High Abundance of Haemoproteus Parasites in Culicoides (Diptera, Ceratopogonidae), with a Confirmation of Culicoides reconditus as a New Vector of These Avian Blood Parasites
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Biting Midges
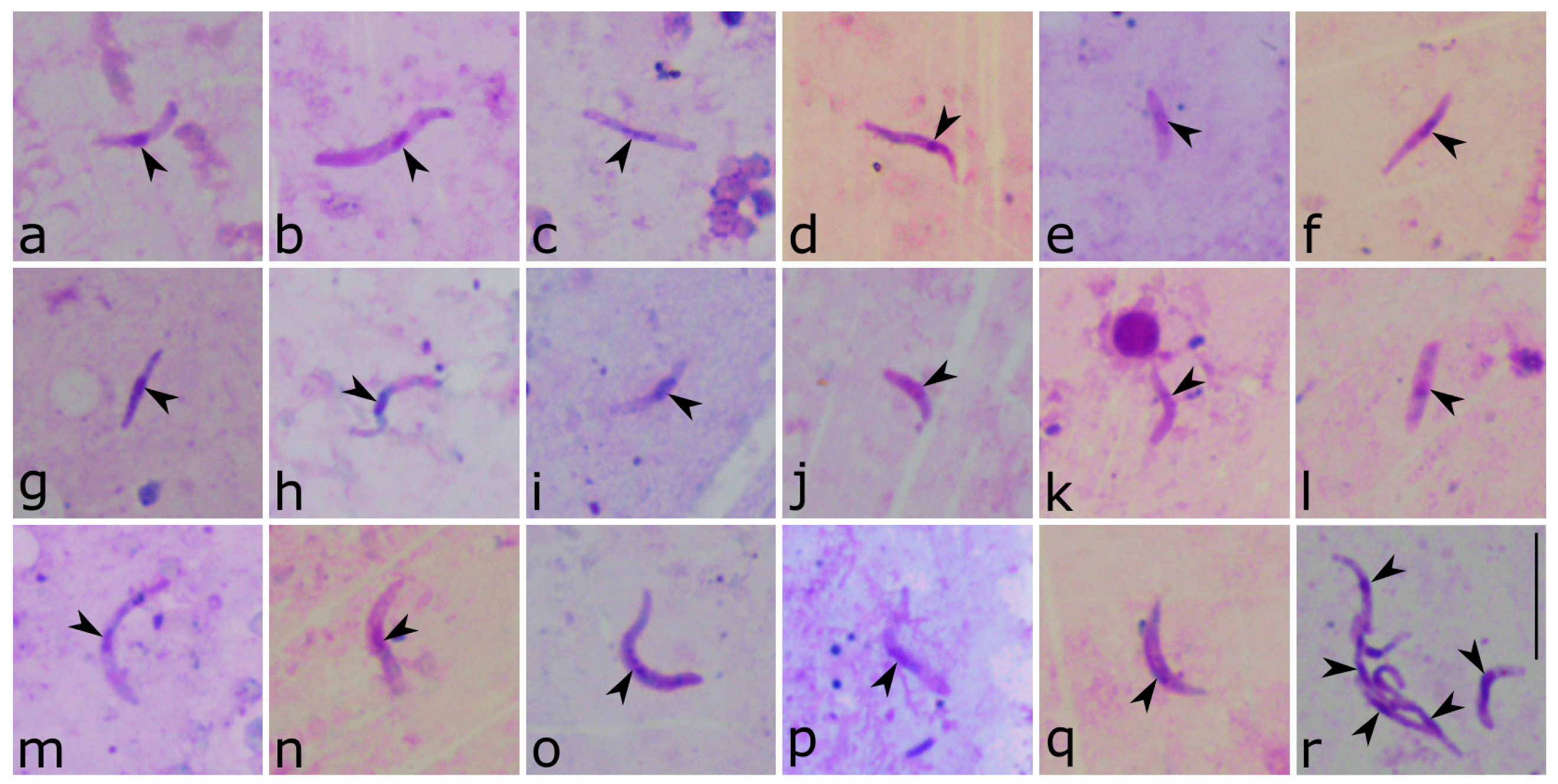
2.2. Biting Midges Dissection, Identification, and Microscopic Examination of Salivary Gland Preparations
2.3. DNA Extraction, PCR, and Sequencing Analysis
2.4. Statistical and Diversity Analyses
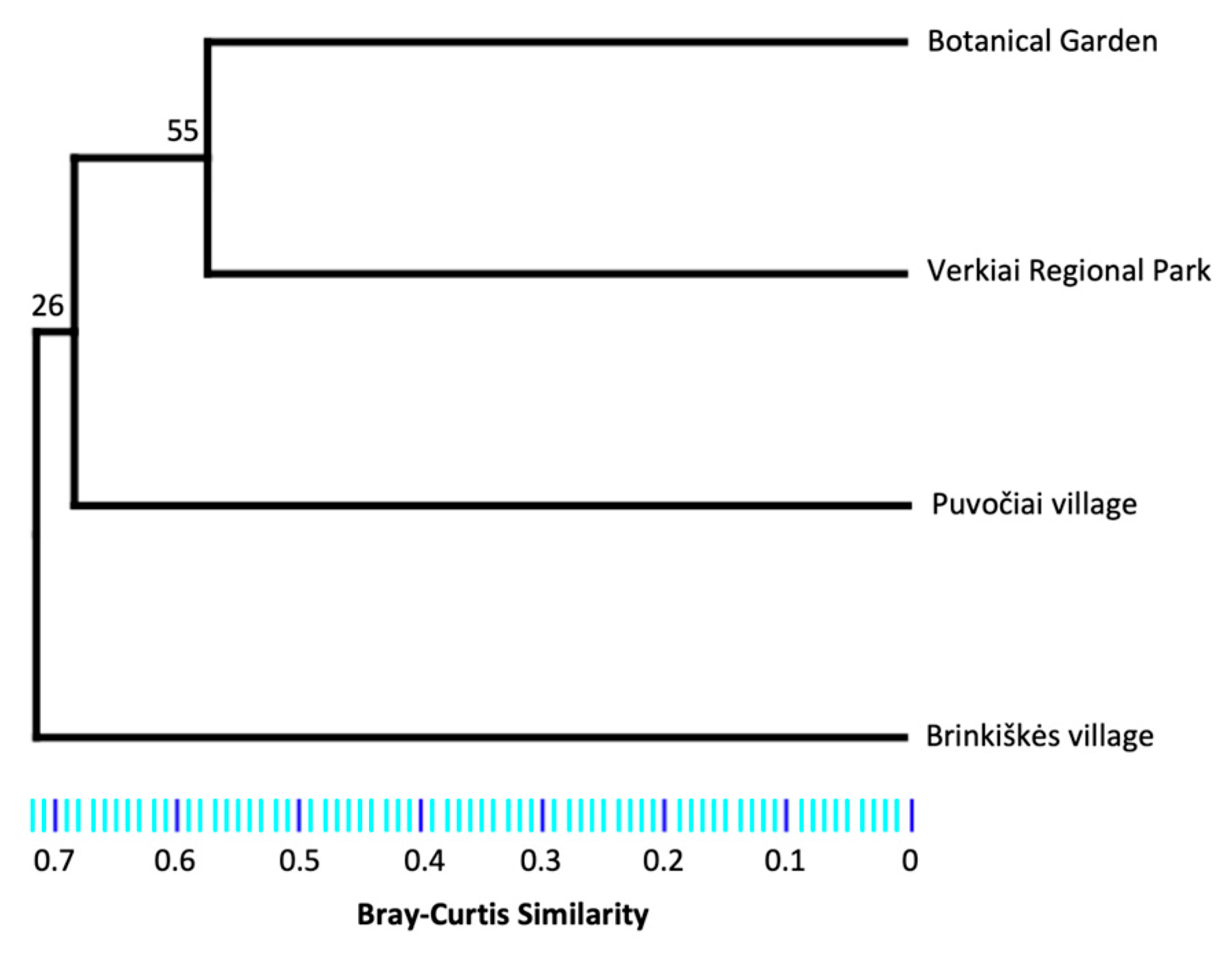
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valkiūnas, G.; Iezhova, T.A. Insights into the biology of Leucocytozoon species (Haemosporida, Leucocytozoidae): Why is there slow research progress on agents of leucocytozoonosis? Microorganisms 2023, 11, 1251. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae). Malar. J. 2022, 21, 269. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef]
- Garnham, P.C.C. Malaria Parasites and Other Haemosporidia; Blackwell Scientific: Oxford, UK, 1966; ISBN 978-0-632-01770-6. [Google Scholar]
- Atkinson, C.T. Vectors, epizootiology, and pathogenicity of avian species of Haemoproteus (Haemosporina: Haemoproteidae). Bull. Soc. Vector Ecol. 1991, 16, 109–126. [Google Scholar]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Lotta, I.A.; Pacheco, M.A.; Escalante, A.A.; González, A.D.; Mantilla, J.S.; Moncada, L.I.; Adler, P.H.; Matta, N.E. Leucocytozoon diversity and possible vectors in the neotropical highlands of Colombia. Protist 2016, 167, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera vectors of avian haemosporidian parasites: Untangling parasite life cycles and their taxonomy. Biol. Rev. 2012, 87, 928–964. [Google Scholar] [CrossRef] [PubMed]
- Bernotienė, R.; Žiegytė, R.; Vaitkutė, G.; Valkiūnas, G. Identification of a new vector species of avian haemoproteids, with a description of methodology for the determination of natural vectors of haemosporidian parasites. Parasites Vectors 2019, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.R.F.; Hernández-Lara, C.; Duc, M.; Valavičiūtė-Pocienė, K.; Bernotienė, R. What can haemosporidian lineages found in Culicoides biting midges tell us about their feeding preferences? Diversity 2022, 14, 957. [Google Scholar] [CrossRef]
- Žiegytė, R.; Palinauskas, V.; Bernotienė, R. Natural vector of avian Haemoproteus asymmetricus parasite and factors altering the spread of infection. Insects 2023, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Žiegytė, R.; Bernotienė, R.; Palinauskas, V. Culicoides segnis and Culicoides pictipennis biting midges (Diptera, Ceratopogonidae), new reported vectors of Haemoproteus parasites. Microorganisms 2022, 10, 898. [Google Scholar] [CrossRef]
- Žiegytė, R.; Platonova, E.; Kinderis, E.; Mukhin, A.; Palinauskas, V.; Bernotienė, R. Culicoides biting midges involved in transmission of haemoproteids. Parasites Vectors 2021, 14, 27. [Google Scholar] [CrossRef]
