Insecticide Effect of Zeolites on the Tomato Leafminer Tuta absoluta (Lepidoptera: Gelechiidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Insects
2.3. Insecticide Materials
2.4. Topical and Residual Bioassays
2.5. Choice Tests for Oviposition Behavior
2.6. Data Analysis
3. Results
3.1. Topical and Residual Bioassays
3.2. Choice Tests for Oviposition Behavior
4. Discussion
4.1. Topical and Residual Toxicity
4.2. Choice Tests for Oviposition Behavior
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moreno, S.C.; Carvalho, G.A.; Picanco, M.C.; Morais, E.G.F.; Pereira, R.M. Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Pest Manag. Sci. 2012, 68, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narvaez-Vasquez, C.A.; Gonzalez-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Seplyarsky, V.; Weiss, M.; Haberman, A. Tuta absoluta Povolny (Lepidoptera: Gelechiidae), a new invasive species in Israel. Phytoparasitica 2010, 38, 445–446. [Google Scholar] [CrossRef]
- Urbaneja, A.; Montón, H.; Mollá, O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Entomol. 2009, 133, 292–296. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Kilic, T. First record of Tuta absoluta in Turkey. Phytoparasitica 2010, 38, 243–244. [Google Scholar] [CrossRef]
- Urbaneja, A.; González-Cabrera, J.; Arnó, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, V.; Moekens, R.; Berckmoes, E.; Wittemans, L.; De Vis, R.; Beck, B.; Nuyttens, D.; Casteels, H.; Tirry, L.; De Clercq, P. Control of the invasive leafminer Tuta absoluta by leaf application of entomopathogenic nematodes. IOBC-WPRS Bull. 2014, 102, 219. [Google Scholar]
- Filho, M.M.; Vilela, E.F.; Jhan, J.M.; Attygalle, A.; Svatos, A.; Meinwald, J. Initial studies of mating disruption of the tomato moth Tuta absoluta (Lepidoptera: Gelechiidae) using synthetic sex phermone. J. Braz. Chem. Soc. 2000, 11, 621–628. [Google Scholar] [CrossRef]
- Siqueira, H.A.A.; Guedes, R.N.C.; Picanço, M.C. Cartap resistance and synergism in populations of Tuta absoluta (Lep., Gelechiidae). J. Appl. Entomol. 2000, 124, 233–238. [Google Scholar] [CrossRef]
- Siqueira, H.A.A.; Guedes, R.N.C.; Picanço, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2001, 2, 147–153. [Google Scholar] [CrossRef]
- Lietti, M.M.M.; Botto, E.; Alzogaray, R.A. Insecticide Resistance in Argentine Populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, P.C.; Picanço, M.C.; Pereira, E.J.G.; Martins, J.C.; Chediak, M.; Guedes, R.N.C. Spatial and temporal variation in the control failure likelihood of the tomato leaf miner, Tuta absoluta. Ann. Appl. Biol. 2013, 162, 50–59. [Google Scholar] [CrossRef]
- Gacemi, A.; Guenaoui, Y. Efficacy of Emamectin Benzoate on Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) Infesting a Protected Tomato Crop in Algeria. Acad. J. Entomol. 2012, 5, 37–40. [Google Scholar]
- López, J.M.; Artín, L.M.; López, A.; Correia, R.; González, F.; Sanz, E.; Gallardo, M.; Cantus, J.M. Affirm® (Emamectin), a new weapon against Tuta absoluta and other lepidopteran caterpillars. Phytoma-Spain 2010, 217, 5. [Google Scholar]
- Campos, M.R.; Silva, T.B.M.; Silva, W.M.; Silva, J.E.; Siqueira, H.A.A. Spinosyn resistance in the tomato borer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2015, 88, 405–412. [Google Scholar] [CrossRef]
- Payra, P.; Dutta, P.K. Zeolites: A primer. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 1–19. [Google Scholar]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Cordeiro, E.M.G.; Rosado, J.F.; Guedes, R.N.C. Egg exposure to pyripoxyfen in the tomato leaf miner Tuta absoluta: Ovicidal activity or behavioural-modulated hatching mortality? Ann. Appl. Biol. 2012, 160, 35–42. [Google Scholar] [CrossRef]
