Molecular Techniques for the Detection and Differentiation of Host and Parasitoid Species and the Implications for Fruit Fly Management
Abstract
:1. Introduction
2. Molecular Methods Employed in Biological Control of Arthropods
2.1. DNA-Barcoding
| Technique | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| DNA barcoding | PCR amplification and sequencing of a genetic marker (usually the mitochondrial COI gene) |
|
| [13,44,45,46] |
| Specific PCR | Targeted assay giving a presence/absence result for a particular genus or species |
|
| [48,62,63,64] |
| Size differential PCR | Employs generic PCR primers but yields amplicons that differ in length. Usually targets the intergenic transcribed spacer regions (ITS) |
|
| [39] |
| PCR-RFLP | Involves discrimination of species based on restriction profile of amplicons. |
|
| [13,48,65,66,67] |
| Multiplex PCR | Combines multiple primer sets with different specificities in a single assay |
|
| [68,69,70,71] |
| RAPD | Uses random primers to generate multiple PCR products resulting in a fingerprint for a particular species |
|
| [72,73,74,75,76,77] |
| AFLP | Involves ligation of adaptors to digested DNA followed by PCR amplification using primers that are partially adaptor and partially gene-specific |
|
| [78,79] |
| Microsatellite analysis | Involves PCR amplification of multiple reiterated repeat-containing loci that are hypervariable due to slipped-strand mispairing mutations |
|
| [32,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95] |
| Quantitative PCR | Short regions of DNA are PCR amplified and products are detected either with SYBR green (double stranded DNA dye) or via specific probes labeled with fluorescent dyes |
|
| [96,97] |
| LAMP | Employs 3 sets of specific primers used for amplification under isothermal conditions. Yields a ladder of amplicons on electrophoresis or amplicons can be detected using SYBR green |
|
| [98,99] |
2.2.PCR-based Approaches: PCR-RFLP, Multiplex PCR, RAPD and AFLP
2.3. Microsatellite Markers

2.4. New Technologies
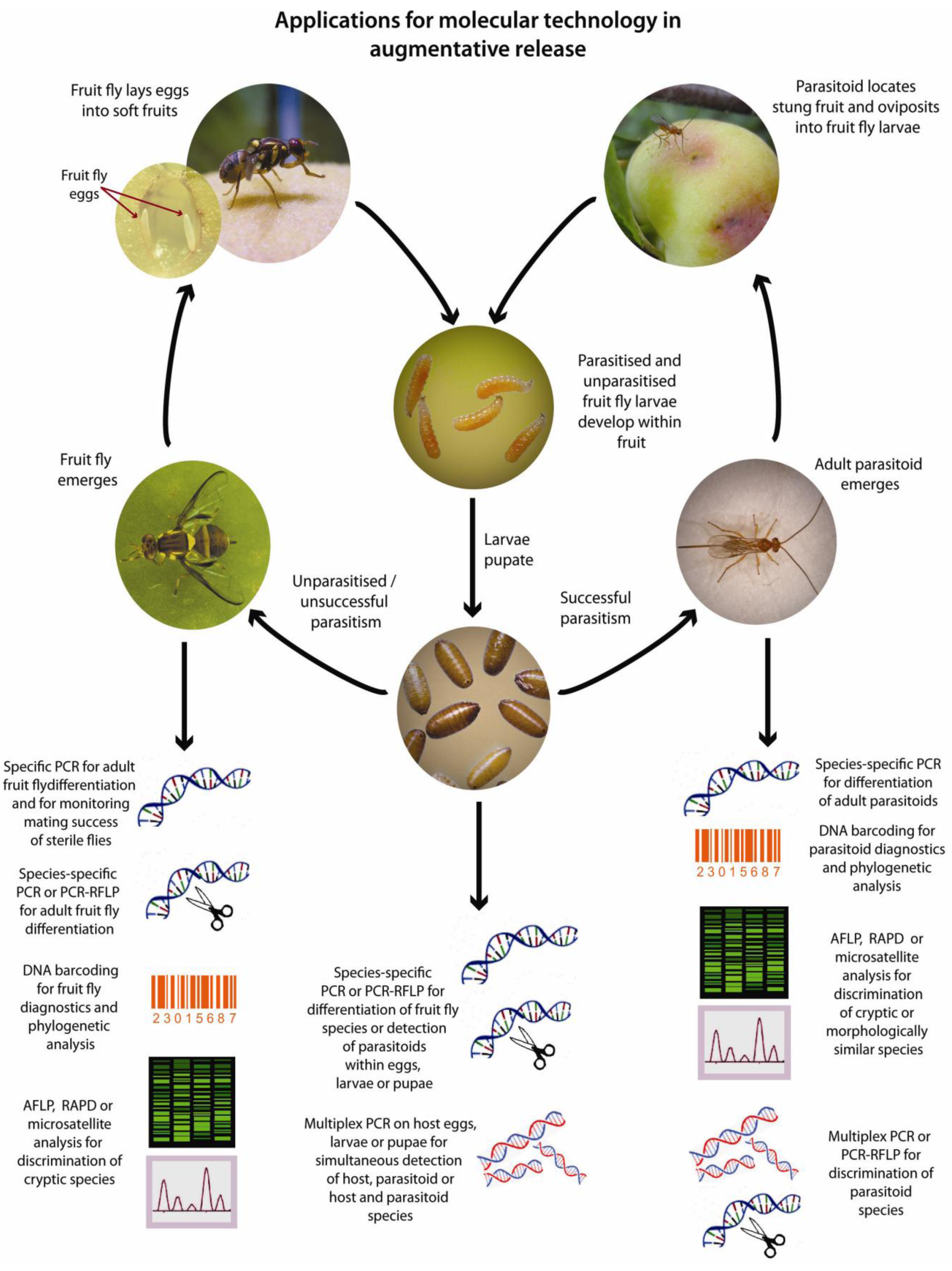
3. Conclusions and Implications of Molecular Technology for Augmentative Fruit Fly Control Programs
Acknowledgements
References
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Binomics; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Compere, G. A few facts concerning the fruit flies of the world. Calif. Dep. Agric. Mon. Bull. 1912, 1, 709–730. [Google Scholar]
- Silvestri, F. Viaggio in africa per cecare parassiti di mosche dei frutti. Boll. Lab. Zool. Gen. Agric. Portici 1913, 8, 1–164. [Google Scholar]
- Montoya, P.; Liedo, P.; Benrey, B.; Cancino, J.; Barrera, J.F.; Sivinski, J.; Aluja, M. Biological control of Anastrepha spp. (diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (ashmead) (hymenoptera: Braconidae). Biol. Control 2000, 18, 216–224. [Google Scholar]
- Sime, K.R.; Daane, K.M.; Wang, X.G.; Johnson, M.W.; Messing, R.H. Evaluation of fopius arisanus as a biological control agent for the olive fruit fly in california. Agric. For. Entomol. 2008, 10, 423–431. [Google Scholar] [CrossRef]
- Rendon, P.; Sivinski, J.; Holler, T.; Bloem, K.; Lopez, M.; Martinez, A.; Aluja, M. The effects of sterile males and two braconid parasitoids, Fopius arisanus (Sonan) and Diachasmimorpha krausii (Fullaway) (Hymenoptera), on caged populations of Mediterranean fruit flies, Ceratitis capitata (wied.) (diptera: Tephritidae) at various sites in Guatemala. Biol. Control 2006, 36, 224–231. [Google Scholar]
- Wong, T.T.Y.; Ramadan, M.M.; Herr, J.C.; McInnis, D.O. Suppression of a Mediterranean fruit fly (diptera: Tephritidae) population with concurrent parasitoid and sterile fly releases in Kula, Maui, Hawaii. J. Econ. Entomol. 1992, 85, 1671–1681. [Google Scholar]
- Clarke, A.R.; Powell, K.S.; Weldon, C.W.; Taylor, P.W. The ecology of Bactrocera tryoni (diptera: Tephritidae): What do we know to assist pest management? Ann. Appl. Biol. 2011, 158, 26–54. [Google Scholar] [CrossRef] [Green Version]
- Wharton, R.A.; Marsh, P.M. New world opiinae (hymenoptera: Braconidae) parasitic on tephritidae (diptera). J. Wash. Acad. Sci. 1978, 68, 147–167. [Google Scholar]
- Wharton, R.A.; Gilstrap, F.E. Key to and status of opiine braconid (hymenoptera) parasitoids used in biological control of Ceratitis and Dacus s. L. (diptera: Tephritidae). Ann. Entomol. Soc. Am. 1983, 76, 721–742. [Google Scholar]