- Borkent, A.; Dominiak, P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa 2020, 4787, 1–377. [Google Scholar] [CrossRef]
- Bukauskaitė, D.; Iezhova, T.A.; Ilgūnas, M.; Valkiūnas, G. High susceptibility of the laboratory-reared biting midges Culicoides nubeculosus to Haemoproteus infections, with review on Culicoides species that transmit avian haemoproteids. Parasitology 2019, 146, 333–341. [Google Scholar] [CrossRef]
- Ayllón, T.; Nijhof, A.M.; Weiher, W.; Bauer, B.; Allène, X.; Clausen, P.-H. Feeding behaviour of Culicoides spp. (Diptera: Ceratopogonidae) on cattle and sheep in northeast Germany. Parasites Vectors 2014, 7, 34. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Figuerola, J.; Soriguer, R. Fur or feather? Feeding preferences of species of Culicoides biting midges in Europe. Trends Parasitol. 2015, 31, 16–22. [Google Scholar] [CrossRef]
- Videvall, E.; Bensch, S.; Ander, M.; Chirico, J.; Sigvald, R.; Ignell, R. Molecular identification of bloodmeals and species composition in Culicoides biting midges. Med. Vet. Entomol. 2013, 27, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides biting midges, arboviruses and public health in Europe. Antivir. Res. 2013, 100, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Bernotienė, R.; Valkiūnas, G. PCR Detection of malaria parasites and related haemosporidians: The sensitive methodology in determining bird-biting insects. Malar. J. 2016, 15, 283. [Google Scholar] [CrossRef] [PubMed]
- González-Olvera, M.; Hernandez-Colina, A.; Himmel, T.; Eckley, L.; Lopez, J.; Chantrey, J.; Baylis, M.; Jackson, A.P. Molecular and epidemiological surveillance of Plasmodium spp. during a mortality event affecting Humboldt Penguins (Spheniscus humboldti) at a Zoo in the UK. Int. J. Parasitol. Parasites Wildl. 2022, 19, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; Havelka, P.; Schaefer, H.M.; Segelbacher, G. Bloodmeal analysis reveals avian Plasmodium infections and broad host preferences of Culicoides (Diptera: Ceratopogonidae) vectors. PLoS ONE 2012, 7, e31098. [Google Scholar] [CrossRef]
- Kasičová, Z.; Schreiberová, A.; Kimáková, A.; Kočišová, A. Blood meal analysis: Host-feeding patterns of biting midges (Diptera, Ceratopogonidae, Culicoides Latreille) in Slovakia. Parasite 2021, 28, 58. [Google Scholar] [CrossRef]
- Augot, D.; Hadj-Henni, L.; Strutz, S.E.; Slama, D.; Millot, C.; Depaquit, J.; Millot, J.-M. Association between host species choice and morphological characters of main sensory structures of Culicoides in the Palaeartic Region. PeerJ 2017, 5, e3478. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Ruiz, S.; Soriguer, R.; Figuerola, J. On the study of the transmission networks of blood parasites from SW Spain: Diversity of avian haemosporidians in the biting midge Culicoides circumscriptus and wild birds. Parasites Vectors 2013, 6, 208. [Google Scholar] [CrossRef]
