Comparative Efficacy of Pyrethroid-Based Paints against Turkestan Cockroaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cockroaches
2.2. Insecticide Paints
2.3. Forced Exposure Bioassays
2.4. Choice-Box Experiments
2.5. Statistical Analysis
3. Results
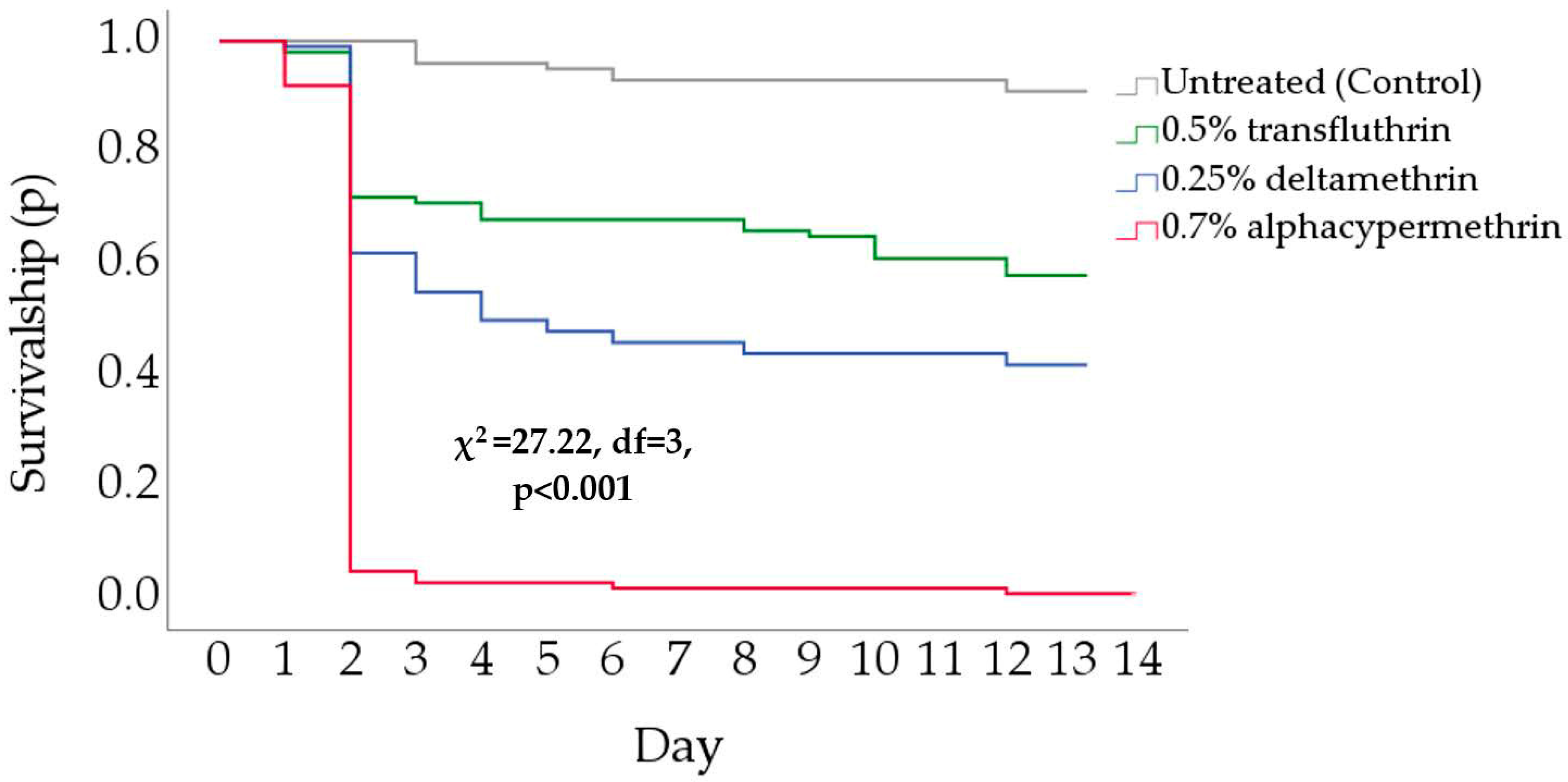
3.1. Forced Exposure
3.2. Choice Box Experiments
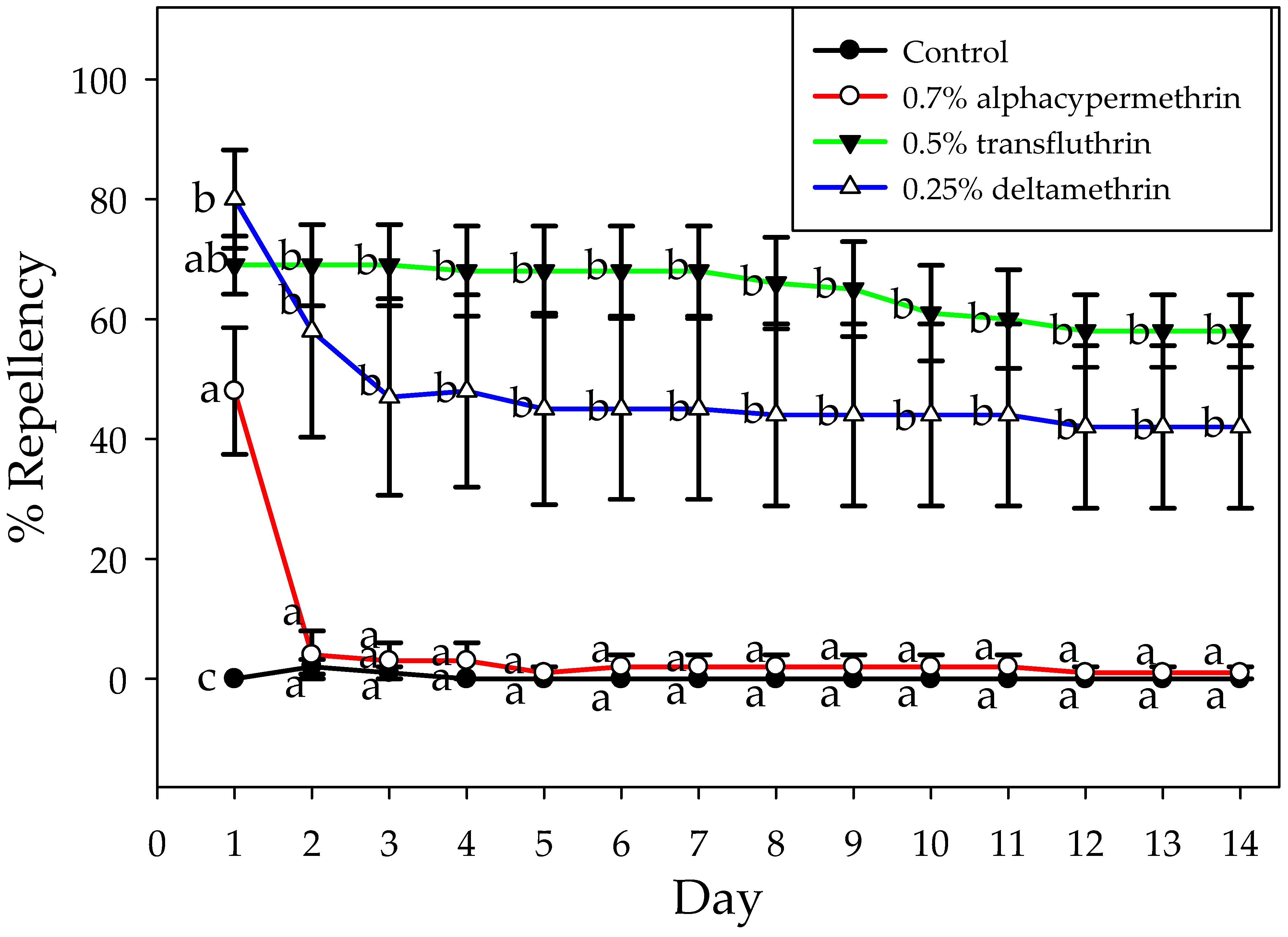
3.2.1. Repellency
3.2.2. Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, C.A. Blatta (Shelfordella) lateralis, the Turkestan cockroach (Blattoidea: Blattidae) recorded from Arizona. Bull. Entomol. Soc. Am. 1985, 31, 30. [Google Scholar] [CrossRef]
- Kassal, S.M.; Mohsen, Z.H. Species of peridomestic cockroach in Iraq. Dirasat 1994, 21, 7–13. [Google Scholar]
- Gaire, S. Toxicity and Repellency of Essential Oils on the Turkestan Cockroach (Blattodea: Blattidae). Master’s Thesis, New Mexico State University, Las Cruces, NM, USA, 2016. [Google Scholar]
- Deng, W.; Luo, X.; Ho, S.Y.W.; Liao, S.; Wang, Z.; Che, Y. Inclusion of rare taxa from Blattidae and Anaplectidae improves phylogenetic resolution in the cockroach superfamily Blattoidea. Syst. Entomol. 2023, 48, 23–39. [Google Scholar] [CrossRef]
- Kim, T.; Rust, M.K. Life history and biology of the invasive Turkestan cockroach (Dictyoptera: Blattidae). J. Econ. Entomol. 2013, 106, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-X.; Li, Q.-Q.; Zamani, A.; Che, Y.-L.; Wang, Z.