- Carmichael, A.E.; Wharton, R.A.; Clarke, A.R. Opiine parasitoids (hymenoptera: Braconidae) of tropical fruit flies (diptera: Tephritidae) of the australian and south pacific region. Bull. Entomol. Res. 2005, 95, 545–569. [Google Scholar]
- Clausen, C.P. Tephritidae (trypetidae, trupaneidae). In Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review. Agricultural Handbook No. 480; Clausen, C.P., Ed.; USDA Agricultural Research Service: Washington, DC, USA, 1978. [Google Scholar]
- Rugman-Jones, P.F.; Wharton, R.A.; van Noort, T.; Stouthamer, R. Molecular differentiation of the Psyttalia concolor (szepligeti) species complex (hymenoptera: Braconidae) associated with olive fly, Bactrocera oleae (rossi) (diptera: Tephritidae), in africa. Biol. Control 2009, 49, 17–26. [Google Scholar] [CrossRef]
- Wharton, R.A. Biological Control of Fruit-Infesting Tephritidae. Fruit Flies Econ. Importance 1989, 87, 323–332. [Google Scholar]
- Wharton, R.A. Classical Biological Control of Fruit-Infesting Tephritidae. In World Crop Pests: Fruit Flies, Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 303–313. [Google Scholar]
- Quimio, G.M.; Walter, G.H. Host preference and host suitability in an egg-pupal fruit fly parasitoid, Fopius arisanus (sonan) (hym., braconidae). J. Appl. Entomol. Z. Angew. Entomol. 2001, 125, 135–140. [Google Scholar]
- Jervis, M.A. Insects as Natural Enemies: A Practical Perspective; Springer: Dordrecht, The Netherlands, 2007; pp. 74–164. [Google Scholar]
- Vreysen, M.J.B.; Robinson, A.S. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. A review. Agron. Sustain. Dev. 2011, 31, 233–250. [Google Scholar] [CrossRef]
- Spinner, J.E.; Cowling, A.M.; Gurr, G.M.; Jessup, A.J.; Reynolds, O.L. Parasitoid fauna of queensland fruit fly, Bactrocera tryoni froggatt (diptera: Tephritidae) in inland new south wales, australia and their potential for use in augmentative biological control. Aust. J. Entomol. 2011, 50, 445–452. [Google Scholar] [CrossRef]
- Argov, Y.; Gazit, Y. Biological control of the mediterranean fruit fly in Israel: Introduction and establishment of natural enemies. Biol. Control 2008, 46, 502–507. [Google Scholar] [CrossRef]
- Baranowski, R.; Glenn, H.; Sivinski, J. Biological control of the caribbean fruit fly (diptera:Tephritidae). Fla. Entomol. 1991, 76, 245–251. [Google Scholar]
- Sivinski, J.M.; Calkins, C.O.; Baranowski, R.; Harris, D.; Brambila, J.; Diaz, J.; Burns, R.E.; Holler, T.; Dodson, G. Suppression of a caribbean fruit fly Anastrepha suspensa (loew) (diptera: Tephritidae) population through augmented releases of the parasitoid Diachasmimorpha longicaudata (ashmead) (hymenoptera: Braconidae). Biol. Control 1996, 6, 177–185. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Gomez, E.; Villasenor, A. Parasitoid releases in the control of Ceratitis capitata (diptera: Tephritidae) outbreaks in coffee growing zones of chiapas, Mexico. Vedalia 2005, 12, 85–89. [Google Scholar]
- Wong, T.T.; Ramadan, M.M.; McInnis, D.O.; Mochizuki, N.; Nishimoto, J.I.; Herr, J.C. Augmentative releases of Diachasmimorpha tryoni (hymenoptera: Braconidae) to suppress a mediterranean fruit fly (diptera: Tephritidae) population in Kula, Maui, Hawaii. Biol. Control 1991, 1, 2–7. [Google Scholar] [CrossRef]
- Sivinski, J.; Jeronimo, F.; Holler, T. Development of aerial releases of Diachasmimorpha tryoni (cameron) (hymenoptera: Braconidae), a parasitoid that attacks the mediterranean fruit fly, Ceratitis capitata (wiedermann) (diptera: Tephritidae), in the guatemalan highlands. Biocontrol Sci. Technol. 2000, 10, 15–25. [Google Scholar] [CrossRef]
- Bautista, R.C.; Mochizuki, N.; Spencer, J.P.; Harris, E.J.; Ichimura, D.M. Mass-rearing of the tephritid fruit fly parasitoid Fopius arisanus (hymenoptera: Braconidae). Biol. Control 1999, 15, 137–144. [Google Scholar] [CrossRef]
- Purcell, M.F. Contribution of biological control to integrated pest management of tephritid fruit flies in the tropics and subtropics. Integr. Pest Manag. Rev. 1998, 3, 63–83. [Google Scholar] [CrossRef]