- Veiga, J.; Martínez-de la Puente, J.; Václav, R.; Figuerola, J.; Valera, F. Culicoides paolae and C. circumscriptus as potential vectors of avian haemosporidians in an arid ecosystem. Parasites Vectors 2018, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Bobeva, A.; Zehtindjiev, P.; Bensch, S.; Radrova, J. A survey of biting midges of the genus Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) in NE Bulgaria, with respect to transmission of avian haemosporidians. Acta Parasitol. 2013, 58, 585–591. [Google Scholar] [CrossRef]
- Bobeva, A.; Ilieva, M.; Dimitrov, D.; Zehtindjiev, P. Degree of associations among vectors of the genus Culicoides (Diptera: Ceratopogonidae) and host bird species with respect to haemosporidian parasites in NE Bulgaria. Parasitol. Res. 2014, 113, 4505–4511. [Google Scholar] [CrossRef]
- Braverman, Y.; Linley, J.R. Effect of light trap height on catch of Culicoides (Diptera: Ceratopogonidae) in Israel. J. Med. Entomol. 1993, 30, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Service, M.W. Adult flight activities of some British Culicoides species. J. Med. Entomol. 1971, 8, 605–609. [Google Scholar] [CrossRef]
- McGregor, B.L.; Runkel, A.E.; Wisely, S.M.; Burkett-Cadena, N.D. Vertical stratification of Culicoides biting midges at a Florida big game preserve. Parasites Vectors 2018, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.A.; Adler, P.H. Vertical distribution of haematophagous Diptera in temperate forests of the Southeastern USA. Med. Vet. Entomol. 2010, 24, 182–188. [Google Scholar] [CrossRef]
- Dyce, A.L. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Aust. J. Entomol. 1969, 8, 11–15. [Google Scholar] [CrossRef]
- Glukhova, V.M. Blood-sucking midges of the genera Culicoides and Forcipomyia (Ceratopogonidae). In Fauna of the USSR; Dipteran Insects; Nauka (Leningradskoe Otdelenie): Leningrad, Russia, 1989; Volume 3. [Google Scholar]
- Gutsevich, A.V. Blood-sucking midges (Ceratopogonidae). In Fauna of the USSR; Nauka Press: Leningrad, Russia, 1973; Volume 3. [Google Scholar]
- Mathieu, B.; Cêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.-L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the western Palaearctic Region. Parasites Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Richardson, D.S.; Jury, F.L.; Blaakmeer, K.; Komdeur, J.; Burke, T. Parentage assignment and extra-group paternity in a cooperative breeder: The Seychelles Warbler (Acrocephalus sechellensis). Mol. Ecol. 2001, 10, 2263–2273. [Google Scholar] [CrossRef]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Örjan, Ö.; Hannson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. Lond. B 2000, 267, 1583–1589. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Hammer, O. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C.H. SpadeR (Species-Richness Prediction and Diversity Estimation in R): An R package in CRAN. 2016. Available online: http://Chao.Stat.Nthu.Edu.Tw/Wordpress/Software_download (accessed on 15 January 2014).
