Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Thrips Parvispinus Colony
2.3. Residue Toxicity Assays
2.4. Direct Contact Assays
2.5. Statistical Analyses
3. Results
3.1. Effect of Conventional and Biorational Insecticides on Thrips Mortality
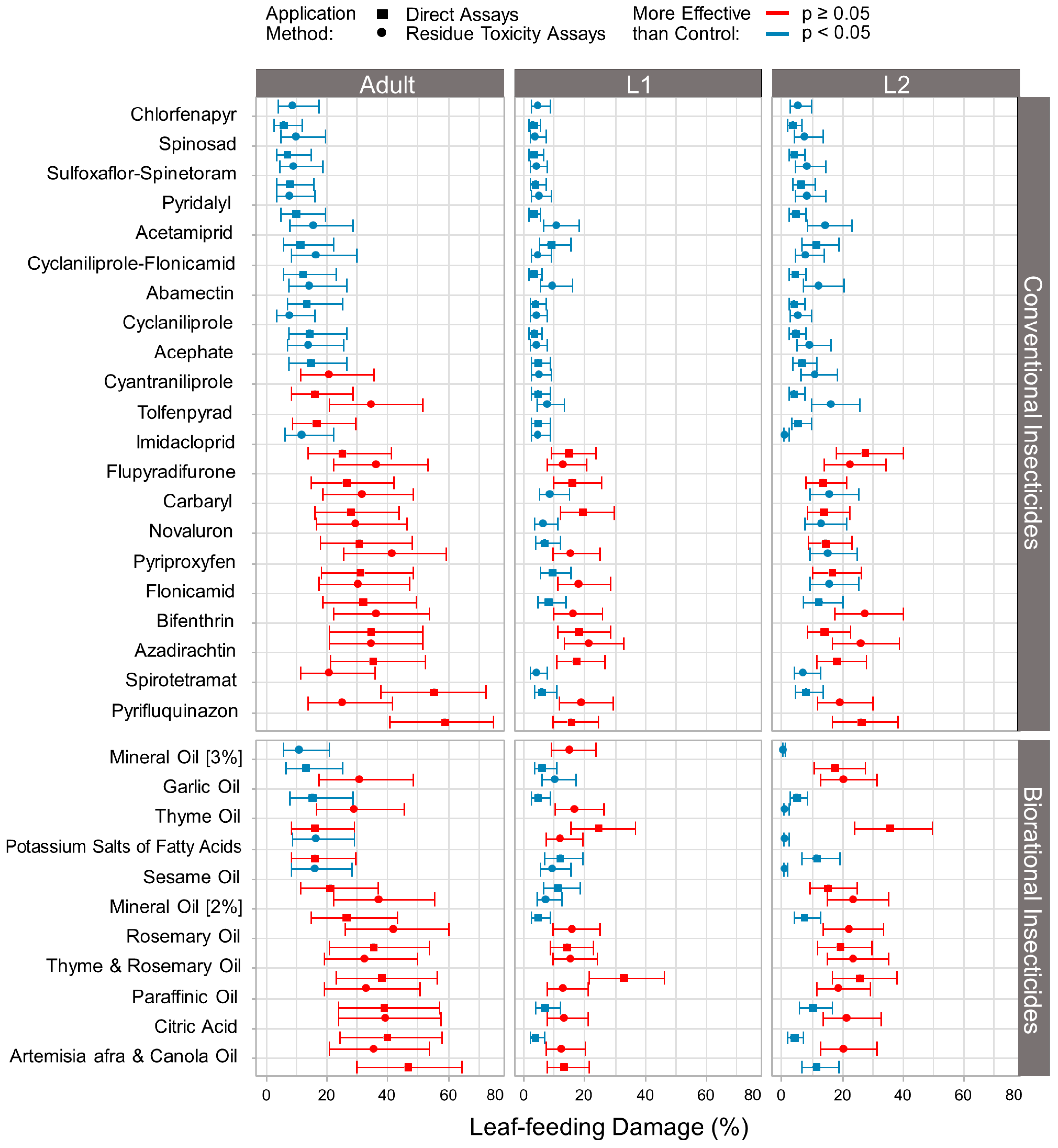
3.2. Effect of Conventional and Biorational Insecticides on Thrips Leaf-Feeding Damage
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes, C.P. Thysanoptera (Hexapoda) of the Philippine Islands. Raffles Bull. Zool. 1994, 42, 107–507. [Google Scholar]
- Johari, A.; Herlinda, S.; Pujiastuti, Y.; Irsan, C.; Sartiami, D. Morphological and Genetic Variation of Thrips parvispinus (Thysanoptera: Thripidae) in Chili Plantation (Capsicum annuum L.) in the Lowland and Highland of Jambi Province, Indonesia. Am. J. Biosci. 2014, 2, 17–21. [Google Scholar]
- Sridhar, V.; Chandana, P.S.; Rachana, R. Global Status of Thrips parvispinus (Karny, 1922), an Invasive Pest. J. Res. PJTSAU 2021, 49, 1–11. [Google Scholar]
- Thorat, S.; Sisodiya, D.; Gangwar, R. Invasive Thrips, Thrips parvispinus (Karny) an Invasive Threat: A Review. Environ. Ecol. 2022, 40, 2170–2175. [Google Scholar]
- Veeranna, D.; Reddy, R.U.; Moguloju, M.; Padmaja, G. Report on Heavy Infestation and Damage by Invasive Thrips Species, Thrips parvispinus (Karny) on Chilli in Telangana State of India. Pharma Innov. 2022, 11, 3845–3848. [Google Scholar]
- Mound, L.A.; Collins, D.W. A South East Asian Pest Species Newly Recorded from Europe: Thrips parvispinus (Thysanoptera: Thripidae), Its Confused Identity and Potential Quarantine Significance. Eur. J. Entomol. 2000, 97, 197–200. [Google Scholar] [CrossRef]
- Murai, T.; Watanabe, H.; Toriumi, W.; Adati, T.; Okajima, S. Damage to Vegetable Crops by Thrips parvispinus Karny (Thysanoptera: Thripidae) and Preliminary Studies on Biology and Control. J. Insect Sci. 2009, 10, 166. [Google Scholar]
- Sugano, J.; Hamasaki, R.; Villalobos, E.; Chou, M.; Wright, M.; Fukuda, S.; Swift, S.; Ferreira, S.; Tsuda, D.; Diaz-Lyke, M. Damage to Papaya Caused by Thrips parvispinus (Karny); University of Hawaii: Honolulu, HI, USA, 2015; 1p. [Google Scholar]
- Soto-Adames, F. Thrips parvispinus (Karny); Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 2020.
- United States Department of Agriculture National Agricultural Statistics Service. USDA-NASS Southern Region News Release Floriculture Production & Sales; United States Department of Agriculture National Agricultural Statistics Service: Washington, DC, USA, 2021.
- United States Department of Agriculture National Agricultural Statistics Service. USDA-NASS Florida Agricultural Facts; United States Department of Agriculture National Agricultural Statistics Service: Washington, DC, USA, 2022.
