.-K.; Hwang, H.-S.; Bunch, H.; Lee, K.-Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Cha, D.H.; Loeb, G.M. Evaluating a push–pull strategy for management of Drosophila suzukii Matsumura in red raspberry. Pest Manag. Sci. 2018, 74, 120–125. [Google Scholar] [CrossRef]
- Cha, D.H.; Roh, G.H.; Hesler, S.P.; Wallingford, A.; Stockton, D.G.; Park, S.K.; Loeb, G.M. 2-Pentylfuran: A novel repellent of Drosophila suzukii. Pest Manag. Sci. 2021, 77, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Diepenbrock, L.M.; Burrack, H.J. Variation of within-crop microhabitat use by Drosophila suzukii (Diptera: Drosophilidae) in blackberry. J. Appl. Entomol. 2017, 141, 1–7. [Google Scholar] [CrossRef]
- FDA. Code of Federal Regulations Title 21—Food and Drugs. Sec. 172.515 Synthetic Flavoring Substances and Adjuvants. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.515 (accessed on 15 November 2023).
- European-Union. Food Flavourings. EU Regulation 1334/2008 & 178/2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=LEGISSUM:sa0006 (accessed on 15 November 2023).
- Bostanian, N.J.; Wise, J.C.; Isaacs, R. Pesticides for arthropod control in vineyards. In Arthropod Management in Vineyards; Bostanian, N., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 139–157. [Google Scholar]
- Wise, J.C.; Vanderpoppen, R.; Vandervoort, C.; O’Donnell, C.; Isaacs, R. Curative activity contributes to control of spotted-wing drosophila (Diptera: Drosophilidae) and blueberry maggot (Diptera: Tephritidae) in highbush blueberry. Can. Entomol. 2015, 147, 109–117. [Google Scholar] [CrossRef]