-Q. Redescription of Periplaneta arabica (Bey-Bienko, 1938) (Blattodea, Blattidae), with a comparative analysis of three species of Periplaneta Burmeister, 1838 (sensu stricto). ZooKeys 2023, 1146, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Schal, C. Cockroaches. In Mallis Handbook of Pest Control, 10th ed.; Hedges, S., Moreland, D., Eds.; Mallis Handbook Co.: Cleveland, OH, USA, 2011; pp. 150–291. [Google Scholar]
- Gondhalekar, A.D.; Appel, A.G.; Thomas, G.M.; Romero, A. A review of alternative management tactics employed for the control of various cockroach species (Order: Blattodea) in the USA. Insects 2021, 12, 550. [Google Scholar] [CrossRef]
- Sutherland, A.M.; Taravati, S.; Hubble, C.; Rust, M.K.; Choe, D.-H. Outdoor baiting to control Turkestan cockroaches (Blattodea: Blattidae). In Proceedings of the Tenth International Conference on Urban Pests, Sant Adrià de Besòs, Spain, 27–29 June 2022; Bueno-Marí, R., Montalvo, T., Robinson, W.H., Eds.; CDM Creador de Motius, S.L., Mare de Deu de Montserrat 53–59. pp. 116–120. [Google Scholar]
- Gaire, S.; Romero, A. Comparative efficacy of residual insecticides against the Turkestan cockroach, Blatta lateralis, (Blattodea: Blattidae) on different substrates. Insects 2020, 11, 477. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Canale, D.M.; Spillmann, C.; Stariolo, R.; Salomón, O.D.; Blanco, S.; Segura, L.S. Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: A district-wide randomized trial. Bull. World Health Organ. 2004, 82, 196–205. [Google Scholar]
- Rust, M.K. Factors affecting control with insecticides. In Understanding and Controlling the German Cockroach; Rust, M.K., Owens, J.M., Reierson, D.A., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 149–170. [Google Scholar]