- Purcell, M.F.; Daniels, K.M.; Whitehand, L.C.; Messing, R.H. Improvement of quality-control methods for augmentative releases ofthe fruit fly parasitoids, Diachasmimorpha longicaudata and Psyttalia fletcheri (hymenoptera: Braconidae). Biocontrol Sci. Technol. 1994, 4, 155–166. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Harvey, J.A.; Kamp, A.F.D.; Wagenaar, R.; Gols, R.; Kostenko, O.; Fortuna, T.; Engelkes, T.; Vet, L.E.M.; van der Putten, W.H.; et al. Behaviour of male and female parasitoids in the field: Influence of patch size, host density, and habitat complexity. Ecol. Entomol. 2010, 35, 341–351. [Google Scholar] [CrossRef]
- Lewis, W.J.; Vet, L.E.M.; Tumlinson, J.H.; Vanlenteren, J.C.; Papaj, D.R. Variations in parasitoid foraging behaviour—Essential element of a sound biological control theory. Environ. Entomol. 1990, 19, 1183–1193. [Google Scholar]
- Garcia-Medel, D.; Sivinski, J.; Diaz-Fleischer, F.; Ramirez-Romero, R.; Aluja, M. Foraging behavior by six fruit fly parasitoids (hymenoptera: Braconidae) released as single- or multiple-species cohorts in field cages: Influence of fruit location and host density. Biol. Control 2007, 43, 12–22. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Stratikopoulos, E.E.; Drosopoulou, E.; Kakani, E.G.; Mavragani-Tsipidou, P.; Zacharopoulou, A.; Mathiopoulos, K.D. Isolation and characterization of microsatellite markers from the olive fly, bactrocera oleae, and their cross-species amplification in the tephritidae family. BMC Genomics 2008, 9, 618. [Google Scholar] [CrossRef]
- Wharton, R.A.; Trostle, M.K.; Messing, R.H.; Copeland, R.S.; Kimani-Njogu, S.W.; Lux, S.; Overholt, W.A.; Mohamed, S.; Sivinski, J. Parasitoids of medfly, ceratitis capitata, and related tephritids in kenyan coffee: A predominantly koinobiont assemblage. Bull. Entomol. Res. 2000, 90, 517–526. [Google Scholar]
- Wang, X.; Messing, R.H. Intra- and interspecific competition by Fopius arisanus and D iachasmimorpha tryoni (hymenoptera: Braconidae), parasitoids of tephritid fruit flies. Biol. Control 2003, 27, 251–259. [Google Scholar] [CrossRef]
- Greenstone, M.H. Molecular methods for assessing insect parasitism. Bull. Entomol. Res. 2006, 96, 1–13. [Google Scholar] [CrossRef]
- Zaldivar-Riveron, A.; Mori, H.; Quicke, D.L.J. Systematics of the cyclostome subfamilies of braconid parasitic wasps (hymenoptera: Ichneumonoidea): A simultaneous molecular and morphological bayesian approach. Mol. Phylogenet. Evol. 2006, 38, 130–145. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Kuhlmann, U.; Gillott, C.; Erlandson, M. Parasitoids, predators and pcr: The use of diagnostic molecular markers in biological control of arthropods. J. Appl. Entomol. 2007, 131, 225–240. [Google Scholar] [CrossRef]
- Malausa, J.-C.; Blanchet, A.; Bon, M.-C.; Cheyppe-Buchmann, S.; Groussier-Bout, G.; Jones, W.; Pickett, C.; Ris, N.; Roche, M.; Thaon, M.; et al. Introductions of the african parasitoid Psyttalia lounsburyi in south of france for classical biological control of Bactrocera oleae. IOBC/WPRS Bull. 2010, 53, 49–55. [Google Scholar]
- Stouthamer, R.; Hu, J.; van Kan, F.J.P.M.; Platner, G.R.; Pinto, J.D. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 1999, 43, 421–440. [Google Scholar] [CrossRef]
- Muirhead, K.A.; Murphy, N.P.; Sallam, N.; Donnellan, S.C.; Austin, A.D. Phylogenetics and genetic diversity of the cotesia flavipes complex of parasitoid wasps (hymenoptera: Braconidae), biological control agents of lepidopteran stemborers. Mol. Phylogenet. Evol. 2012, 63, 904–914. [Google Scholar] [CrossRef]
- Mills, N.J.; Kean, J.M. Behavioural studies, molecular approaches and modeling: Methodological contributions to biological control success. Biol. Control 2010, 52, 255–262. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar]
- Liu, L.; Liu, J.; Wang, Q.; Ndayiragije, P.; Ntahimpera, A.; Nkubaye, E.; Yang, Q.; Li, Z. Identification of Bactrocera invadens (diptera: Tephritidae) from burundi, based on morphological characteristics and DNA barcode. Afr. J. Biotechnol. 2011, 10, 13623–13630. [Google Scholar]
- Blacket, M.J.; Semeraro, L.; Malipatil, M.B. Barcoding queensland fruit flies (Bactrocera tryoni): Impediments and improvements. Mol. Ecol. Resour. 2012, 12, 428–436. [Google Scholar] [CrossRef]
- Barr, N.B.; Islam, M.S.; de Meyer, M.; McPheron, B.A. Molecular identification of Ceratitis capitata (diptera: Tephritidae) using DNA sequences of the coi barcode region. Ann. Entomol. Soc. Am. 2012, 105, 339–350. [Google Scholar] [CrossRef]