- Beament, J.W.L. The role of cuticle and egg-shell membranes in the penetration of insecticides. Ann. Appl. Biol. 1952, 39, 142–143. [Google Scholar] [CrossRef]
- Smith, E.H.; Salkeld, E.H. The use and action of ovicides. Annu. Rev. Entomol. 1966, 11, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.E.; Lopez, J.D.; Lan, Y.; Fritz, B.K.; Hoffman, W.C.; Duke, S.E. Novaluron as an ovicide for bollworm on cotton: Deposition and efficacy of fieldscale aerial applications. J. Cotton Sci. 2010, 14, 99–106. [Google Scholar]
- Koppel, A.L.; Herbert, D.A., Jr.; Kuhar, T.P.; Malone, S.; Arrington, M. Efficacy of selected insecticides against eggs of Euchistus servus and Acrosternum hilare (Hemiptera: Pentatomidae) and the egg parasitoid Telenomus podisi (Hymenopera: Scelionidae). J. Econ. Entomol. 2011, 104, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zschintzsch, J.; O’Brien, R.D.; Smith, E.H. The relation between uptake and toxicity of organophosphates for eggs of the large milkweed bug. J. Econ. Entomol. 1965, 58, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Furneaux, P.J.S.; Mackay, A.L. The composition, structure, and formation of the chorion and the vitelline membrane of the insect egg-shell. In The Insect Integument; Hepburn, H.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 157–176. [Google Scholar]
- Al-Dosary, M.M.; Al-Bekairi, A.M.; Moursy, E.B. Morphology of the egg shell and the developing embryo of the Red Palm Weevil, Rhynchophorus ferrugineus. Saudi J. Biol. Sci. 2010, 17, 177–183. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, C.; Ferrer, F.; Leus, K.; Spanoghe, P. Removal of pesticides from aqueous solutions by adsorption on zeolites as solid adsorbents. Adsorpt. Sci. Technol. 2015, 33, 457–485. [Google Scholar] [CrossRef] [Green Version]
- Van Der Walt, A.; Du Plessis, H.; Van den Berg, J. Using morphological characteristics to distinguish between male and female larvae and pupae of the groundnut leafminer, Aproaerema modicella (Deventer) (Lepidoptera: Gelechiidae). South Afr. J. Plant Soil 2008, 25, 182–184. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Efficacy Testing of Household Insecticide Products; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Kramer, L.W.; Mulla, S.M. Oviposition attractants and repellents of mosquitoes: Oviposition responses of Culex mosquito to organic infusions. Environ. Entomol. 1979, 8, 1111–1117. [Google Scholar] [CrossRef]
- Trisyono, A.; Puttler, B.; Chippendale, G.M. Effect of the ecdysone agonists, methoxyfenozide and tebufenozide, on the lady beetle, Coleomegilla maculata. Entomol. Exp. Appl. 2000, 94, 103–105. [Google Scholar] [CrossRef]
- Consoli, F.L.; Botelho, P.S.M.; Parra, J.R.P. Selectivity of insecticides to the egg parasitoid Trichogramma. galloi Zucchi, 1988, (Hym., Trichogrammatidae). J. Appl. Entomol. 2001, 125, 37–43. [Google Scholar] [CrossRef]
- Galvan, T.L.; Koch, R.L.; Hutchison, W.D. Toxicity of commonly used insecticides in sweet corn and soybean to multicolored Asian lady beetle (Coleoptera: Coccinellidae). J. Econ. Entomol. 2005, 98, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Nation, J.L. Reproduction. In Insect Physiology and Biochemistry; Nation, J.L., Ed.; CRC Press: Boca, FL, USA, 2008; pp. 497–498. [Google Scholar]
- Fogel, M.N.; Schneider, M.I. Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Dagli, F.; Bahsi, S.U. Topical and residual toxicity of six pesticides to Orius majusculus. Phytoparasitica 2009, 37, 399–405. [Google Scholar] [CrossRef]