- EPPO Global Database. Thrips parvispinus (THRIPV): Distribution. 2023. Available online: https://gd.eppo.int/taxon/THRIPV/distribution (accessed on 10 November 2023).
- Morse, J.G.; Hoddle, M.S. Invasion Biology of Thrips. Annu. Rev. Entomol. 2006, 51, 67–89. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion Biology, Ecology, and Management of Western Flower Thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef]
- Rodríguez, D.; Coy-Barrera, E. Overview of Updated Control Tactics for Western Flower Thrips. Insects 2023, 14, 649. [Google Scholar] [CrossRef]
- Cloyd, R.A. Western Flower Thrips (Frankliniella occidentalis) Management on Ornamental Crops Grown in Greenhouses: Have We Reached an Impasse. Pest Technol. 2009, 3, 1–9. [Google Scholar]
- Reitz, S.R. Biology and Ecology of the Western Flower Thrips (Thysanoptera: Thripidae): The Making of a Pest. Fla. Entomol. 2009, 92, 7–13. [Google Scholar] [CrossRef]
- Seaton, K.A.; Cook, D.F.; Hardie, D.C. The Effectiveness of a Range of Insecticides against Western Flower Thrips (Frankliniella occidentalis) (Thysanoptera: Thripidae) on Cut Flowers. Aust. J. Agric. Res. 1997, 48, 781–788. [Google Scholar] [CrossRef]
- Gao, Y.; Lei, Z.; Reitz, S.R. Western Flower Thrips Resistance to Insecticides: Detection, Mechanisms and Management Strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef]
- Daughtrey, M.L.; Jones, R.K.; Moyer, J.W.; Daub, M.E.; Baker, J.R. Tospoviruses Strike the Greenhouse Industry: INSV Has Become a Major Pathogen on Flower Crops. Plant Dis. 1997, 81, 1220–1230. [Google Scholar] [CrossRef]
- Lewis, T. Chemical Control. In Thrips Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, CT, USA, 1997; pp. 567–593. [Google Scholar]
- Hutasoit, R.; Triwidodo, H.; Anwar, R. Biology and Demographic Statistic of Thrips parvispinus Karny (Thysanoptera: Thripidae) in Chili Pepper (Capsicum annuum Linnaeus). Indones. J. Entomol. 2017, 14, 107–116. [Google Scholar] [CrossRef]
- Cowles, R.S.; Eitzer, B.D. Residues of Neonicotinoid Insecticides in Pollen and Nectar from Model Plants. J. Environ. Hortic. 2017, 35, 24–34. [Google Scholar] [CrossRef]
- Joseph, S.V. Transovarial Effects of Insect Growth Regulators on Stephanitis pyrioides (Hemiptera: Tingidae). Pest Manag. Sci. 2019, 75, 2182–2187. [Google Scholar] [CrossRef]
- Bogran, C.E.; Ludwig, S.; Metz, B. Using Oils as Pesticides. In Texas Farmer Collectionl. Agrilife Extension Texas A&M System. 2006. Available online: https://hdl.handle.net/1969.1/86885 (accessed on 10 November 2023).