- Andika, I.P.; Vandervoort, C.; Wise, J.C. Curative activity of insecticides used to control spotted-wing Drosophila (Diptera: Drosophilidae) in tart cherry productions. J. Econ. Entomol. 2020, 113, 2372–2379. [Google Scholar] [CrossRef]
- van Timmeren, S.; Mota-Sanchez, D.; Wise, J.C.; Isaacs, R. Baseline susceptibility of spotted wing Drosophila (Drosophila suzukii) to four key insecticide classes. Pest Manag. Sci. 2018, 74, 78–87. [Google Scholar] [CrossRef]
- Wong, J.S.; Wallingford, A.K.; Loeb, G.M.; Lee, J.C. Physiological status of Drosophila suzukii (Diptera: Drosophilidae) affects their response to attractive odours. J. Appl. Entomol. 2018, 142, 473–482. [Google Scholar] [CrossRef]
- Clymans, R.; van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; de Clercq, P.; Beliën, T.; Bylemans, D. Olfactory preference of Drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. Insects 2019, 10, 200. [Google Scholar] [CrossRef]
- Cloonan, K.R.; Hernández-Cumplido, J.; de Sousa, A.L.V.; Ramalho, D.G.; Burrack, H.J.; Della Rosa, L.; Diepenbrock, L.M.; Ballman, E.; Drummond, F.A.; Gut, L.J.; et al. Laboratory and field evaluation of host-related foraging odor-cue combinations to attract Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2019, 112, 2850–2860. [Google Scholar] [CrossRef]

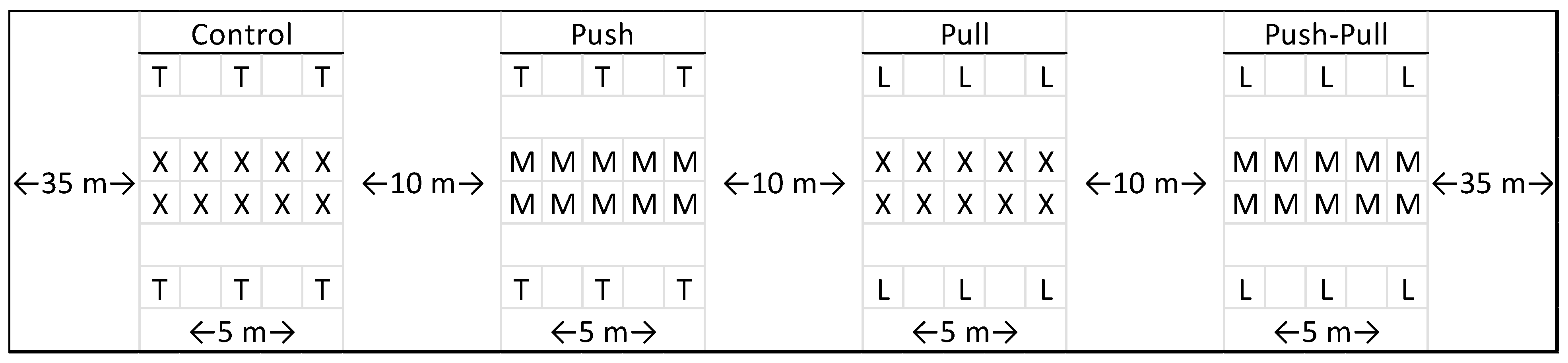
| Dispenser | Compound | Loading Volume (Blend Proportion) | Polyethylene Tubing Thickness |
|---|---|---|---|
| Push (repellent) | Methyl benzoate | 8 mL | 2 MIL |
| Pull (attractant) | Acetic acid | 5 mL | 2 MIL |