- Hoffmann, E.J.; Middleton, S.M.; Wise, J.C. Ovicidal Activity of Organophosphate, Oxadiazine, Neonicotinoid and Insect Growth Regulator Chemistries on Northern Strain Plum Curculio, Conotrachelus nenupha. J. Insect Sci. 2008. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Lazzari, C.; Diotaiuti, L.; Lorenzo, M.G. The effect of relative humidity on the behaviour and development of Triatoma brasiliensis. Phys. Entomol. 2002, 27, 142–147. [Google Scholar] [CrossRef]
- Norhisham, A.R.; Abood, F.; Rita, M.; Hakeem, K.R. Effect of humidity on egg hatchability and reproductive biology of the bamboo borer (Dinoderus minutus Fabricius). Springerplus 2013, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A.; Singer, M.S. Contrasting responses to desiccation and starvation by eggs and neonates of two Lepidoptera. Phys. Biochem. Zoo 2001, 74, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Adiyodi, K.G.; Adiyodi, R.G. Morphology and cytology of the accessory sex glands in invertebrates. Int. Rev. Cytol. 1976, 43, 353–398. [Google Scholar]
- Hilker, M.; Stein, C.; Schroeder, R.; Varama, M.; Mumm, R. Insect egg deposition induces defence responses in Pinus sylvestris: Characterisation of the elicitor. J. Exp. Biol. 2005, 20, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Meiners, T. Early herbivore alert: Insect eggs induce plant defense. J. Chem. Ecol. 2006, 32, 1379–1397. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.A.; Davidowitz, G.; Woods, H.A. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 2009, 212, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A. Water loss and gas exchange by eggs of Manduca sexta: Trading off costs and benefits. J. Insect Physiol. 2010, 56, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Balbyshev, N.F.; Lorenzen, J.H. Hypersensitivity and egg drop, a novel mechanism of host–plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1997, 90, 652–657. [Google Scholar] [CrossRef]
- Little, D.; Gouhier-Darimont, C.; Bruessow, F.; Reymond, P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant. Physiol. 2007, 143, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; De Vay, J.E. Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera: Pieridae). Oecologia 1987, 71, 631–632. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hortic. Rev. 2005, 31, 1–44. [Google Scholar]
- Unruh, T.R.; Knight, A.L.; Upton, J.; Glenn, D.M.; Puterka, G.J. Particle films for suppression of the Codling Moth (Lepidoptera: Tortricidae) in Apple and Pear Orchards. J. Econ. Entomol. 2000, 93, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Larentzaki, E.; Shelton, A.M.; Plate, J. Effect of kaolin particle film on Thrips tabaci (Thysanoptera: Thripidae), oviposition, feeding and development on onions: A lab and field case study. Crop Prot. 2008, 27, 727–734. [Google Scholar] [CrossRef]
- Bengochea, P.; Saelices, R.; Amor, F.; Adan, A.; Budia, F.; del Estal, P.; Vinuela, E.; Medina, P. Non-target effects of kaolin and coppers applied on olive trees for the predatory lacewing Chrysoperla carnea. Biocontrol. Sci. Technol. 2014, 24, 625–640. [Google Scholar] [CrossRef]
- Temerak, S.A. Ovicidal activity of the natural bio-product spinosad through field observation of tagged egg masses of Spodoptera littoralis on cotton in five Governorates of Egypt Assiut. J. Agric. Sci. 2005, 36, 85–95. [Google Scholar]
- Hanan, S.A.; Samya, Z.S. Effects of certain insecticides on eggs of Spodoptera Littoralis. Egypt J. Agric. Res. 2014, 92, 875–883. [Google Scholar]
- Boiteau, G.; Noronha, C. Topical, residual and ovicidal contact toxicity of three reduced-risk insecticides against the European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae), on potato. Pest Manag. Sci. 2007, 63, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Dow AgroSciences. Conserve SC Turf and Ornamental. Available online: http://www.dowagro.com/turf/products/insecticides/conserve.htm (accessed on 5 September 2015).