- MacGregor-Fors, I.; Payton, M.E. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS ONE 2013, 8, e56794. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (H Ill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Pakalniškis, S.; Rimšaitė, J.; Sprangauskaitė-Bernotienė, R.; Butautaitė, R.; Podėnas, S. Checklist of Lithuanian Diptera. Acta Zool. Litu. 2000, 10, 3–58. [Google Scholar] [CrossRef]
- Cuéllar, A.C.; Jung Kjær, L.; Baum, A.; Stockmarr, A.; Skovgard, H.; Nielsen, S.A.; Andersson, M.G.; Lindström, A.; Chirico, J.; Lühken, R.; et al. Monthly variation in the probability of presence of adult Culicoides populations in nine European countries and the implications for targeted surveillance. Parasites Vectors 2018, 11, 608. [Google Scholar] [CrossRef]
- Cuéllar, A.C.; Kjær, L.J.; Kirkeby, C.; Skovgard, H.; Nielsen, S.A.; Stockmarr, A.; Andersson, G.; Lindstrom, A.; Chirico, J.; Lühken, R.; et al. Spatial and temporal variation in the abundance of Culicoides biting midges (Diptera: Ceratopogonidae) in nine European countries. Parasites Vectors 2018, 11, 112. [Google Scholar] [CrossRef]
- Saroya, Y.; Gottlieb, Y.; Klement, E. The Effect of ambient temperature fluctuations on Culicoides biting midges population dynamics and activity in dairy farms: A longitudinal study. Med. Vet. Entomol. 2021, 35, 68–78. [Google Scholar] [CrossRef]
- Bernotienė, R.; Bartkevičienė, G.; Bukauskaitė, D. The flying activity of biting midges (Ceratopogonidae: Culicoides) in Verkiai Regional Park, Southeastern Lithuania. Parasitol. Res. 2021, 120, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Mullen, G.R.; Murphree, C.S. Biting midges (Ceratopogonidae). In Medical and Veterinary Entomology; Elsevier: San Diego, CA, USA, 2019; pp. 213–236. ISBN 978-0-12-814043-7. [Google Scholar]
- Valkiūnas, G.; Liutkevičius, G.; Iezhova, T.A. Complete development of three species of Haemoproteus (Haemosporida, Haemoproteidae) in the biting midge Culicoides impunctatus (Diptera, Ceratopogonidae). J. Parasitol. 2002, 88, 864–868. [Google Scholar] [CrossRef]
- Groschupp, S.; Kampen, H.; Werner, D. Occurrence of putative Culicoides biting midge vectors (Diptera: Ceratopogonidae) inside and outside barns in Germany and factors influencing their activity. Parasites Vectors 2023, 16, 307. [Google Scholar] [CrossRef] [PubMed]
- Barceló, C.; del Río, R.; Miranda, M.A. Seasonal and nocturnal activity of Culicoides spp. (Diptera: Ceratopogonidae) adapted to different environments in the Balearic Islands. Diversity 2023, 15, 690. [Google Scholar] [CrossRef]
- Uslu, U.; Dik, B. Chemical characteristics of breeding sites of Culicoides species (Diptera: Ceratopogonidae). Vet. Parasitol. 2010, 169, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Foxi, C.; Delrio, G. Larval habitats and seasonal abundance of Culicoides biting midges found in association with sheep in northern Sardinia, Italy. Med. Vet. Entomol. 2010, 24, 199–209. [Google Scholar] [CrossRef]
- González, M.; López, S.; Mullens, B.A.; Baldet, T.; Goldarazena, A. A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Vet. Parasitol. 2013, 191, 81–93. [Google Scholar] [CrossRef]
- Černý, O.; Votýpka, J.; Svobodová, M. Spatial feeding preferences of ornithophilic mosquitoes, blackflies and biting midges. Med. Vet. Entomol. 2011, 25, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Synek, P.; Munclinger, P.; Albrecht, T.; Votýpka, J. Avian haemosporidians in haematophagous insects in the Czech Republic. Parasitol. Res. 2013, 112, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Martínez-de la Puente, J.; Martínez, J.; Rivero-de Aguilar, J.; Herrero, J.; Merino, S. On the specificity of avian blood parasites: Revealing specific and generalist relationships between haemosporidians and biting midges. Mol. Ecol. 2011, 20, 3275–3287. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-López, R.; Martínez-de La Puente, J.; Gangoso, L.; Yan, J.; Soriguer, R.C.; Figuerola, J. Do mosquitoes transmit the avian malaria-like parasite Haemoproteus? An experimental test of vector competence using mosquito saliva. Parasites Vectors 2016, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New insights from molecular data. Int. J. Parasitol. 2014, 44, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Chagas, C.R.F.; Bernotienė, R.; Himmel, T.; Harl, J.; Weissenböck, H.; Iezhova, T.A. Molecular characterization of six widespread avian haemoproteids, with description of three new Haemoproteus species. Acta Trop. 2019, 197, 105051. [Google Scholar] [CrossRef] [PubMed]
- Šujanová, A.; Špitalská, E.; Václav, R. Seasonal dynamics and diversity of haemosporidians in a natural woodland bird community in Slovakia. Diversity 2021, 13, 439. [Google Scholar] [CrossRef]
- Ellis, V.A.; Huang, X.; Westerdahl, H.; Jönsson, J.; Hasselquist, D.; Neto, J.M.; Nilsson, J.; Nilsson, J.; Hegemann, A.; Hellgren, O.; et al. Explaining prevalence, diversity and host specificity in a community of avian haemosporidian parasites. Oikos 2020, 129, 1314–1329. [Google Scholar] [CrossRef]
- Benmazouz, I.; Jokimäki, J.; Lengyel, S.; Juhász, L.; Kaisanlahti-Jokimäki, M.-L.; Kardos, G.; Paládi, P.; Kövér, L. Corvids in urban environments: A systematic global literature review. Animals 2021, 11, 3226. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Duc, M.; Iezhova, T.A. Description of Haemoproteus asymmetricus n. sp. (Haemoproteidae), with remarks on predictability of the DNA haplotype networks in haemosporidian parasite taxonomy research. Acta Trop. 2021, 218, 105905. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species; Version 2023-1. Available online: https://www.iucnredlist.org (accessed on 20 December 2023).