- Weston, D.P.; Holmes, R.W.; Lydy, M.J. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ. Pollut. 2009, 157, 287–294. [Google Scholar] [CrossRef]
- Antwi, F.B.; Reddy, G.V. Toxicological effects of pyrethroids on non-target aquatic insects. Environ. Toxicol. Pharmacol. 2015, 40, 915–923. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- Yazikov, D.F. The preparation and use of insecticidal lacquers and paints II. Insecticidal paints containing dieldrin and aldrin. J. Microbiol. Epidemiol. Immunobiol. 1961, 32, 711–718. [Google Scholar]
- Ogg, C.L.; Gold, R.E. Laboratory evaluation of lacquer formulated fenvalerate against German cockroaches. Insectic. Acaric. Test 1990, 15, 380. [Google Scholar] [CrossRef]
- Adetuyi, O.A.; Ajekwene, K.K.; Jabar, J.M. Production and performance evaluation of insecticide-based coatings. J. Chem. Soc. Nigeria. 2012, 37, 20–26. [Google Scholar]
- Srivastava, B.C.; Jaiswal, A.K. Evaluation of lac-based slow release pesticide formulation for the effective control of cockroach. Nat. Acad. Sci. Lett. 1999, 22, 142–145. [Google Scholar]
- Wickham, J.C. Conventional insecticides. In Understanding and Controlling the German Cockroach, 1st ed.; Rust, M.K., Owens, J.M., Reierson, D.A., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 109–148. [Google Scholar]
- Inesfly Corporation. Control of Aedes Genus Mosquitoes. Dengue, Yellow Fever, Chikungunya, Zika. Valencia, Spain. Available online: https://inesfly.com/wp-content/uploads/2021/08/INESFLY-SOLUTIONS-FOR-AEDES.pdf (accessed on 1 March 2024).