- Huang, J.; Walker, E.D.; Giroux, P.Y.; Vulule, J.; Miller, J.R. Ovipositional site selection by Anopheles gambiae: Influences of substrate moisture and texture. Med. Vet. Entomol. 2005, 19, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Guha, L.; Seenivasagan, T.; Bandyopadhyay, P.; Iqbal, S.T.; Sathe, M.; Sharma, P.; Parashar, B.D.; Kaushik, M.P. Oviposition and flight orientation response of Aedes aeqypti to certain aromatic aryl hydrazono esters. Parasitol. Res. 2012, 111, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Städler, E. Plant chemicals important for egg deposition in herbivorous insects. In Chemoecology of Insect Eggs and Egg Deposition; Hilker, M., Meiners, T., Eds.; Blackwell Publishing Ltd.: Berlin, Germany, 2002; pp. 171–197. [Google Scholar]
- Reddy, P. Disguising the leaf surface. In Recent Advances in Crop Protection; Reddy, P., Ed.; Springer: New York, NY, USA, 2012; pp. 91–102. [Google Scholar]
 ,
,  ), 4000 (
), 4000 (  ,
,  ), and 20,000 (
), and 20,000 (  ,
,  ) mg·L−1. Asterisks indicate no significant differences (p < 0.05) between eggs and eggs + larvae mortality (n = 8).
) mg·L−1. Asterisks indicate no significant differences (p < 0.05) between eggs and eggs + larvae mortality (n = 8).
 ,
,  ), 4000 (
), 4000 (  ,
,  ), and 20,000 (
), and 20,000 (  ,
,  ) mg·L−1. Asterisks indicate no significant differences (p < 0.05) between eggs and eggs + larvae mortality (n = 8).
) mg·L−1. Asterisks indicate no significant differences (p < 0.05) between eggs and eggs + larvae mortality (n = 8).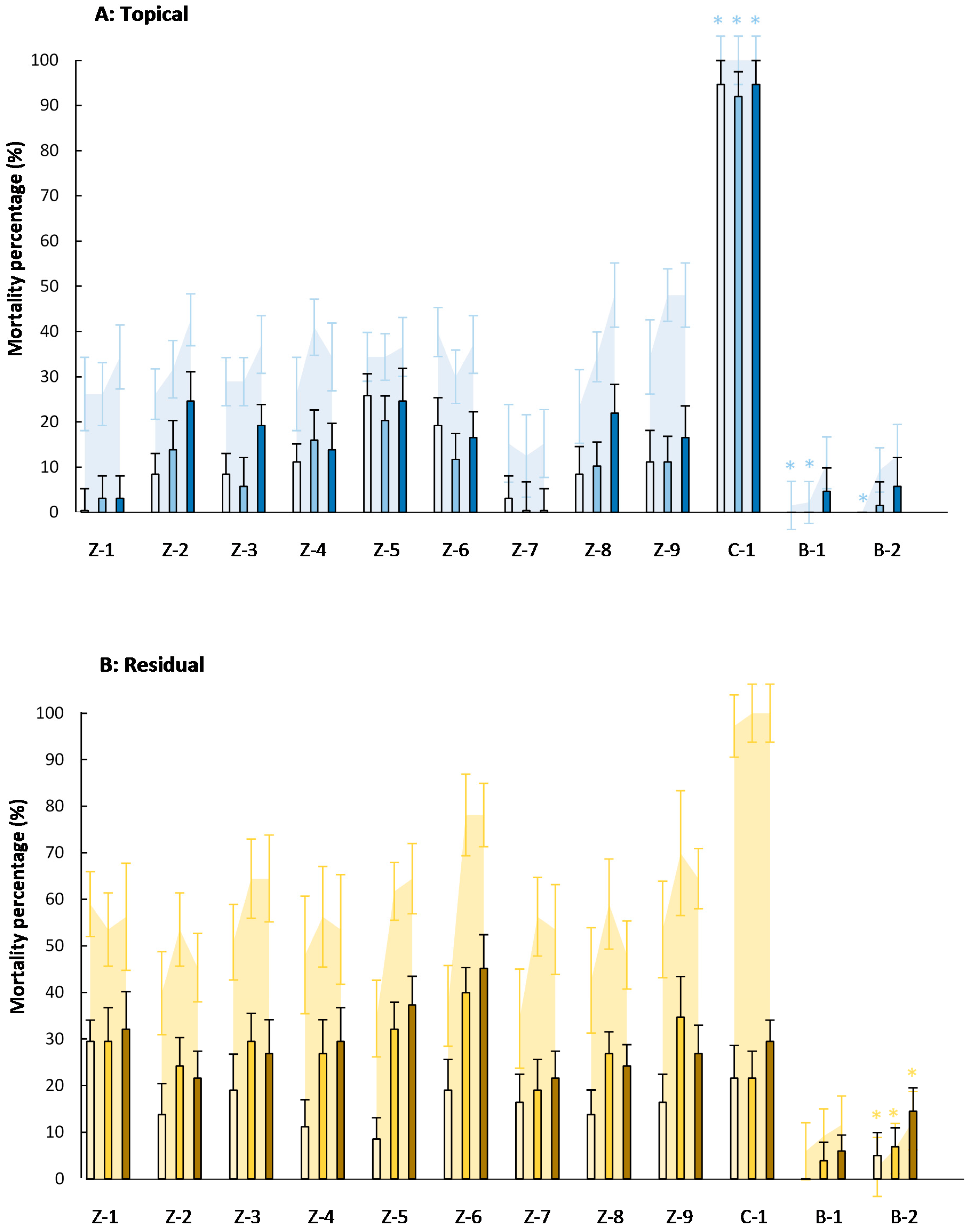
 ), 4000 (
), 4000 (  ), and 20,000 (
), and 20,000 (  ) mg·L−1. Asterisks indicate significant differences (p < 0.05) in oviposition activity between treated and control leaves (n = 4). OAI: oviposition activity index.
