1. Introduction
The nests of eusocial paper wasps often support a diverse array of insect parasites and symbionts [
1]. Understanding these insects with which paper wasps have co-evolved can ultimately help us understand the paper wasp ecology. The sooty-winged Chalcoela moth,
Chalcoela iphitalis (Walker), is a brood parasite in the family Crambidae [
2] which attacks Polistine wasps, including at least ten species of
Polistes and one species of
Mischocyttarus in the US and Central America [
3]. However, evidence from a ten-year study indicated that
C. iphitalis did not show a host preference between
P. fuscatus and
P. dominula [
4]. Moth larvae feed on pupal or pre-pupal wasps before spinning silken cocoons containing layers of air pockets within wasp brood cells. Moth silk is not chewed through or removed by the wasps, and newly laid eggs and early instar wasp larvae can sometimes be observed on top of moth silk [
3].
C. iphitalis overwinter inside the abandoned wasp nest and are typically bivoltine, with an adult emergence in the spring as well as late summer when nests containing brood are present. Infestations can be commonly observed, with up to 73% of nests parasitized in a population of one wasp species [
5]. Parasitism of a population of
Polistes exclamans in Texas by
C. iphitalis has been detailed by Strassmann [
5] over the course of three years. The average number of mature cells infested per nest ranged from 19–34% and varied significantly between years. Infestations usually peaked around July or August, and
C. iphitalis may avoid ovipositing in previously infested nests. Each successful
C. iphitalis larva kills one wasp pupa, but holes between cells were observed, indicating that some moth larvae may be feeding on multiple pupae.
The ability of
C. iphitalis to destroy a large percentage of wasp pupae in a small period of time may have a large impact on worker replacement at the nest. Strassmann and Thomas [
6] conducted a principal component analysis on a population of
P. exclamans and found that nest decline was correlated with
C. iphitalis infestation. A similar study concluded that heavy infestations of
C. iphitalis can be a primary cause of colony failure. Moth diapause inside of old nests may be a cause for the rarity of nest re-use by paper wasps [
7].
Paper wasp nests attach to a variety of substrates that include natural vegetation and manmade structures, and nest location is thought to be an important aspect related to moth invasion [
1]. In one study, approximately 60% of the
Polistes nests located on manmade structures in Illinois were parasitized by
C. iphitalis, whereas only 20% of nests built on trees or shrubs were parasitized, suggesting that nests in vegetation may be less preferred or difficult for moths to locate [
8].
Strassmann [
5] notes that this moth lays eggs in wasp nests at night. Strassmann [
5] and West-Eberhard [
9] also described the parasite alarm reaction of adult wasps when encountering a moth, during which wasps will react violently by biting or stinging the area where the moth had been and subsequently alarming other wasps through vibrations and wing flipping on the nest. Wasps continuing the alarm behavior will sometimes leave the nest and walk over the substrate for up to 10 h after initial detection of the moth [
5]. Mechanisms by which the moth parasite is able to bypass the host defense is still unknown.
The objective of this study was to determine parasitism rates in wasp species found in the Baton Rouge, Louisiana area, factors related to the parasitism, as well as oviposition behavior in the laboratory. To achieve this, we examined the behavior of both host and parasite in the laboratory, as well as the occurrence of C. iphitalis in field populations of Polistine wasps in southern Louisiana.
2. Materials and Methods
2.1. Field Study
Southern Louisiana study sites encompassed areas in Baton Rouge and St. Gabriel including Bluebonnet swamp, Burden Research Station, LSU Reproductive Biology Center, and LSU main campus. Areas were systematically searched for Polistine nests throughout the spring and summer of 2016. Because air temperature is a major factor affecting wasp activity [
10], searches were carried out mostly on warm and sunny days when wasps were active, with search increments of 3–4 h each day. Areas for visual search included sheltered sites such as palm fronds, picnic shelters, eaves of buildings, as well as low shrub vegetation. Observations of wasps flying directly into a location sometimes led to the discovery of a nest in that manner. Upon discovery, each colony was identified to species and visited weekly for the duration of the season through colony decline. Presence of
C. iphitalis infestation as well as percentage of cells infested were recorded. We used total cell number of nests that reached the pupal stage to calculate the percentage of cells with webbing (infested) for each species. Only nests that reached the pupal stage were included in our calculations due to the fact that
C. iphitalis feeds only on pupal or pre-pupal wasp stages [
3].
2.2. Laboratory Study
In July of 2016, two moth-infested wasp nests (P. fuscatus and P. bellicosus) which had been abandoned by the wasps were collected in the field. The nests were brought back to the laboratory and placed together in the bottom of a 12.7 × 10.16 cm cylindrical clear plastic container, whereupon 29 C. iphitalis adults eclosed within two days. Two active Polistes dorsalis nests without evidence of previous C. iphitalis infestation were also collected from the field and glued to the top of identical separate plastic containers as noted above; however, these containers were inverted such that the lid served as the bottom of the cage. Both nests were of similar size, and one nest (A) consisted of two adult females while the other nest (B) consisted of four adult females. All brood stages (eggs, larvae, and pupae) were present in both nests, which were maintained in the laboratory and given access to honey, water, and wax worms daily under a natural photoperiod. The two moth-infested wasp nests and two active P. dorsalis nests were collected at Burden Research Station in Baton Rouge, LA, USA.