- Acharya, B.N.; Rajkumar, A.; Sunil, D.; Kavita, Y.; Pratibha, P.; Devanathan, S. Deltamethrin microencapsulation in emulsion paint binder and its long-term efficacy against dengue vector Aedes aegypti. Front. Public Health 2021, 9, 686122. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, B.; Chabi, J.; Chandre, F.; Akogbeto, M.; Hougard, J.; Carnevale, P.; Mas-Coma, S. Efficacy of an insecticide paint against malaria vectors and nuisance in West Africa—Part 2: Field evaluation. Malar. J. 2010, 9, 341. [Google Scholar] [CrossRef]
- Gorla, D.E.; Vargas-Ortiz, R.; Catalá, S.S. Control of rural house infestation by Triatoma infestans in the Bolivian Chaco using a microencapsulated insecticide formulation. Parasit. Vectors 2015, 8, 255. [Google Scholar] [CrossRef]
- Schiøler, K.L.; Alifrangis, M.; Kitron, U.; Konradsen, F. Insecticidal paints: A realistic approach to vector control? PLoS Negl. Trop. Dis. 2016, 10, e0004518. [Google Scholar] [CrossRef]
- Ebeling, W.; Wagner, R.E.; Reierson, D.A. Influence of repellency on the efficacy of blatticides. I. Learned modification of behavior of the German cockroach. J. Econ. Entomol. 1966, 59, 1374–1388. [Google Scholar]
- Machin, D.; Cheung, Y.B.; Parmar, M. Survival Analysis: A Practical Approach, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Lewis, C.T. The penetration of cuticle by insecticides. In Cuticle Techniques in Arthropods; Miller, T.A., Ed.; Springer: New York, NY, USA, 1980; pp. 367–399. [Google Scholar]
- Yako, A.B.; Hassan, S.C.; Olayinka, M.D.; Igboanugo, S.I. The Bio-efficacy of Inesfly Vesta 50 (transfluthrin 0.5% w/w) insecticidal paint: An alternative vector control intervention against Anopheles gambiae mosquitoes under laboratory condition, Keffi, Nigeria. Int. J. Mosq. Res. 2023, 10, 21–28. [Google Scholar] [CrossRef]
- Aznar-Alemany, Ò.; Eljarrat, E. Introduction to pyrethroid insecticides: Chemical structures, properties, mode of action and use. In Pyrethroid Insecticides: The Handbook of Environmental Chemistry; Eljarrat, E., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar]
- Malaithong, N.; Tisgratog, R.; Tainchum, K.; Prabaripai, A.; Juntarajumnong, W.; Bangs, M.J.; Chareonviriyaphap, T. Locomotor behavioral responses of Anopheles minimus and Anopheles harrisoni to alpha-cypermethrin in Thailand. J. Am. Mosq. Control Assoc. 2011, 27, 217–226. [Google Scholar] [CrossRef]
- Kennedy, J.S. The excitant and repellent effects on mosquitoes of sub-lethal contacts with DDT. Bull. Entomol. Res. 1947, 37, 593–607. [Google Scholar] [CrossRef]
- Smith, J.L.; Rust, M.K. Vapor activity of insecticides used for subterranean termite (Isoptera: Rhinotermitidae) control. J. Econ. Entomol. 1991, 84, 181–184. [Google Scholar] [CrossRef]
- Aiello, F.; Simons, M.G.; van Velde, J.W.; Dani, P. New insights into the degradation path of deltamethrin. Molecules 2021, 26, 3811. [Google Scholar] [CrossRef]
- Haynes, K.F. Sublethal effects of insecticides on the behavioral responses of insects. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef]
- Romero, A.; Potter, M.F.; Haynes, K.F. Behavioral responses of the bed bug to insecticide residues. J. Med. Entomol. 2009, 46, 51–57. [Google Scholar] [CrossRef]