- Santos, A.M.; Besnard, G.; Quicke, D.L. Applying DNA barcoding for the study of geographical variation in host-parasitoid interactions. Mol. Ecol. Resour. 2011, 11, 46–59. [Google Scholar] [CrossRef]
- Quicke, D.L.; Alex Smith, M.; Janzen, D.H.; Hallwachs, W.; Fernandez-Triana, J.; Laurenne, N.M.; Zaldivar-Riveron, A.; Shaw, M.R.; Broad, G.R.; Klopfstein, S.; et al. Utility of the DNA barcoding gene fragment for parasitic wasp phylogeny (hymenoptera: Ichneumonoidea): Data release and new measure of taxonomic congruence. Mol. Ecol. Resour. 2012, 12, 676–685. [Google Scholar] [CrossRef]
- Jenkins, C.; Micallef, J.L.; Fell, S.A.; Reynolds, O.E. Department of Primary Industries New South Wales: Sydney, Australia, 2012; Unpublished work.
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ceccarelli, F.S.; Sharkey, M.J.; Zaldivar-Riveron, A. Species identification in the taxonomically neglected, highly diverse, neotropical parasitoid wasp genus Notiospathius (braconidae: Doryctinae) based on an integrative molecular and morphological approach. Mol. Phylogenet. Evol. 2012, 62, 485–495. [Google Scholar] [CrossRef]
- Derocles, S.A.P.; Le Ralec, A.; Plantegenest, M.; Chaubet, B.; Cruaud, C.; Cruaud, A.; Rasplus, J.Y. Identification of molecular markers for DNA barcoding in the aphidiinae (hym. Braconidae). Mol. Ecol. Resour. 2012, 12, 197–208. [Google Scholar]
- Smith, M.A.; Wood, D.M.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D. DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (diptera, Tachinidae) are not all generalists. Proc. Natl. Acad. Sci. USA 2007, 104, 4967–4972. [Google Scholar]
- Smith, M.A.; Woodley, N.E.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D. DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (diptera: Tachinidae). Proc. Natl. Acad. Sci. USA 2006, 103, 3657–3662. [Google Scholar]
- Janzen, D.H.; Hajibabaei, M.; Burns, J.M.; Hallwachs, W.; Remigio, E.; Hebert, P.D. Wedding biodiversity inventory of a large and complex lepidoptera fauna with DNA barcoding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1835–1845. [Google Scholar] [CrossRef]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Cox, A.J.; Hebert, P.D. Colonization, extinction, and phylogeographic patterning in a freshwater crustacean. Mol. Ecol. 2001, 10, 371–386. [Google Scholar] [CrossRef]
- Wu, Y.; McPheron, B.A.; Wu, J.-J.; Li, Z.-H. Genetic relationship of the melon fly, Bactrocera cucurbitae (diptera: Tephritidae) inferred from mitochondrial DNA. Insect Sci. 2012, 19, 195–204. [Google Scholar] [CrossRef]
- Moritz, C.; Cicero, C. DNA barcoding: Promise and pitfalls. PLoS Biol. 2004, 2, e354. [Google Scholar] [CrossRef] [Green Version]
- Tautz, D.; Arctander, P.; Minelli, A.; Thomas, R.H.; Vogler, A.P. DNA points the way ahead in taxonomy. Nature 2002, 418, 479. [Google Scholar]
- Blaxter, M.L. The promise of a DNA taxonomy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 669–679. [Google Scholar] [CrossRef]
- Saccone, C.; de Giorgi, C.; Gissi, C.; Pesole, G.; Reyes, A. Evolutionary genomics in metazoa: The mitochondrial DNA as a model system. Gene 1999, 238, 195–209. [Google Scholar] [CrossRef]
- Amornsak, W.; Gordh, G.; Graham, G. Detecting parasitized eggs with polymerase chain reaction and DNA sequence of Trichogramma australicum girault (hymenoptera: Encyrtidae). Aust. J. Entomol. 1998, 37, 174–179. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Riddick, E.W.; Williams III, L.; Schotozko, D.J.; Logarzo, G.A.; Jackson, C.G. Potential of detection and identification of nymphal parasitoids (hymenoptera: Braconidae) of Lygus bugs (heteroptera: Miridae) by using polymerase chain reaction and ITS2 sequence analysis techniques. Ecol. Popul. Biol. 2004, 97, 743–752. [Google Scholar]
- Zhu, Y.C.; Williams, L., III. Detecting the egg parasitoid Anaphes iole (hymenoptera: Mymaridae) in tarnished plant bug (heteroptera: Miridae) eggs by using a molecular approach. Ecol. Popul. Biol. 2002, 95, 359–365. [Google Scholar]
- Ashfaq, M.; Braun, L.; Hegedus, D.; Erlandson, M. Estimating parasitism levels in Lygus spp. (hemiptera: Miridae) field populations using standard and molecular techniques. Biocontrol Sci. Technol. 2004, 14, 731–735. [Google Scholar]