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef]
- Himmel, T.; Harl, J.; Pfanner, S.; Nedorost, N.; Nowotny, N.; Weissenböck, H. Haemosporidioses in wild Eurasian Blackbirds (Turdus merula) and Song Thrushes (T. philomelos): An in situ hybridization study with emphasis on exo-erythrocytic parasite burden. Malar. J. 2020, 19, 69. [Google Scholar] [CrossRef]
- Van Rooyen, J.; Lalubin, F.; Glaizot, O.; Christe, P. Avian haemosporidian persistence and co-infection in great tits at the individual level. Malar. J. 2013, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.; Ellis, V.A.; Ricklefs, R.E. Co-infections of haemosporidian and trypanosome parasites in a North American Songbird. Parasitology 2016, 143, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Marchand, R. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, Southern Vietnam. Emerg. Infect. Dis. 2011, 17, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.S.; Nelms, B.; Barker, C.M.; Reisen, W.K.; Sehgal, R.N.M.; Cornel, A.J. Avian malaria co-infections confound infectivity and vector competence assays of Plasmodium homopolare. Parasitol. Res. 2018, 117, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; Mettler, R.; Segelbacher, G.; Schaefer, H.M. Haemosporidian parasitism in the Blackcap Sylvia atricapilla in relation to spring arrival and body condition. J. Avian Biol. 2013, 44, 521–530. [Google Scholar] [CrossRef]
- Garrido-Bautista, J.; Martínez-de la Puente, J.; Ros-Santaella, J.L.; Pintus, E.; Lopezosa, P.; Bernardo, N.; Comas, M.; Moreno-Rueda, G. Habitat-dependent Culicoides species composition and abundance in Blue Tit (Cyanistes caeruleus) nests. Parasitology 2022, 149, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Bukauskaitė, D.; Žiegytė, R.; Palinauskas, V.; Iezhova, T.A.; Dimitrov, D.; Ilgūnas, M.; Bernotienė, R.; Markovets, M.Y.; Valkiūnas, G. Biting midges (Culicoides, Diptera) transmit Haemoproteus parasites of owls: Evidence from sporogony and molecular phylogeny. Parasites Vectors 2015, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.; Iezhova, T.A.; Zehtindjiev, P.; Bobeva, A.; Ilieva, M.; Kirilova, M.; Bedev, K.; Sjöholm, C.; Valkiūnas, G. Molecular characterisation of three avian haemoproteids (Haemosporida, Haemoproteidae), with the description of Haemoproteus (Parahaemoproteus) palloris n. sp. Syst. Parasitol. 