- Robinson, J.V. Horticultural Oils and Pest Control. Texas Agricultural Extension Service, Texas A&M University System. 2000. Available online: https://kendall.agrilife.org (accessed on 10 November 2023).
- Wright, P.J.; Walker, G.P.; MacDonald, F.H.; Gardner-Gee, R.; Hedderley, D.I. Mineral Oil Foliar Applications in Combination with Insecticides Affect Tomato Potato Psyllid (Bactericera cockerelli) and Beneficial Insects in Potato Crops. N. Z. J. Crop Hortic. Sci. 2017, 45, 263–276. [Google Scholar] [CrossRef]
- Picard, I.; Hollingsworth, R.G.; Salmieri, S.; Lacroix, M. Repellency of Essential Oils to Frankliniella occidentalis (Thysanoptera: Thripidae) as Affected by Type of Oil and Polymer Release. J. Econ. Entomol. 2012, 105, 1238–1247. [Google Scholar] [CrossRef]
- Stepanycheva, E.; Petrova, M.; Chermenskaya, T.; Pavela, R. Fumigant Effect of Essential Oils on Mortality and Fertility of Thrips Frankliniella occidentalis Perg. Environ. Sci. Pollut. Res. Int. 2019, 26, 30885–30892. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food and Agriculture Organization of the United Nations Guidelines on Prevention and Management of Pesticide Resistance; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’ (2.0.0). J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Brooks, M.; Bolker, B.; Kristensen, K.; Maechler, M.; Magnusson, A.; McGillycuddy, M.; Skaug, H.; Nielsen, A.; Berg, C.; van Bentham, K. glmmTMB: Generalized Linear Mixed Models Using Template Model Builder. R Package. 2022. [Computer Software]. Available online: https://cran.r-project.org/package=glmmTMB (accessed on 10 November 2023).
- Bolker, B.M. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 1-4008-4090-2. [Google Scholar]
- Stroup, W.W. Rethinking the Analysis of Non-normal Data in Plant and Soil Science. Agron. J. 2015, 107, 811–827. [Google Scholar] [CrossRef]
- Smithson, M.; Verkuilen, J. A Better Lemon Squeezer? Maximum-Likelihood Regression with Beta-Distributed Dependent Variables. Psychol. Methods 2006, 11, 54. [Google Scholar] [CrossRef]
- Albert, A.; Anderson, J.A. On the Existence of Maximum Likelihood Estimates in Logistic Regression Models. Biometrika 1984, 71, 1–10. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package. 2022. [Computer Software]. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 10 November 2023).
- IRAC. Insecticide Resistance Action Committee. 2023. Available online: https://irac-online.org/mode-of-action/classification-online/ (accessed on 10 November 2023).