- Barr, N.B.; Copeland, R.S.; de Myer, M.; Masiga, D.; Kibogo, H.G.; Billah, M.K.; Osir, E.O.; Wharton, R.A.; McPheron, B.A. Molecular diagnostics of economically important Ceratitis fruit fly species (diptera: Tephritidae) in africa using pcr and RFLP analyses. Bull. Entomol. Res. 2006, 96, 505–521. [Google Scholar]
- Ratcliffe, S.T.; Robertson, H.M.; Jones, C.J.; Bollero, G.A.; Weinzierl, R.A. Assessment of parasitism of house fly and stable fly (diptera: Muscidae) pupae by pteromalid (hymenoptera: Pteromalidae) parasitoids using a polymerase chain reaction assay. J. Med. Entomol. 2002, 39, 52–60. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Kuhlmann, U.; Haye, T.; Gillott, C.; Erlandson, M. A single-step multiplex PCR assay for the detection of european Peristenus spp., parasitoids of Lygus spp. Biocontrol Sci. Technol. 2005, 15, 481–495. [Google Scholar]
- Traugott, M.; Symondson, W.O. Molecular analysis of predation on parasitized hosts. Bull. Entomol. Res. 2008, 98, 223–231. [Google Scholar]
- Traugott, M.; Bell, J.R.; Raso, L.; Sint, D.; Symondson, W.O. Generalist predators disrupt parasitoid aphid control by direct and coincidental intraguild predation. Bull. Entomol. Res. 2012, 102, 239–247. [Google Scholar] [CrossRef]
- Moreno-Ripoll, R.; Gabarra, R.; Symondson, W.O.; King, R.A.; Agusti, N. Trophic relationships between predators, whiteflies and their parasitoids in tomato greenhouses: A molecular approach. Bull. Entomol. Res. 2012, 102, 415–423. [Google Scholar] [CrossRef]
- Baruffi, L.; Damiani, G.; Guglielmino, C.R.; Bandi, C.; Malacrida, A.R.; Gasperi, G. Polymorphism within and between populations of Ceratitis capitata: Comparison between rapd and multilocus enzyme electrophoresis data. Heredity (Edinb) 1995, 74, 425–437. [Google Scholar]
- Alaoui, A.; Imoulan, A.; El Alaoui-Talibi, Z.; El Meziane, A. Genetic structure of mediterranean fruit fly (Ceratitis capitata) populations from moroccan endemic forest of Argania spinosa. Int. J. Agric. Biol. 2010, 12, 291–298. [Google Scholar]
- Gasperi, G.; Bonizzoni, M.; Gomulski, L.M.; Murelli, V.; Torti, C.; Malacrida, A.R.; Guglielmino, C.R. Genetic differentiation, gene flow and the origin of infestations of the medfly, Ceratitis capitata. Genetica 2002, 116, 125–135. [Google Scholar]
- Sonvico, A.; Manso, F.; Quesada-Allue, L.A. Discrimination between the immature stages of Ceratitis capitata and Anastrepha fraterculus (diptera:Tephritidae) populations by random amplified polymorphic DNA polymerase chain reaction. J. Econ. Entomol. 1996, 89, 1208–1212. [Google Scholar]
- Zahran, M.M.; El-Fandary, O.O.; Mahmoud, Y.A. Assessment of genetic variability and genotyping of Ceratitis capitata and Bactrocera zonata by molecular techniques. Arch. Phytopathol.Plant Prot. 2009, 42, 92–98. [Google Scholar] [CrossRef]
- Karam, N.; Guglielmino, C.R.; Bertin, S.; Gomulski, L.M.; Bonomi, A.; Baldacchino, F.; Simeone, V.; Malacrida, A.R. Rapd analysis in the parasitoid wasp Psyttalia concolor reveals mediterranean population structure and provides scar markers. Biol. Control 2008, 47, 22–27. [Google Scholar] [CrossRef]
- Billah, M.K.; Kimani-Njogu, S.W.; Wharton, R.A.; Woolley, J.B.; Masiga, D. Comparison of five allopatric fruit fly parasitoid populations (Psyttalia species) (hymenoptera: Braconidae) from coffee fields using morphometric and molecular methods. Bull. Entomol. Res. 2008, 98, 63–75. [Google Scholar]
- Kakouli-Duarte, T.; Casey, D.G.; Burnell, A.M. Development of a diagnostic DNA probe for the fruit flies ceratitis capitata and ceratitis rosa (diptera: Tephritidae) using amplified fragment-length polymorphism. J. Econ. Entomol. 2001, 94, 989–997. [Google Scholar] [CrossRef]
- Kinnear, M.W.; Bariana, H.S.; Sved, J.A.; Frommer, M. Polymorphic microsatellite markers for population analysis of a tephritid pest species, Bactrocera tryoni. Mol. Ecol. 1998, 7, 1489–1495. [Google Scholar] [CrossRef]
- Yu, H.; Frommer, M.; Robson, M.K.; Meats, A.W.; Shearman, D.C.; Sved, J.A. Microsatellite analysis of the queensland fruit fly Bactrocera tryoni (diptera: Tephritidae) indicates spatial structuring: Implications for population control. Bull. Entomol. Res. 2001, 91, 139–147. [Google Scholar]
- Gilchrist, A.S.; Meats, A.W. The genetic structure of populations of an invading pest fruit fly, Bactrocera tryoni, at the species climatic range limit. Heredity (Edinb) 2010, 105, 165–172. [Google Scholar]