2016, 93, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Križanauskienė, A.; Pérez-Tris, J.; Palinauskas, V.; Hellgren, O.; Bensch, S.; Valkiūnas, G. Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European Songbird, the Blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology 2010, 137, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Strehmann, F.; Becker, M.; Lindner, K.; Masello, J.F.; Quillfeldt, P.; Schumm, Y.R.; Farwig, N.; Schabo, D.G.; Rösner, S. Half of a forest bird community infected with haemosporidian parasites. Front. Ecol. Evol. 2023, 11, 1107736. [Google Scholar] [CrossRef]
- Bobeva, A.; Zehtindjiev, P.; Ilieva, M.; Dimitrov, D.; Mathis, A.; Bensch, S. Host preferences of ornithophilic biting midges of the genus Culicoides in the Eastern Balkans. Med. Vet. Entomol. 2015, 29, 290–296. [Google Scholar] [CrossRef]
- González, M.A.; Goiri, F.; Prosser, S.W.J.; Cevidanes, A.; Hernández-Triana, L.M.; Barandika, J.F.; Hebert, P.D.N.; García-Pérez, A.L. Culicoides species community composition and feeding preferences in two aquatic ecosystems in Northern Spain. Parasites Vectors 2022, 15, 199. [Google Scholar] [CrossRef]
- Tomazatos, A.; Jöst, H.; Schulze, J.; Spînu, M.; Schmidt-Chanasit, J.; Cadar, D.; Lühken, R. Blood-meal analysis of Culicoides (Diptera: Ceratopogonidae) reveals a broad host range and new species records for Romania. Parasites Vectors 2020, 13, 79. [Google Scholar] [CrossRef]
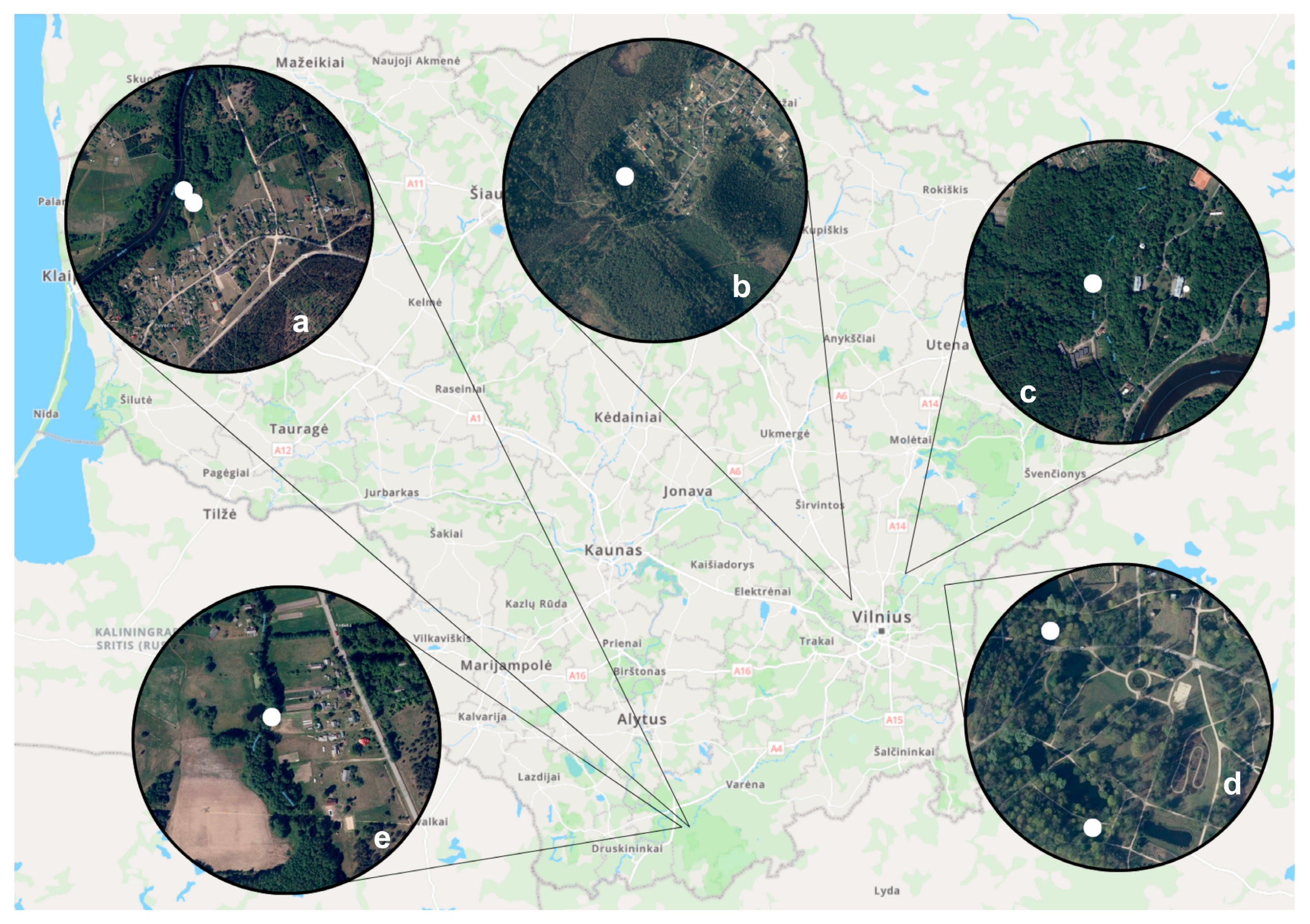

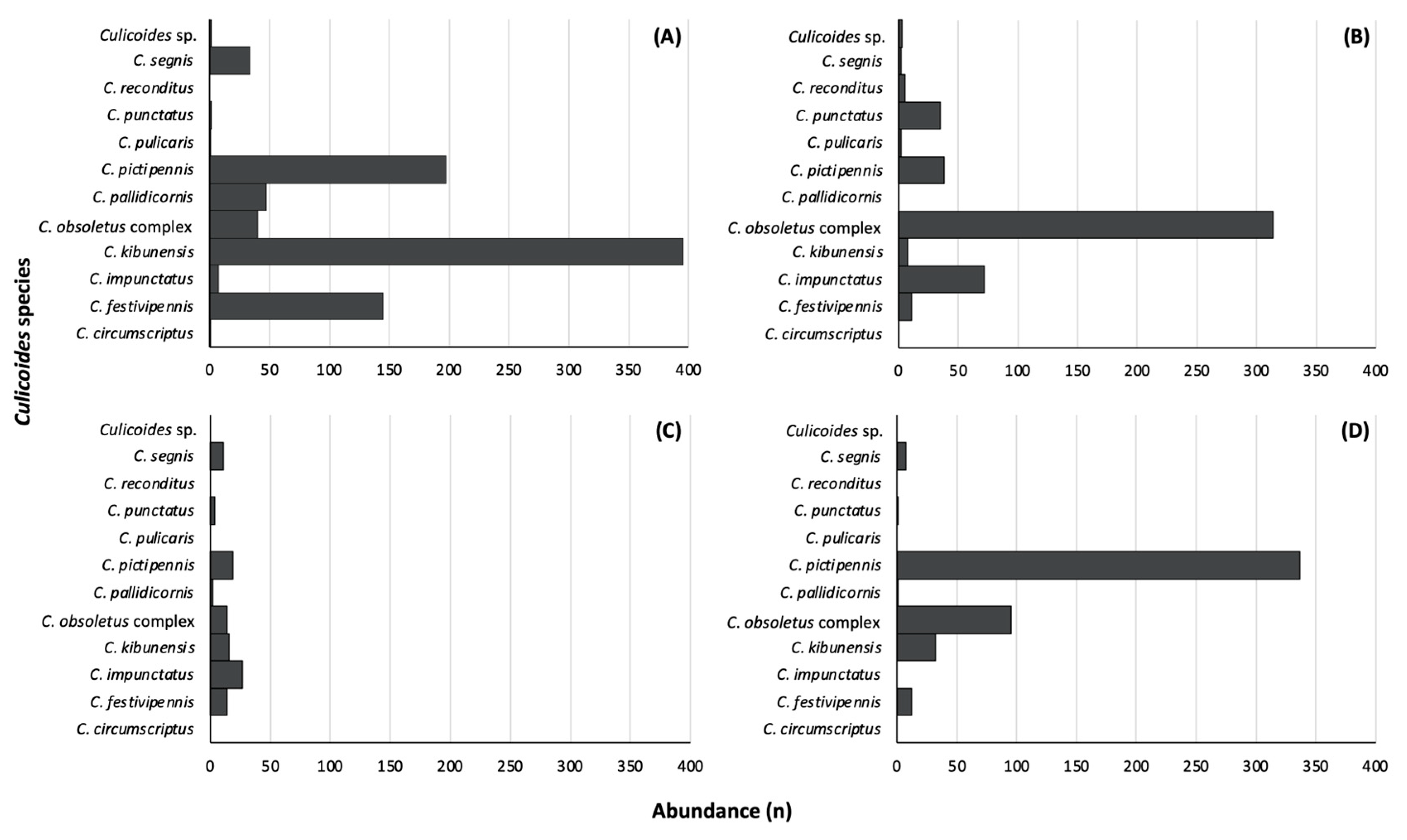
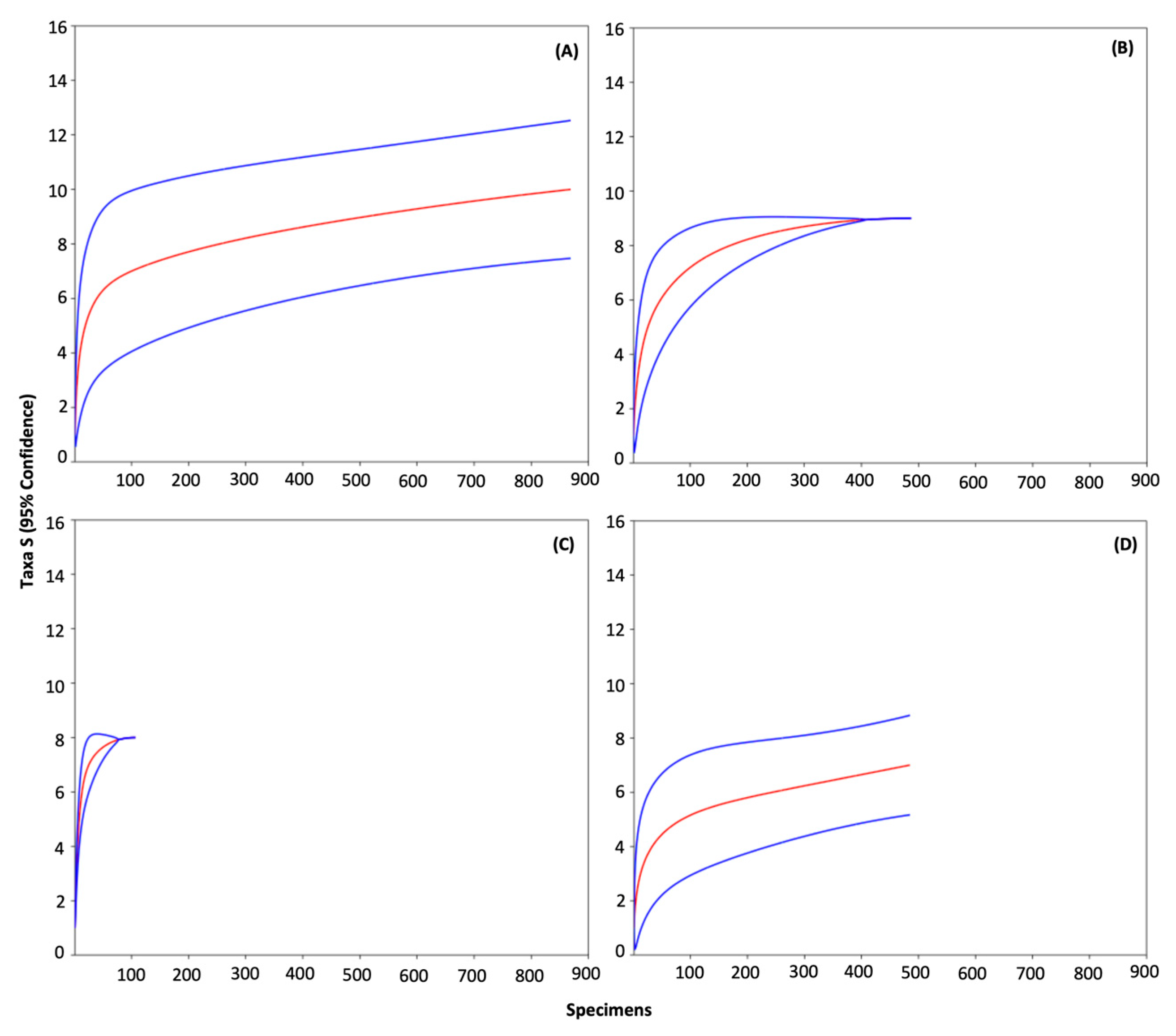

| Pi | O | K | F | I | S | Pa | Pn | R | Sp | Pu | C | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. asymmetricus TUPHI01 | 64 (10) | 19 (10) | 1 | 2 (1) | 80 (20) | ||||||||
| H. minutus TURDUS2 | 15 (2) | 10 (5) | 1 (1) | 26 (8) | |||||||||
| H. parabelopolskyi SYAT02 | 4 (3) | 1 (1) | 5 (4) | ||||||||||
| H. belopolskyi HIICT1 | 1 | 1 | 2 (2) | 4 (2) | |||||||||
| H. parabelopolskyi SYAT01 | 2 (2) | 1 (1) | 3 (3) | ||||||||||
| Haemoproteus sp. SYAT13 | 1 (1) | 1 (1) | 2 (2) | ||||||||||
| H. homominutus CUKI1 | 2 (1) | 2 (1) | |||||||||||
| Haemoproteus sp. CULPIC02 | 1 (1) | 1 (1) | |||||||||||
| H. magnus ROFI1 | 1 (1) | 1 (1) | |||||||||||