To release moths from their cage to one of the two active
P. dorsalis nests, container lids were removed and containers were placed together. This allowed the 29 moths in the lower cage to fly freely to the upper cage housing an active
P. dorsalis nest for an immediate observation of 30 min. Two daytime (temperature = 23.1 °C and luminosity = 59) and two night-time (temperature = 21.9 °C and luminosity = 0) observations (28 and 29 July, data obtained from Onset HOBO
® UA-002 Pendant Temperature Light Data Logger) were conducted on each wasp nest. After the first 30-min daytime observation concluded, any moths remaining in the wasp cage area were placed back into their own cage, which was then placed underneath the next wasp nest for a 30-min observation. The behavior and interactions between wasp and moth were recorded. Because red light is not visible to most insects but provides light for observation by humans [
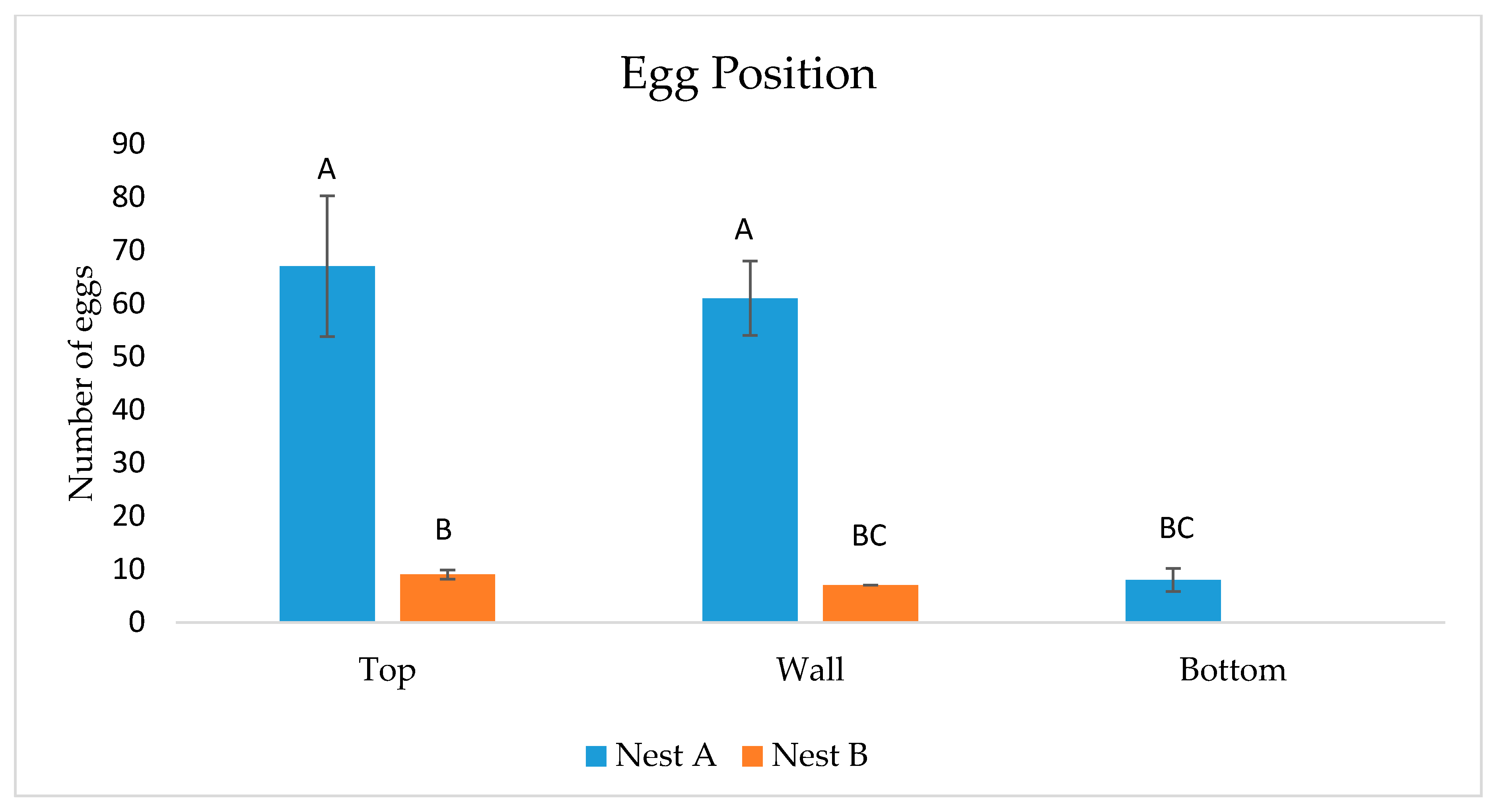
11], a red light was utilized during night-time trials. After both 30-min night-time observations terminated, cages were separated and seven adult moths (from the 29 total) were placed in each wasp cage. Moths were then allowed further oviposition for 10 additional hours before being removed the next morning. This created a standard number of moths and oviposition time in each wasp cage. Removal of adult moths from wasp cages was facilitated by physical stimulation from a small paintbrush which encouraged downward flight into the moth cage. After each 10.5 h period, number of moth eggs and egg locations (wasp nest surface or cage surface including top, wall, or bottom) were noted in each wasp cage. In total, moths were permitted to oviposit in each wasp cage for 22 h (including daytime and night-time observational periods). Fourteen days after trials ended, two late instar wasp larvae were removed from each nest for examination of moth parasitism. Moth larval silk was noted in cells as evidence.