| Ethyl acetate | 5 mL | 6 MIL | |
| Phenethyl alcohol | 1 mL | 2 MIL | |
| Ethyl octanoate, Acetoin | 2 mL (1:1) | 2 MIL |
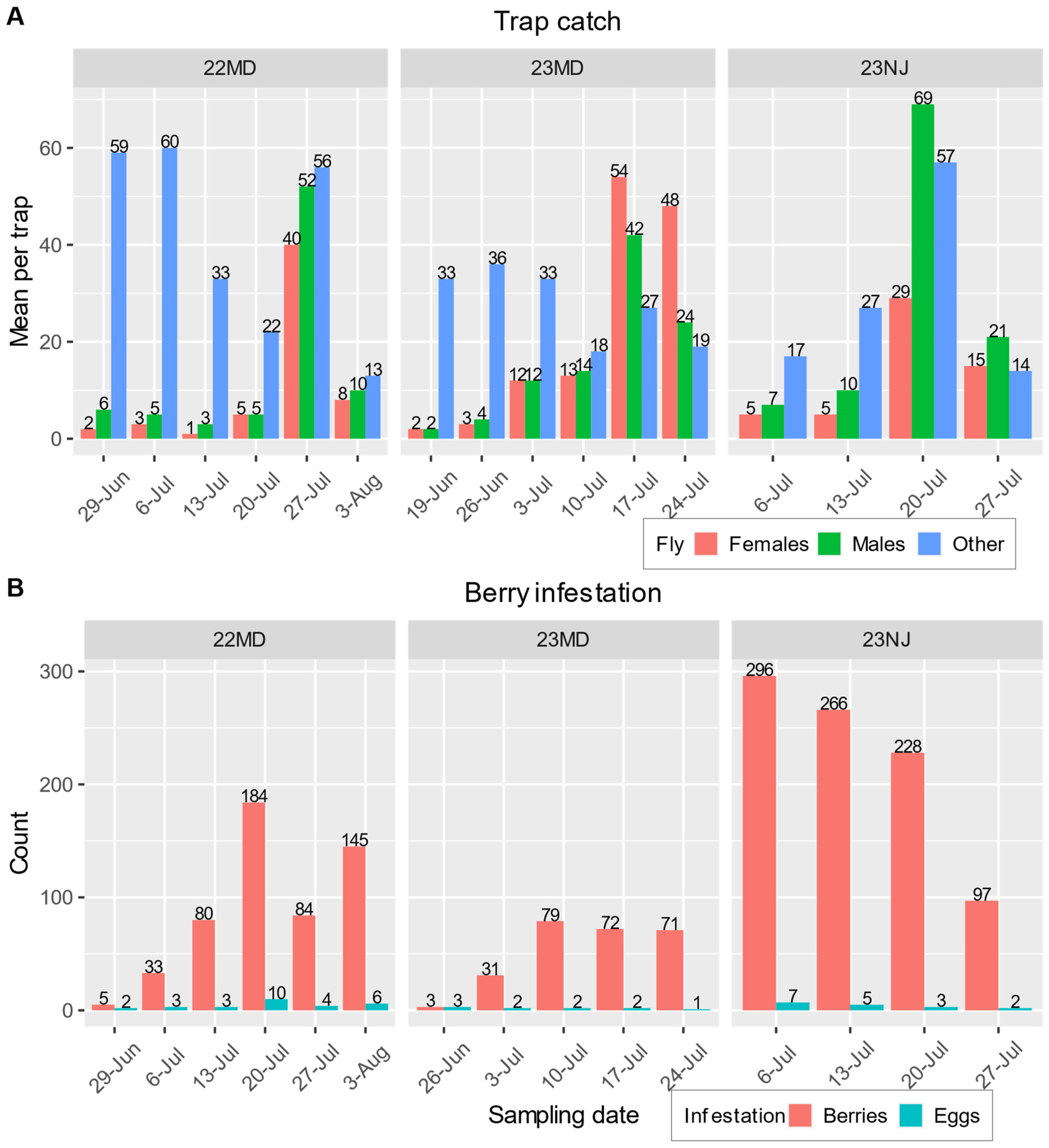
| Field Test | Date | Activities |
|---|---|---|
| 22 MD | 22 June 2022 | Set up traps and dispensers |
| 29 June 2022 | Sampled traps and berries | |
| 6 July 2022 | Sampled traps and berries, replaced dispensers | |
| 13 July 2022 | Sampled traps and berries | |
| 20 July 2022 | Sampled traps and berries, replaced dispensers | |
| 27 July 2022 | Sampled traps and berries | |
| 3 August 2022 | Sampled traps and berries | |
| 23 MD | 12 June 2023 | Set up traps and dispensers |
| 19 June 2023 | Sampled traps and berries | |
| 26 June 2023 | Sampled traps and berries, replaced dispensers | |
| 3 July 2023 | Sampled traps and berries | |
| 10 July 2023 | Sampled traps and berries, replaced dispensers | |
| 17 July 2023 | Sampled traps and berries | |
| 24 July 2023 | Sampled traps and berries | |
| 23 NJ | 29 June 2023 | Set up traps and dispensers |
| 6 July 2023 | Sampled traps and berries | |
| 13 July 2023 | Sampled traps and berries, replaced dispensers | |
| 20 July 2023 | Sampled traps and berries | |
| 27 July 2023 | Sampled traps and berries |
| Field Test | Response | Factor | χ2 | Df | p |
|---|---|---|---|---|---|
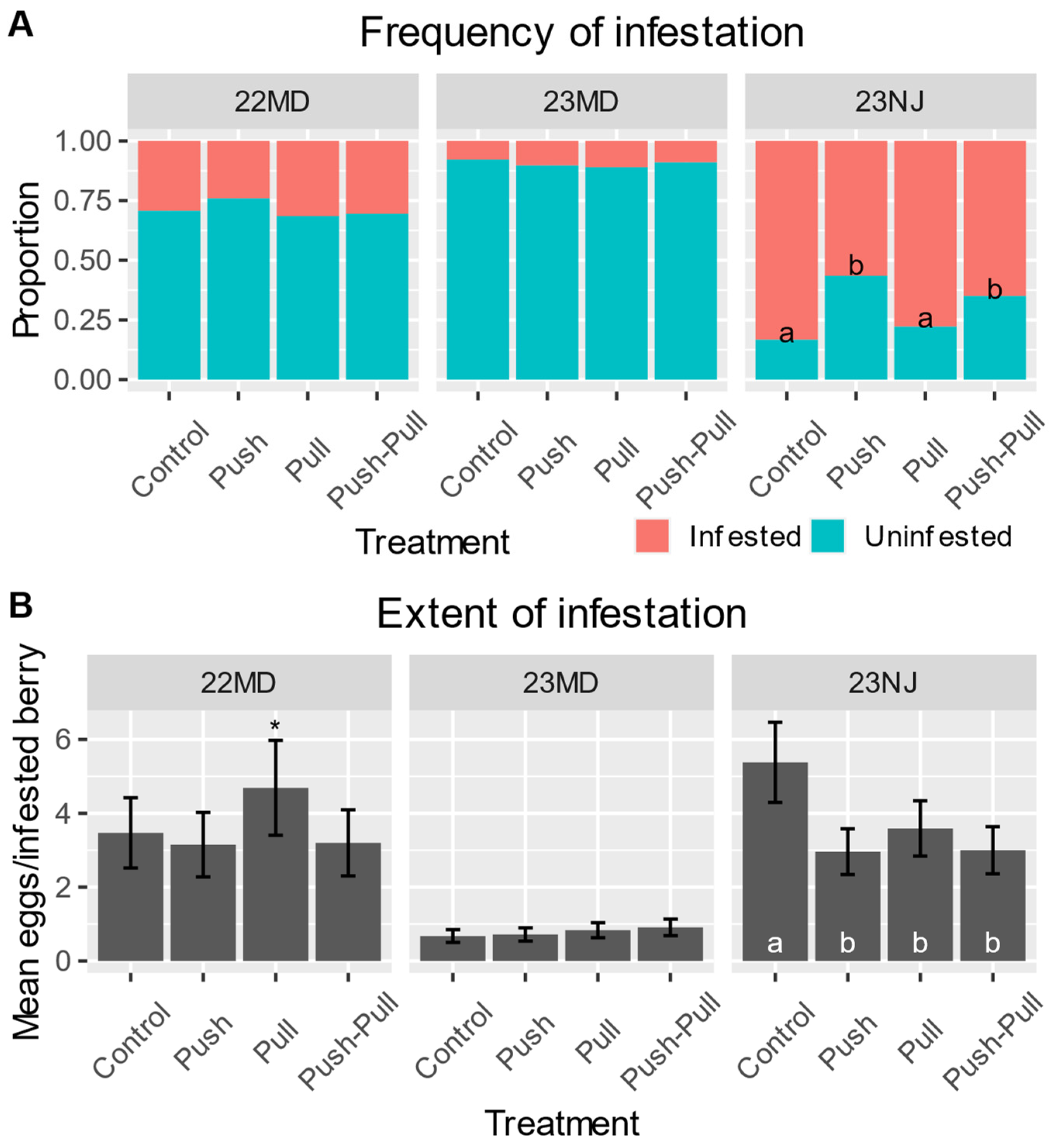
| 2022 MD | Frequency of infestation | Treatment | 3.958 | 3 | 0.266032 |
| Position | 7.5626 | 1 | 0.005959 *** | ||
| Treatment:Position | 0.5013 | 3 | 0.918615 | ||