- Manideep, S.; Muthuswami, M.; Shanmugam, P.S.; Suganthi, A.; Boopathi, N.M. Field Evaluation of Biorationals and Chemical Insecticides against Thrips parvispinus (Karny) (Thysanoptera: Thripidae), in Chrysanthemum. Int. J. Plant Soil Sci. 2023, 35, 179–186. [Google Scholar] [CrossRef]
- Seal, D.; Jangra, S.; Kanchupati, N.; Adeleye, V.; Sabines, C. Efficacy of various insecticides for controlling Thrips parvispinus, spring 2023. Arthropod Manag. Tests 2023, 48, 1–2. [Google Scholar] [CrossRef]
- Eger, J.; Stavisky, J.; Funderburk, J. Comparative Toxicity of Spinosad to Frankliniella Spp. (Thysanoptera: Thripidae), with Notes on a Bioassay Technique. Fla. Entomol. 1998, 81, 547–551. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Sadof, C.S. Effects of Spinosad and Acephate on Western Flower Thrips inside and Outside a Greenhouse. HortTechnology 2000, 10, 359–362. [Google Scholar] [CrossRef]
- Herron, G.; James, T. Insecticide Resistance in Australian Populations of Western Flower Thrips, ‘Frankliniella occidentalis’ (Pergande) (Thysanoptcra: Thripidae). Gen. Appl. Entomol. J. Entomol. Soc. N. S. W. 2007, 36, 1–5. [Google Scholar]
- Bielza, P. Insecticide Resistance Management Strategies against the Western Flower Thrips, Frankliniella occidentalis. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 1131–1138. [Google Scholar] [CrossRef]
- Kirst, H.A. The Spinosyn Family of Insecticides: Realizing the Potential of Natural Products Research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef]
- Bret, B.; Larson, L.; Schoonover, J.; Sparks, T.; Thompson, G. Biological Properties of Spinosad. Down Earth 1997, 52, 6–13. [Google Scholar]
- Tillman, P.G.; Mulrooney, J.E. Effect of Selected Insecticides on the Natural Enemies Coleomegilla maculata and Hippodamia convergens (Coleoptera: Coccinellidae), Geocoris punctipes (Hemiptera: Lygaeidae), and Bracon mellitor, Cardiochiles nigriceps, and Cotesia marginiventris (Hymenoptera: Braconidae) in Cotton. J. Econ. Entomol. 2000, 93, 1638–1643. [Google Scholar] [CrossRef]
- Williams, T.; Valle, J.; Viñuela, E. Is the Naturally Derived Insecticide Spinosad® Compatible with Insect Natural Enemies? Biocontrol Sci. Technol. 2003, 13, 459–475. [Google Scholar] [CrossRef]
- International Organisation for Biological and Integrated Control. IOBC-WPRS Pesticide Side Effect Database. 2023. Available online: https://iobc-wprs.org/ip-tools/pesticide-side-effect-database/ (accessed on 20 November 2023).
- Herron, G.A.; Rophail, J. First Detection of Chlorfenapyr (Secure®) Resistance in Two-Spotted Spider Mite (Acari: Tetranychidae) from Nectarines in an Australian Orchard. Exp. Appl. Acarol. 2003, 31, 131–134. [Google Scholar] [CrossRef]
- Bhuyain, M.M.H.; Lim, U.T. Relative Susceptibility to Pesticides and Environmental Conditions of Frankliniella intonsa and F. occidentalis (Thysanoptera: Thripidae), an Underlying Reason for Their Asymmetrical Occurrence. PLoS ONE 2020, 15, e0237876. [Google Scholar] [CrossRef]
- Corteva Agriscience XXpire® Insecticide—Turf & Ornamental. Available online: https://www.corteva.us/products-and-solutions/turf-and-ornamental/xxpire.html (accessed on 6 December 2023).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Greene, A.D.; Yang, X.; Velazquez-Hernandez, Y.; Vargas, G.; Kendra, P.E.; Mannion, C.; Revynthi, A.M. Lethal and Sublethal Effects of Contact Insecticides and Horticultural Oils on the Hibiscus Bud Weevil, Anthonomus testaceosquamosus Linell (Coleoptera: Curculionidae). Insects 2023, 14, 544. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the Sulfoximine Insecticides: Chemistry, Mode of Action and Basis for Efficacy on Resistant Insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef]