- Dai, S.M.; Lin, C.C.; Chang, C. Polymorphic microsatellite DNA markers from the oriental fruit fly Bactrocera dorsalis (hendel). Mol. Ecol. Notes 2004, 4, 629–631. [Google Scholar] [CrossRef]
- Zygouridis, N.E.; Augustinos, A.A.; Zalom, F.G.; Mathiopoulos, K.D. Analysis of olive fly invasion in california based on microsatellite markers. Heredity (Edinb) 2009, 102, 402–412. [Google Scholar]
- Wu, Y.; Li, Y.; Ruiz-Arce, R.; McPheron, B.A.; Wu, J.; Li, Z. Microsatellite markers reveal population structure and low gene flow among collections of Bactrocera cucurbitae (diptera: Tephritidae) in asia. J. Econ. Entomol. 2011, 104, 1065–1074. [Google Scholar] [CrossRef]
- Fritz, A.H.; Schable, N. Microsatellite loci from the caribbean fruit fly, Anastrepha suspensa (diptera: Tephritidae). Mol. Ecol. Notes 2004, 4, 443–445. [Google Scholar] [CrossRef]
- Boykin, L.M.; Shatters, R.C.; Hall, D.C.; Dean, D.; Beerli, P. Genetic variation of Anastrepha suspensa (diptera: Tephritidae) in florida and the caribbean using microsatellite DNA markers. Mol. Entomol. 2010, 103, 2214–2222. [Google Scholar]
- Augustinos, A.A.; Stratikopoulos, E.E.; Zacharopoulou, A.; Mathiopoulos, K.D. Polymorphic microsatellite markers in the olive fruit fly, Bactrocera oleae. Mol. Ecol. Notes 2002, 2, 278–280. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Mamuris, Z.; Stratikopoulos, E.E.; D’Amelio, S.; Zacharopoulou, A.; Mathiopoulos, K.D. Microsatellite analysis of olive fly populations in the mediterranean indicates a westward expansion of the species. Genetica 2005, 125, 231–241. [Google Scholar] [CrossRef]
- Silva, J.G.; Meixner, M.D.; McPheron, B.A.; Steck, G.J.; Sheppard, W.S. Recent mediterranean fruit fly (diptera: Tephritidae) infestations in florida—a genetic perspective. J. Econ. Entomol. 2003, 96, 1711–1718. [Google Scholar] [CrossRef]
- Baliraine, F.N.; Bonizzoni, M.; Osir, E.O.; Lux, S.A.; Mulaa, F.J.; Zheng, L.; Gomulski, L.M.; Gasperi, G.; Malacrida, A.R. Comparative analysis of microsatellite loci in four fruit fly species of the genus Ceratitis (diptera: Tephritidae). Bull. Entomol. Res. 2003, 93, 1–10. [Google Scholar]
- Forbes, A.A.; Powell, T.H.; Lobo, N.F.; Noor, M.A.; Feder, J.L. Permanent genetic resources: Polymorphic microsatellite loci for Diachasma alloeum (hymenoptera: Braconidae). Mol. Ecol. Resour. 2008, 8, 373–376. [Google Scholar] [CrossRef]
- Vorsino, A.; Wieczorek, A.; Wright, M.; Messing, R. Isolation and characterization of 12 microsatellite loci from the fruit fly (diptera: Tephritidae) parasitoid Diachasmimorpha tryoni (hymenoptera: Braconidae). Mol. Ecol. Resour. 2010, 10, 1106–1108. [Google Scholar] [CrossRef]
- Bon, M.C.; Jones, W.; Hurard, C.; Loiseau, A.; Ris, N.; Pickett, C.; Estoup, A.; Fauvergue, X. Identification of 21 polymorphic microsatellites in the african parasitoid wasp, Psyttalia lounsburyi (silvestri) (hymenoptera: Braconidae). Mol. Ecol. Resour. 2008, 8, 930–932. [Google Scholar] [CrossRef]
- Forbes, A.A.; Powell, T.H.; Stelinski, L.L.; Smith, J.J.; Feder, J.L. Sequential sympatric speciation across trophic levels. Science 2009, 323, 776–779. [Google Scholar] [CrossRef]
- Yu, D.J.; Zhang, G.M.; Chen, Z.L.; Zhang, R.J.; Yin, W.Y. Rapid identification of Bactrocera latifrons (dipt., tephritidae) by real-time pcr using sybr green chemistry. Green Chem. 2004, 128, 670–676. [Google Scholar]
- Yu, D.J.; Chen, Z.-L.; Zhang, R.-J.; Yin, W.-Y. Real-time qualitative pcr for the inspection and identification of Bactrocera philippinensis and Bactrocera occipitalis (diptera: Tephritidae) using sybr green assay. System 2005, 53, 73–78. [Google Scholar]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar]
- Huang, C.-G.; Hsu, J.-C.; Haymer, D.S.; Lin, G.-C.; Wu, W.-J. Rapid identification of the mediterranean fruit fly (diptera: Tephritidae) by loop-mediated isothermal amplification. Mol. Entomol. 2009, 102, 1239–1246. [Google Scholar]
- Derocles, S.A.; Plantegenest, M.; Simon, J.C.; Taberlet, P.; LE Ralec, A. A universal method for the detection and identification of Aphidiinae parasitoids within their aphid hosts. Mol. Ecol. Resour. 2012, 12, 634–645. [Google Scholar] [CrossRef]
- Lim, P.E.; Tan, J.; Suana, I.W.; Eamsobhana, P.; Yong, H.S. Distinct genetic lineages of bactrocera caudata (insecta: Tephritidae) revealed by coi and 16s DNA sequences. PLoS One 2012, 7, e37276. [Google Scholar]