| H. homogeneae SYAT16 | 1 (1) | 1 (1) | |||||||||||
| H. majoris CCF5 | 2 | 2 | |||||||||||
| H. majoris PARUS1 | 1 | 1 | 2 | ||||||||||
| Haemoproteus sp. CCF2 | 1 | 1 | |||||||||||
| Haemoproteus sp. CULKIB04 | 1 | 1 | |||||||||||
| Haemoproteus sp. CULKIB05 | 1 | 1 | |||||||||||
| Haemoproteus sp. HAWF6 | 1 | 1 | |||||||||||
| Haemoproteus sp. CIRCUM05 | 1 | 1 | |||||||||||
| H. syrnii CULKIB01 | 1 | 1 | |||||||||||
| Haemoproteus sp. CULPIC01 | 1 | 1 | |||||||||||
| H. majoris CWT4 | 1 | 1 | |||||||||||
| Plasmodium sp. CULFES01 | 1 | 1 | |||||||||||
| Plasmodium sp. CULOBS01 | 1 | 1 | |||||||||||
| P. vaughani SYAT05 | 1 | 1 | |||||||||||
| Co-infection | 4 (1) | 1 | 1 | 1 | 1 | 8 | |||||||
| Negative | 496 | 459 | 409 | 176 | 105 | 49 | 50 | 42 | 4 | 5 | 3 | 1 | 1799 |
| Total positive | 95 (21) | 4 | 42 (19) | 6 (2) | 1 | 5 (2) | 0 | 0 | 1 (1) | 0 | 0 | 0 | 154 (45) |
| Total sampled | 591 | 463 | 451 | 182 | 106 | 54 | 50 | 42 | 5 | 5 | 3 | 1 | 1953 |
| Prevalence (%) | 15.8 | 0.9 | 9.4 | 3.3 | 1.0 | 9.3 | 0 | 0 | 20 | 0 | 0 | 0 | 7.9 |
| Study Site | n | D | C | CV | eH’ (95% CI) |
|---|---|---|---|---|---|
| Botanical Garden | 871 | 10 | 0.99 | 1.39 | 4.46 (4.20–4.72) |
| Puvočiai village | 490 | 9 | 1 | 1.74 | 3.35 (2.96–3.73) |
| Brinkiškės village | 107 | 8 | 1 | 0.50 | 6.93 (6.31–7.54) |
| Verkiai Regional Park | 485 | 7 | 0.99 | 1.64 | 2.56 (2.36–2.76) |
| BG | P | VRP | B | |||||
|---|---|---|---|---|---|---|---|---|
| Culicoides species | PCR | MIC | PCR | MIC | PCR | MIC | PCR | MIC |
| C. pictipennis | 30/197 | 12/30 | 8/38 | 0/8 | 53/337 | 7/53 | 4/19 | 2/4 |
| C. obsoletus complex | 0/40 | - a | 4/314 | 0/4 | 0/95 | - a | 0/14 | - a |
| C. kibunensis | 37/395 | 17/37 | 1/8 | 0/1 | 2/32 | 0/2 | 2/16 | 2/2 |
| C. festivipennis | 3/145 | 1/3 | 0/11 | - a | 0/12 | - a | 3/14 | 1/3 |
| C. impunctatus | 0/7 | - a | 1/73 | 0/1 | - b | - b | 0/27 | - a |
| C. segnis | 3/34 | 2/3 | 0/2 | - | 1/7 | 0/1 | 1/11 | 0/1 |
| C. pallidicornis | 0/47 | - a | - b | - b | 0/1 | - a | 0/2 | - a |
| C. punctatus | 0/2 | - a | 0/35 | - a | 0/1 | - a | 0/4 | - a |
| C. reconditus | - b | - | 1/5 | 1/1 | - b | - b | - b | - b |
| Culicoides sp. | 0/3 | - a | 0/2 | - a | - b | - b | - b | - b |
| C. pulicaris | 0/1 | - a | 0/2 | - a | - b | - b | - b | - b |