) mg·L−1. Asterisks indicate significant differences (p < 0.05) in oviposition activity between treated and control leaves (n = 4). OAI: oviposition activity index.
 ), 4000 (
), 4000 (  ), and 20,000 (
), and 20,000 (  ) mg·L−1. Asterisks indicate significant differences (p < 0.05) in oviposition activity between treated and control leaves (n = 4). OAI: oviposition activity index.
) mg·L−1. Asterisks indicate significant differences (p < 0.05) in oviposition activity between treated and control leaves (n = 4). OAI: oviposition activity index.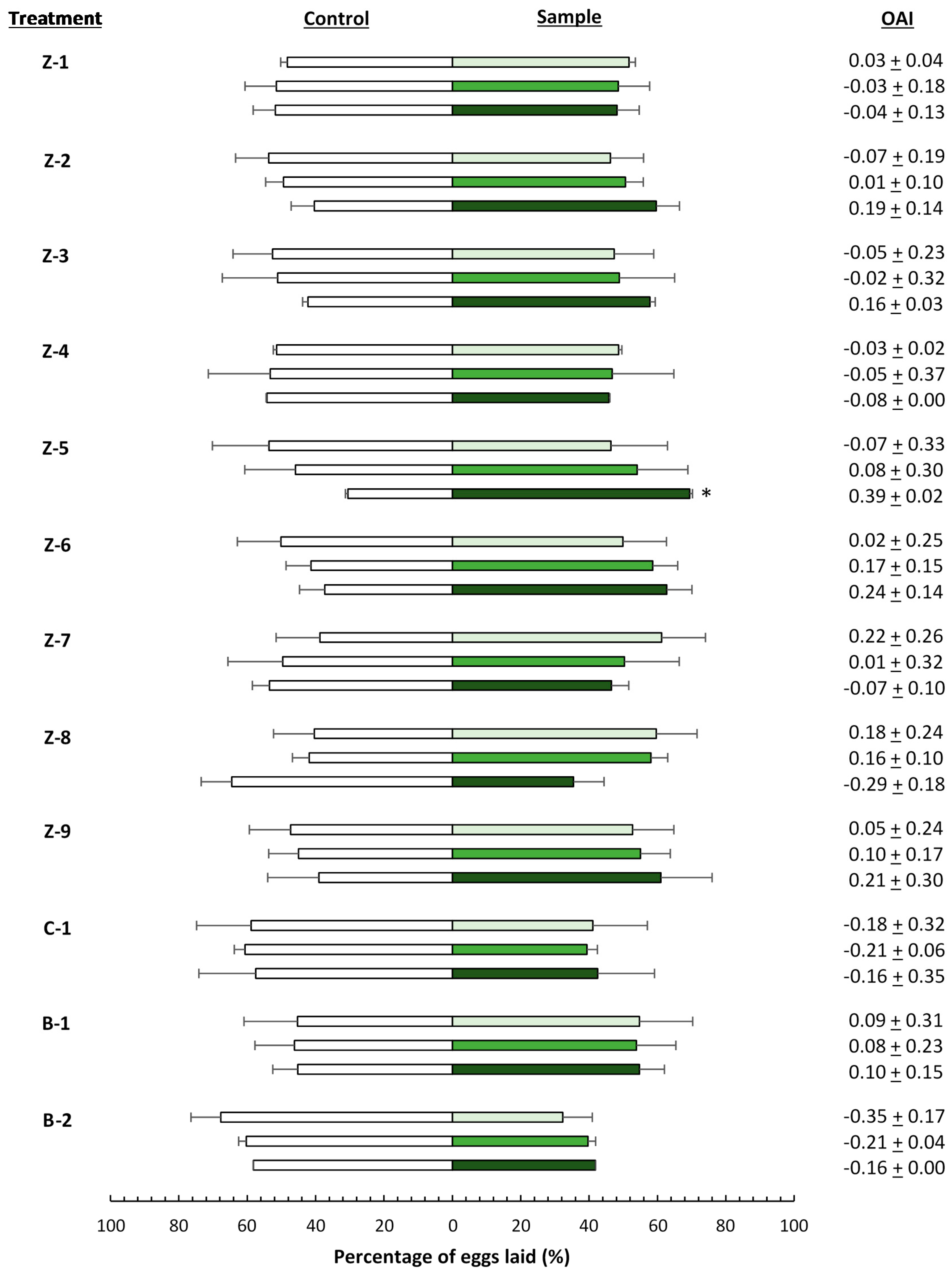
| Treatment | Material Name | Formulation Type | Manufacturers |
|---|---|---|---|
| Z-1 | BEA (Beta polymorph A) | Technical product | Clariant |
| Z-2 | BEA 850 a | WP c | Fitofarmacia |
| Z-3 | BEA 950 b | WP | Fitofarmacia |
| Z-4 | FAU (Faujasite) | Technical product | Zeolyst |
| Z-5 | FAU 850 a | WP | Fitofarmacia |
| Z-6 | FAU 920 b | WP | Fitofarmacia |
| Z-7 | LTA (Linde type A) | Technical product | FMC |
| Z-8 | LTA 800 | SC c | Fitofarmacia |
| Z-9 | LTA 850 | WP | Fitofarmacia |
| C-1 | Spinosad (Conserve Pro) | SC | Dow Agrosciences B.V. |
| B-1 | Kaolin | Technical product | Sigma Aldrich |
| B-2 | Kaolin (Surround) | WP | Tessenderlo Group |
| Factor | F | df | p |
|---|---|---|---|
| Dose | 3.234 | 1 | 0.074 |
| Application method | 6.925 | 1 | 0.010 b |
| Product | 1.057 | 1 | 0.306 |