- Virgilio, M.; Backeljau, T.; Barr, N.; Meyer, M.D. Molecular evaluation of nominal species in the Ceratitis fasciventris, c. Anonae, c. Rosa complex (diptera: Tephritidae). Mol. Phylogenet. Evol. 2008, 48, 270–280. [Google Scholar]
- Virgilio, M.; de Meyer, M.; White, I.M.; Backeljau, T. African Dacus (diptera: Tephritidae: Molecular data and host plant associations do not corroborate morphology based classifications. Mol. Phylogenet. Evol. 2009, 51, 531–539. [Google Scholar] [CrossRef]
- Boykin, L.M.; Shatters, R.G.; Hall, D.G.; Burns, R.E.; Franqui, R.A. Analysis of host preference and geographical distribution of Anastrepha suspensa (diptera: Tephritidae) using phylogenetic analyses of mitochondrial cytochrome oxidase I DNA sequence data. Bull. Entomol. Res. 2006, 96, 457–469. [Google Scholar]
- Connect, Barcode of life; Project: Tephritid Barcode Initiative (TBI). Available online: http://connect.barcodeoflife.net/profiles/blogs/project-tephritid-barcode (accessed on 8 August 2012).
- Wei, S.J.; Shi, M.; Sharkey, M.J.; van Achterberg, C.; Chen, X.X. Comparative mitogenomics of braconidae (insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to holometabolous insects. BMC Genomics 2010, 11, 371. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar]
- Chamberlain, J.S.; Gibbs, R.A.; Ranier, J.E.; Nguyen, P.N.; Caskey, C.T. Deletion screening of the duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988, 16, 11141–11156. [Google Scholar] [CrossRef]
- Jones, D.B.; Giles, K.L.; Chen, Y.; Shufran, K.A. Estimation of hymenopteran parasitism in cereal aphids by using molecular markers. J. Econ. Entomol. 2005, 98, 217–221. [Google Scholar]
- Weathersbee, A.A., III; Shufran, K.A.; Panchal, T.D.; Dang, P.M.; Evans, G.A. Detection and differentiation of parasitoids (hymenoptera: Aphidiidae and aphelinidae) of the brown citrus aphid (homoptera: Aphididae): Species-specific polymerase chain reaction amplification of 18s rdna. Ecol. Popul. Biol. 2004, 97, 286–292. [Google Scholar]
- Agusti, N.; Bourguet, D.; Spataro, T.; Delos, M.; Eychenne, N.; Folcher, L.; Arditi, R. Detection, identification and geographical distribution of european corn borer larval parasitoids using molecular markers. Mol. Ecol. 2005, 14, 3267–3274. [Google Scholar] [CrossRef]
- Hrcek, J.; Miller, S.E.; Quicke, D.L.J.; Smith, M.A. Molecular detection of trophic links in a complex insect host-parasitoid food web. Mol. Ecol. Resour. 2011, 11, 786–794. [Google Scholar] [CrossRef]
- San Andres, V.; Urbaneja, A.; Sabater-Munoz, B.; Castanera, P. A novel molecular approach to assess mating success of sterile Ceratitis capitata (diptera: Tephritidae) males in sterile insect technique programs. Mol. Entomol. 2007, 100, 1444–1449. [Google Scholar]
- Selkoe, K.A.; Toonen, R.J. Microsatellites for ecologists: A practical guide to using and evaluating microsatellite markers. Ecol. Lett. 2006, 9, 615–629. [Google Scholar] [CrossRef]
- Levinson, G.; Gutman, G.A. Slipped-strand mispairing: A major mechanism for DNA sequence evolution. Mol. Biol. Evol. 1987, 4, 203–221. [Google Scholar]
- Hoshino, A.A.; Bravo, J.P.; Nobile, P.M.N.; Morelli, K.A. Microsatellites as Tools for Genetic Diversity Analysis. In Genetic Diversity in Microorganisms; Caliskan, M., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Temnykh, S.; DeClerck, G.; Lukashova, A.; Lipovich, L.; Cartinhour, S.; McCouch, S. Computational and experimental analysis of microsatellites in rice (oryza sativa l.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001, 11, 1441–1452. [Google Scholar]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting est databases for the development and characterization of gene-derived ssr-markers in barley (Hordeum vulgare L.). Theor. App. Genet. 2003, 106, 411–422. [Google Scholar]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Kofler, R.; Schlotterer, C.; Lelley, T. Sciroko: A new tool for whole genome microsatellite search and investigation. Bioinformatics 2007, 13, 1683–1685. [Google Scholar]
- Sharma, P.C.; Grover, A.; Kahl, G. Mining microsatellites in eukaryotic genomes. Trends Biotechnol. 2007, 25, 490–498. [Google Scholar] [CrossRef]