| Extent of infestation | Treatment | 6.3418 | 3 | 0.09612 * | |
| Position | 21.9587 | 1 | <0.0001 *** | ||
| Treatment:Position | 1.0299 | 3 | 0.79401 | ||
| 2023 MD | Frequency of infestation | Treatment | 4.9245 | 3 | 0.1774 |
| Position | 26.8748 | 1 | <0.0001 *** | ||
| Treatment:Position | 1.7799 | 3 | 0.6193 | ||
| Extent of infestation | Treatment | 3.3753 | 3 | 0.3372961 | |
| Position | 12.6701 | 1 | 0.0003715 *** | ||
| Treatment:Position | 4.4535 | 3 | 0.2164736 | ||
| 2023 NJ | Frequency of infestation | Treatment | 61.3336 | 3 | <0.0001 *** |
| Position | 3.0326 | 1 | 0.08161 * | ||
| Treatment:Position | 3.4441 | 3 | 0.32808 | ||
| Extent of infestation | Treatment | 38.7119 | 3 | <0.0001 *** | |
| Position | 0.3125 | 1 | 0.5762 | ||
| Treatment:Position | 0.5517 | 3 | 0.9074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gale, C.C.; Ferguson, B.; Rodriguez-Saona, C.; Shields, V.D.C.; Zhang, A. Evaluation of a Push–Pull Strategy for Spotted-Wing Drosophila Management in Highbush Blueberry. Insects 2024, 15, 47. https://doi.org/10.3390/insects15010047
Gale CC, Ferguson B, Rodriguez-Saona C, Shields VDC, Zhang A. Evaluation of a Push–Pull Strategy for Spotted-Wing Drosophila Management in Highbush Blueberry. Insects. 2024; 15(1):47. https://doi.org/10.3390/insects15010047
Chicago/Turabian StyleGale, Cody C., Beth Ferguson, Cesar Rodriguez-Saona, Vonnie D. C. Shields, and Aijun Zhang. 2024. "Evaluation of a Push–Pull Strategy for Spotted-Wing Drosophila Management in Highbush Blueberry" Insects 15, no. 1: 47. https://doi.org/10.3390/insects15010047