A two-way ANOVA procedure (SAS®9.4, ©2016 Proc Mixed, SAS Institute Inc., Cary, NC, USA) was utilized to analyze differences between moth egg positioning (either top, wall, or bottom of cage) and egg number in both wasp cages.
4. Discussion
Although
Chalcoela iphitalis attacks many different paper wasp species [
3], our data suggest that the moth attacks
Polistes bellicosus most often in this area, especially as compared with
Mischocyttarus mexicanus. The only available host record for
C. iphitalis from the genus
Mischocyttarus is in
M. basimacula, a species occurring in Central America [
3]. In our study,
Polistes bellicosus was the most commonly observed species and had the highest
C. iphitalis infestation rate, while
Mischocyttarus mexicanus was second most commonly observed species and had no infestations. This lack of infested
M. mexicanus nests is of great interest, and several possibilities exist: enhanced behavioral and chemical defenses against the moth, insufficient host quality, more concealed nesting locations [
8], or morphology of the two proximal abdominal segments, which are more elongate and stalk-like in
Mischocyttarus than in
Polistes. We speculate that this elongate petiole may add to the body flexibility for use of Van der Vecht’s gland—a sternal gland which secretes repellant compounds that protect the nest against ants, yellowjackets, and house flies [
12]. Van der Vecht’s gland secretions have yet to be tested for repellency against parasitic Lepidoptera. It is plausible that the allomone produced by this gland in
Mischocyttarus—which is typically applied to the nest petiole—prevents moth larvae from traveling down the petiole and into the comb in the same manner in which it prevents ants from doing so [
13].
C. iphitalis has been noted to have no known host specificity within the
Polistes genus [
1]; however, a closely related species (
C. pegasalis) with a similar life history to that of
C. iphitalis was shown to have differential parasitism rates among
Polistes species in Jamaica [
14]. We found that
Polistes species in our study were not attacked equally as often, and the percentage of infested cells also differed between species. However, the most frequently attacked species did not have the highest percentage of infested cells. This discrepancy may be due to the variation in the average number of cells built by each species [
15]. If moths lay similar quantities of eggs at each nest, nests with fewer cells should have a higher percentage of total cells infested, which was indeed the case with
P. metricus. A tradeoff between parasite vigilance behavior and foraging or brood care by adult female wasps was suggested as a possible explanation to differential parasitism among
Polistes species [
14]. However, oviposition of
Chalcoela moths occurs mainly at night when wasps are not foraging [
5], suggesting that other possible factors are at play. In the laboratory, we found that individual adult wasps exhibited a varying degree of alarm behavior after the detection of an adult moth. The wasp exhibiting the highest degree of alarm was also preforming abdominal wagging—a behavior observed more often in queens than in workers [
16]. Avoidance of an alarmed individual by callow individuals in another instance was also observed, suggesting that alarm behavior may be correlated with social rank of the individual, as has been shown with aggression behavior [
17].
Laboratory experiments also demonstrated that
Chalcoela iphitalis oviposit on substrates surrounding the host nest rather than on the nest itself. This makes the substrate which the nest is built upon an important factor in avoiding predation by
C. iphitalis. A substrate which has a large surface area (such as
S. palmetto leaves or manmade structures) may be more conducive for moth oviposition, while a narrower substrate (such as twigs or small tree branches) may inhibit oviposition by the moth. In this study, we saw no infestation of nests built on twigs or branches in the field, supporting similar observations made by Reed and Vinson [
8]. Antennal contact by a female moth with either an adult wasp or the wasp nest itself appeared to be a prerequisite for oviposition in the laboratory, but moths avoided walking onto or next to the nest. Antennal contact most likely provides host checking and oviposition stimulation, while avoidance behavior serves a function of remaining at a safe distance from defending wasps while ovipositing. Although the ectoparasitic moth larvae typically attack only pupae and prepupae [
5], last instar wasp larvae were attacked in the laboratory. This could be due to the confined setting and lack of available pupae once all wasp pupae had matured. Field observations supported the basis of an exclusion of pre-pupal nests from the study, in that infestation of a pre-pupal nest was not observed. It is presumable that upon hatching, caterpillars located the nest pedicel and traveled into the nest, although this was not directly observed. Neonates likely go undetected by adult wasps due to their small size. Our results provide the first report of oviposition behavior of
C. iphitalis and suggest differential host parasitism in field populations. Future studies focusing on behavioral differences of individual wasps during episodes of parasite invasion may provide the basis for differential alarm behavior among individuals within the colony, and provide further explanation for the differential parasitism by
Chalcoela moths.