- Gardner, M.G.; Fitch, A.J.; Bertozzi, T.; Lowe, A.J. Rise of the machines—Recommendations for ecologists when using next generation sequencing for microsatellite development. Mol. Ecol. Resour. 2011, 11, 1093–1101. [Google Scholar] [CrossRef]
- Guichous, E.; Lagache, L.; Wagner, S.; Chaumeil, P.; Leger, P.; Lepais, O.; Lepoittevin, C.; Malausa, T.; Revardel, E.; Salin, F.; Petit, R.J. Current trends in microsatellite genotyping. Mol. Ecol. Resour. 2011, 11, 591–611. [Google Scholar] [CrossRef]
- Miller, M.R.; Dunham, J.P.; Amores, A.; Cresko, W.A.; Johnson, E.A. Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Res. 2007, 17, 240–248. [Google Scholar] [CrossRef]
- Baird, N.A.; Etter, P.D.; Atwood, T.S.; Currey, M.C.; Shiver, A.L.; Lewis, Z.A.; Selker, E.U.; Cresko, W.A.; Johnson, E.A. Rapid snp discovery and genetic mapping using sequenced rad markers. PLoS One 2008, 3, e3376. [Google Scholar]
- Hohenlohe, P.A.; Bassham, S.; Etter, P.D.; Stiffler, N.; Johnson, E.A.; Cresko, W.A. Population genomics of parallel adaptation in threespine stickleback using sequenced radtags. PLoS Genet. 2010, 6, e1000862. [Google Scholar] [CrossRef]
- Peterson, B.K.; Hare, E.E.; Iyer, V.N.; Storage, S.; Conner, L.; Papaj, D.R.; Kurashima, R.; Jang, E.; Eisen, M.B. Big genomes facilitate the comparative identification of regulatory elements. PLoS One 2009, 4, e4688. [Google Scholar]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest radseq: An inexpensive method for de novo snp discovery and genotyping in model and non-model species. PLoS One 2012, 7, e37135. [Google Scholar]
- Spanos, L.; Koutroumbas, G.; Kotsyfakis, M.; Louis, C. The mitochondrial genome of the mediterranean fruit fly, Ceratitis capitata. Insect Mol. Biol. 2000, 9, 139–144. [Google Scholar] [CrossRef]
- Nardi, F.; Carapelli, A.; Boore, J.L.; Roderick, G.K.; Dallai, R.; Frati, F. Domestication of olive fly through a multi-regional host shift to cultivated olives: Comparative dating using complete mitochondrial genomes. Mol. Phylogenet. Evol. 2010, 57, 678–686. [Google Scholar] [CrossRef]
- Bactrocera dorsalis Mitochondrion, Complete Genome. Available online: http://www.ncbi.nlm.nih.gov/nuccore/DQ845759.1 (accessed 8 August 2012).
- Ragland, G.J.; Egan, S.P.; Feder, J.L.; Berlocher, S.H.; Hahn, D.A. Developmental trajectories of gene expression reveal candidates for diapause termination: A key life-history transition in the apple maggot fly Rhagoletis pomonella. J. Exp. Biol. 2011, 214, 3948–3959. [Google Scholar] [CrossRef]
- Gomulski, L.M.; Dimopoulos, G.; Xi, Z.; Soares, M.B.; Bonaldo, M.F.; Malacrida, A.R.; Gasperi, G. Gene discovery in an invasive tephritid model pest species, the mediterranean fruit fly, Ceratitis capitata. BMC Genomics 2008, 9, 243. [Google Scholar]
- Werren, J.H.; Richards, S.; Desjardins, C.A.; Niehuis, O.; Gadau, J.; Colbourne, J.K. The Nasonia Genome Working Group. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 2010, 327, 343–348. [Google Scholar]
- Rivers, D.B.; Ruggiero, L.; Yoder, Y.A. Venom from Nasonia vitripennis: A model for understanding the roles of venom during parasitism by ectoparasitoids. Trends Entomol. 1999, 2, 1–17. [Google Scholar]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2009, 55, 151–169. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jenkins, C.; Chapman, T.A.; Micallef, J.L.; Reynolds, O.L. Molecular Techniques for the Detection and Differentiation of Host and Parasitoid Species and the Implications for Fruit Fly Management. Insects 2012, 3, 763-788. https://doi.org/10.3390/insects3030763
Jenkins C, Chapman TA, Micallef JL, Reynolds OL. Molecular Techniques for the Detection and Differentiation of Host and Parasitoid Species and the Implications for Fruit Fly Management. Insects. 2012; 3(3):763-788. https://doi.org/10.3390/insects3030763
Chicago/Turabian StyleJenkins, Cheryl, Toni A. Chapman, Jessica L. Micallef, and Olivia L. Reynolds. 2012. "Molecular Techniques for the Detection and Differentiation of Host and Parasitoid Species and the Implications for Fruit Fly Management" Insects 3, no. 3: 763-788. https://doi.org/10.3390/insects3030763
APA StyleJenkins, C., Chapman, T. A., Micallef, J. L., & Reynolds, O. L. (2012). Molecular Techniques for the Detection and Differentiation of Host and Parasitoid Species and the Implications for Fruit Fly Management. Insects, 3(3), 763-788. https://doi.org/10.3390/insects3030763