- Powell, G.F.; Ward, D.A.; Prescott, M.C.; Spiller, D.G.; White, M.R.H.; Turner, P.C.; Earley, F.G.P.; Phillips, J.; Rees, H.H. The Molecular Action of the Novel Insecticide, Pyridalyl. Insect Biochem. Mol. Biol. 2011, 41, 459–469. [Google Scholar] [CrossRef]
- Isayama, S.; Saito, S.; Kuroda, K.; Umeda, K.; Kasamatsu, K. Pyridalyl, a Novel Insecticide: Potency and Insecticidal Selectivity. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2005, 58, 226–233. [Google Scholar] [CrossRef]
- Sakamoto, N.; Saito, S.; Hirose, T.; Suzuki, M.; Matsuo, S.; Izumi, K.; Nagatomi, T.; Ikegami, H.; Umeda, K.; Tsushima, K. The Discovery of Pyridalyl: A Novel Insecticidal Agent for Controlling Lepidopterous Pests. Pest Manag. Sci. Former. Pestic. Sci. 2004, 60, 25–34. [Google Scholar] [CrossRef]
- Saito, S.; Isayama, S.; Sakamoto, N.; Umeda, K. Insecticidal Activity of Pyridalyl: Acute and Sub-Acute Symptoms in Spodoptera litura Larvae. J. Pestic. Sci. 2004, 29, 372–375. [Google Scholar] [CrossRef]
- Pes, M.P.; Melo, A.A.; Stacke, R.S.; Zanella, R.; Perini, C.R.; Silva, F.M.A.; Carús Guedes, J.V. Translocation of Chlorantraniliprole and Cyantraniliprole Applied to Corn as Seed Treatment and Foliar Spraying to Control Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2020, 15, e0229151. [Google Scholar] [CrossRef]
- Zilnik, G.; Kraus, D.A.; Burrack, H.J. Translocation and Persistence of Soil Applied Chlorantraniliprole as a Control Measure for Chloridea virescens in Tobacco Plant Nicotiana Tabacum. Crop Prot. 2021, 140, 105413. [Google Scholar] [CrossRef]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Neonicotinoids Working Group Alternatives to Neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential Oil Compounds for Thrips Control—A Review. Nat. Prod. Commun. 2008, 3, 1934578X0800300726. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; Messeha, S.; El-Gengaihi, S. Botanical Biocides. 4. Mosquitocidal Activity of Certain Thymus capitatus Constituents. J. Nat. Toxins 2000, 9, 49–62. [Google Scholar] [PubMed]
- Hummelbrunner, L.A.; Isman, M.B. Acute, Sublethal, Antifeedant, and Synergistic Effects of Monoterpenoid Essential Oil Compounds on the Tobacco Cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Dusi, R.G.; da Silva Morais, L.; Magalhães, N.M.G.; Albernaz, L.C.; Hamilton, C.J.; Espindola, L.S. Potential of Garlic Oil as a Biopesticide against All Aedes Aegypti Life Stages. Ind. Crops Prod. 2022, 181, 114780. [Google Scholar] [CrossRef]
- Tremblay, É.; Bélanger, A.; Brosseau, M.; Boivin, G. Toxicity Effects of an Insecticidal Soap on the Green Peach Aphid [Homoptera: Aphididae]. Phytoprotection 2009, 90, 35–39. [Google Scholar] [CrossRef]
- Raudonis, L.; Duchovskiene, L.; Valiuskaite, A.; Surviliene, E. Toxicity of Biopesticides to Green Apple Aphid, Predatory Insects and Mite in an Apple-Tree Orchard. Zemdirb. Agric. 2010, 97, 49–54. [Google Scholar]
- Robb, K.L.; Parrella, M.P. IPM of Western Flower Thrips. In Thrips Biology and Management; Plenum Press: New York, NY, USA, 1995; pp. 365–370. [Google Scholar]

| Trade Name | Active Ingredient(s) | Insecticide Group a | Rate b | Rate in 1 L Solution | Site c | EPA Registration Number d | |
|---|---|---|---|---|---|---|---|
| Conventional Insecticides | Acephate 97 UP WDG | Acephate | 1B | 91.8 g/ha | 599 mg | G, N, L | 70506-8 |
| Altus | Flupyradifurone | 4D | 167.6 mL/ha | 1.09 mL | G, N, L | 432-1575 | |