| C. circumscriptus | 0/1 | - a | - b | - b | - b | - b | - b | - b |
| Total | 73/871 | 32/73 | 15/490 | 1/15 | 485 | 7/49 | 107 | 5/5 |
| Parasite Species and Lineage | BG | P | VRP | B |
|---|---|---|---|---|
| Haemoproteus asymmetricus TUPHI01 | 37 | 6 | 39 | 4 |
| H. minutus TURDUS2 | 11 | 3 | 9 | 3 |
| H. parabelopolskyi SYAT02 | 2 | 3 | ||
| H. belopolskyi HIICT1 | 3 | 1 | ||
| H. parabelopolskyi SYAT01 | 1 | 2 | ||
| Haemoproteus sp. SYAT13 | 2 | |||
| H. homominutus CUKI1 | 2 | |||
| Haemoproteus sp. CULPIC02 | 1 | |||
| H. magnus ROFI1 | 1 | |||
| H. homogeneae SYAT16 | 1 | |||
| H. majoris CCF5 | 2 | |||
| H. majoris PARUS1 | 1 | 1 | ||
| Haemoproteus sp. CCF2 | 1 | |||
| Haemoproteus sp. CULKIB04 | 1 | |||
| Haemoproteus sp. CULKIB05 | 1 | |||
| Haemoproteus sp. HAWF6 | 1 | |||
| Haemoproteus sp. CIRCUM05 | 1 | |||
| H. syrnii CULKIB01 | 1 | |||
| Haemoproteus sp. CULPIC01 | 1 | |||
| H. majoris CWT4 | 1 | |||
| Plasmodium sp. CULFES01 | 1 | |||
| Plasmodium sp. CULOBS01 | 1 | |||
| P. vaughani SYAT05 | 1 | |||
| Co-infection | 4 | 2 | 1 | 1 |
| Negative | 798 | 475 | 429 | 97 |
| Total | 871 | 490 | 485 | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagas, C.R.F.; Duc, M.; Kazak, M.; Valavičiūtė-Pocienė, K.; Bukauskaitė, D.; Hernández-Lara, C.; Bernotienė, R. High Abundance of Haemoproteus Parasites in Culicoides (Diptera, Ceratopogonidae), with a Confirmation of Culicoides reconditus as a New Vector of These Avian Blood Parasites. Insects 2024, 15, 157. https://doi.org/10.3390/insects15030157
Chagas CRF, Duc M, Kazak M, Valavičiūtė-Pocienė K, Bukauskaitė D, Hernández-Lara C, Bernotienė R. High Abundance of Haemoproteus Parasites in Culicoides (Diptera, Ceratopogonidae), with a Confirmation of Culicoides reconditus as a New Vector of These Avian Blood Parasites. Insects. 2024; 15(3):157. https://doi.org/10.3390/insects15030157
Chicago/Turabian StyleChagas, Carolina Romeiro Fernandes, Mélanie Duc, Margarita Kazak, Kristina Valavičiūtė-Pocienė, Dovilė Bukauskaitė, Carolina Hernández-Lara, and Rasa Bernotienė. 2024. "High Abundance of Haemoproteus Parasites in Culicoides (Diptera, Ceratopogonidae), with a Confirmation of Culicoides reconditus as a New Vector of These Avian Blood Parasites" Insects 15, no. 3: 157. https://doi.org/10.3390/insects15030157