- Knight, R.L.; Rust, M.K. Repellency and efficacy of various insecticides against foraging ant workers in laboratory colonies of the Argentine ant, Iridomyrmex humilis (Mayr) (Hymenoptera: Formicidae). J. Econ. Entomol. 1990, 83, 1402–1408. [Google Scholar] [CrossRef]
- Diotaiuti, L.; Marques-Penido, C.; Saraiva de Araujo, H.; Schofield, C.J.; Teixeira-Pinto, C. Excito-repellency effect of deltamethrin on triatomines under laboratory conditions. Rev. Soc. Bras. Med. Trop. 2000, 33, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Hii, J.; Chareonviriyaphap, T. Transfluthrin and metofluthrin as effective repellents against pyrethroid susceptible and pyrethroid-resistant Aedes aegypti (L.) (Diptera: Culicidae). Insects 2023, 14, 767. [Google Scholar] [CrossRef]
- Maloney, K.M.; Ancca-Juarez, J.; Salazar, R.; Borrini-Mayori, K.; Niemierko, M.; Yukich, J.O.; Naquira, C.; Keating, J.A.; Levy, M.Z. Comparison of insecticidal paint and deltamethrin against Triatoma infestans (Hemiptera: Reduviidae) feeding and mortality in simulated natural conditions. J. Vector Ecol. 2013, 38, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, M.C.; Sanders, C.J.; Hladik, M.L. Measured efficacy, bioaccumulation, and leaching of a transfluthrin-based insecticidal paint: A case study with a nuisance, nonbiting aquatic insect. Pest Manag. Sci. 2022, 78, 5413–5422. [Google Scholar] [CrossRef]
- Trask, J.R.; Harbourt, C.M.; Miller, P.; Cox, M.; Jones, R.; Hendley, P.; Lam, C. Washoff of cypermethrin residues from slabs of external building material surfaces using simulated rainfall. Environ. Toxicol. Chem. 2014, 33, 302–307. [Google Scholar] [CrossRef]
- Chadwick, P.R. The activity of some pyrethroids against Periplaneta americana and Blattela germanica. Pestic. Sci. 1979, 10, 32–38. [Google Scholar] [CrossRef]
- Metcalf, R.L. Insecticides in pest management. In Introduction to Pest Management, 2nd ed.; Metcalf, R.L., Luckman, W.H., Eds.; John Wiley & Sons: New York, NY, USA, 1982; pp. 217–277. [Google Scholar]



| Substrate | Treated Area (cm2) | Total Paint (g) | Insecticide | mg Active Ingredient/Treated Area |
|---|---|---|---|---|
| PVC | 50.2 | 0.88 | 0.25% deltamethrin | 2.2 |
| 0.5% transfluthrin | 4.4 | |||
| 0.7% alphacypermethrin | 6.16 | |||
| Metal | 50.2 | 0.7 | 0.25% deltamethrin | 1.75 |
| 0.5% transfluthrin | 3.5 | |||
| 0.7% alphacypermethrin | 4.9 | |||
| Concrete | 50.2 | 1.76 | 0.25% deltamethrin | 4.4 |
| 0.5% transfluthrin | 8.8 | |||
| 0.7% alphacypermethrin | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, M.; Agnew, J.L.; Romero, A. Comparative Efficacy of Pyrethroid-Based Paints against Turkestan Cockroaches. Insects 2024, 15, 171. https://doi.org/10.3390/insects15030171
Salazar M, Agnew JL, Romero A. Comparative Efficacy of Pyrethroid-Based Paints against Turkestan Cockroaches. Insects. 2024; 15(3):171. https://doi.org/10.3390/insects15030171
Chicago/Turabian StyleSalazar, Miguel, John L. Agnew, and Alvaro Romero. 2024. "Comparative Efficacy of Pyrethroid-Based Paints against Turkestan Cockroaches" Insects 15, no. 3: 171. https://doi.org/10.3390/insects15030171