| Aria | Flonicamid | 29 | 33.3 g/ha | 211 mg | G, N, L | 279-3287 | |
| AzaSol | Azadirachtin | Unknown | 68.8 g/ha | 898 mg | G, N, I, L | 81899-4-74578 | |
| Conserve SC | Spinosad | 5 | 1.20 mL/ha | 0.78 mL | G, N, L | 62719-291 | |
| Fulcrum | Pyriproxyfen | 7C | 143.6 mL/ha | 0.94 mL | G, N, L, S | 59807-14 | |
| Hachi-Hachi SC | Tolfenpyrad | 21A | 323.1 mL/ha | 2.11 mL | G, N, S, L | 71711-31-67690 | |
| Kontos | Spirotetramat | 23 | 40.7 mL/ha | 0.26 mL | G, N, I | 432-1471 | |
| Mainspring GNL | Cyantraniliprole | 28 | 95.7 mL/ha | 0.63 mL | G, N, I, L | 10015-43 | |
| Merit 75 WSP | Imidacloprid | 4A | 18 g/ha | 37 mg | N, L, I | 432-1318 | |
| Overture 35 WP | Pyridalyl | Unclassified | 91.8 g/ha | 599 mg | G | 59639-125 | |
| Pedestal | Novaluron | 15 | 95.7 mL/ha | 0.63 mL | G, N, S | 53883-419-59807 | |
| Piston TR | Chlorfenapyr | 13 | 119.7 mL/ha | 0.78 mL | G | 91234-19 | |
| Pradia | Cyclaniliprole-Flonicamid | 28–29 | 209.4 mL/ha | 1.37 mL | G, N, S | 71512-33-59807 | |
| Rycar | Pyrifluquinazon | 9B | 38.3 mL/ha | 0.25 mL | G | 71711-37-67690 | |
| Sarisa | Cyclaniliprole | 28 | 323.1 mL/ha | 2.11 mL | G, N, S | 71512-32-59807 | |
| Sevin SL | Carbaryl | 1A | 383 mL/ha | 2.5 mL | G, N, L | 432-1227 | |
| Talstar P | Bifenthrin | 3A | 259.7 mL/ha | 1.70 mL | G, N, L | 279-3206 | |
| Timectin 0.15 EC | Abamectin | 6 | 95.7 mL/ha | 0.63 mL | S, G, N | 84229-1 | |
| Tristar 8.5 SL | Acetamiprid | 4A | 302.8 mL/ha | 1.98 mL | G, N, S, L | 8033-106-1001 | |
| Xxpire | Sulfoxaflor-Spinetoram | 4C-5 | 31.5 g/ha | 206 mg | G, N | 62719-676 | |
| Biorational Insecticides | Agropest | Thyme + rosemary oil | Unclassified | 0.5% | 5 mL | S, G, N, L | FIFRA 25 (b) exempt |
| Arte + Guard | Artemisia afra + canola oil | Unclassified | 12.0 mL/ha | 7.81 mL | G, N, I, L | FIFRA 25 (b) exempt | |
| Bee Safe 3-in-1 | Sesame oil | Unclassified | 35.9 mL/ha | 23.02 mL | S, G, N, L | FIFRA 25 (b) exempt | |
| Bush Doctor Force of Nature | Garlic oil | Unclassified | 1531.9 mL/ha | 9.99 mL | S, G, N, L | FIFRA 25 (b) exempt | |
| M-Pede | Potassium salts of fatty acids | Unclassified | 29.9 mL/ha | 20.07 mL | G, N, L, I | 10163-324 | |
| Nuke EM | Citric acid | Unclassified | 95.7 mL/ha | 62.5 mL | S, G, N, L | FIFRA 25 (b) exempt | |
| Sierra Natural Science 209 | Rosemary oil | Unclassified | 646.3 mL/ha | 8.44 mL | S, G, N, S | FIFRA 25 (b) exempt | |
| Stylet-Oil JMS | Paraffinic oil | Unclassified | 12.0 mL/ha | 7.81 mL | G, N, I, L | 65564-1 | |
| SuffOil-X | Mineral oil | Unclassified | 2% | 20 mL | G, N, L | 48813-1-68539 | |
| Thyme Guard | Thyme oil | Unclassified | 0.5% | 5 mL | S, G, N, L | FIFRA 25 (b) exempt | |
| Ultra-fine | Mineral oil | Unclassified | 3% | 30 mL | G, N, L, I | 86330-11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataide, L.M.S.; Vargas, G.; Velazquez-Hernandez, Y.; Reyes-Arauz, I.; Villamarin, P.; Canon, M.A.; Yang, X.; Riley, S.S.; Revynthi, A.M. Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions. Insects 2024, 15, 48. https://doi.org/10.3390/insects15010048
Ataide LMS, Vargas G, Velazquez-Hernandez Y, Reyes-Arauz I, Villamarin P, Canon MA, Yang X, Riley SS, Revynthi AM. Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions. Insects. 2024; 15(1):48. https://doi.org/10.3390/insects15010048
Chicago/Turabian StyleAtaide, Livia M. S., German Vargas, Yisell Velazquez-Hernandez, Isamar Reyes-Arauz, Paola Villamarin, Maria A. Canon, Xiangbing Yang, Simon S. Riley, and Alexandra M. Revynthi. 2024. "Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions" Insects 15, no. 1: 48. https://doi.org/10.3390/insects15010048