| Life stage | 45.472 | 1 | 0.000 c |
| Dose × application method | 0.043 | 1 | 0.836 |
| Dose × product | 0.001 | 1 | 0.971 |
| Dose × life stage | 0.004 | 1 | 0.947 |
| Application method × product | 4.883 | 1 | 0.029 a |
| Application method × life stage | 3.938 | 1 | 0.049 a |
| Product × life stage | 0.772 | 1 | 0.381 |
| Dose × application method × product | 0.000 | 1 | 0.996 |
| Dose × application method × life stage | 0.043 | 1 | 0.836 |
| Dose × product × life stage | 0.003 | 1 | 0.958 |
| Application method × product × life stage | 0.144 | 1 | 0.705 |
| Dose × application method × product × life stage | 0.000 | 1 | 0.989 |
| Error | 128 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Smedt, C.; Van Damme, V.; De Clercq, P.; Spanoghe, P. Insecticide Effect of Zeolites on the Tomato Leafminer Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2016, 7, 72. https://doi.org/10.3390/insects7040072
De Smedt C, Van Damme V, De Clercq P, Spanoghe P. Insecticide Effect of Zeolites on the Tomato Leafminer Tuta absoluta (Lepidoptera: Gelechiidae). Insects. 2016; 7(4):72. https://doi.org/10.3390/insects7040072
Chicago/Turabian StyleDe Smedt, Caroline, Veerle Van Damme, Patrick De Clercq, and Pieter Spanoghe. 2016. "Insecticide Effect of Zeolites on the Tomato Leafminer Tuta absoluta (Lepidoptera: Gelechiidae)" Insects 7, no. 4: 72. https://doi.org/10.3390/insects7040072






