Influence of Grapevine Cultivar on the Second Generations of Lobesia botrana and Eupoecilia ambiguella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampled Vineyard
2.2. Larval Population Levels of the Two Vine Moths
2.3. Age Composition of L. botrana Larvae
2.4. Bunch Traits during L. botrana Second-Generation Egg Hatching
2.5. Correlation between First- and Second-Generation Demographic Parameters
3. Results
3.1. Proportion of the Two Vine Moths on Larval Population
3.2. Influence of Cultivar on Larval Population Levels
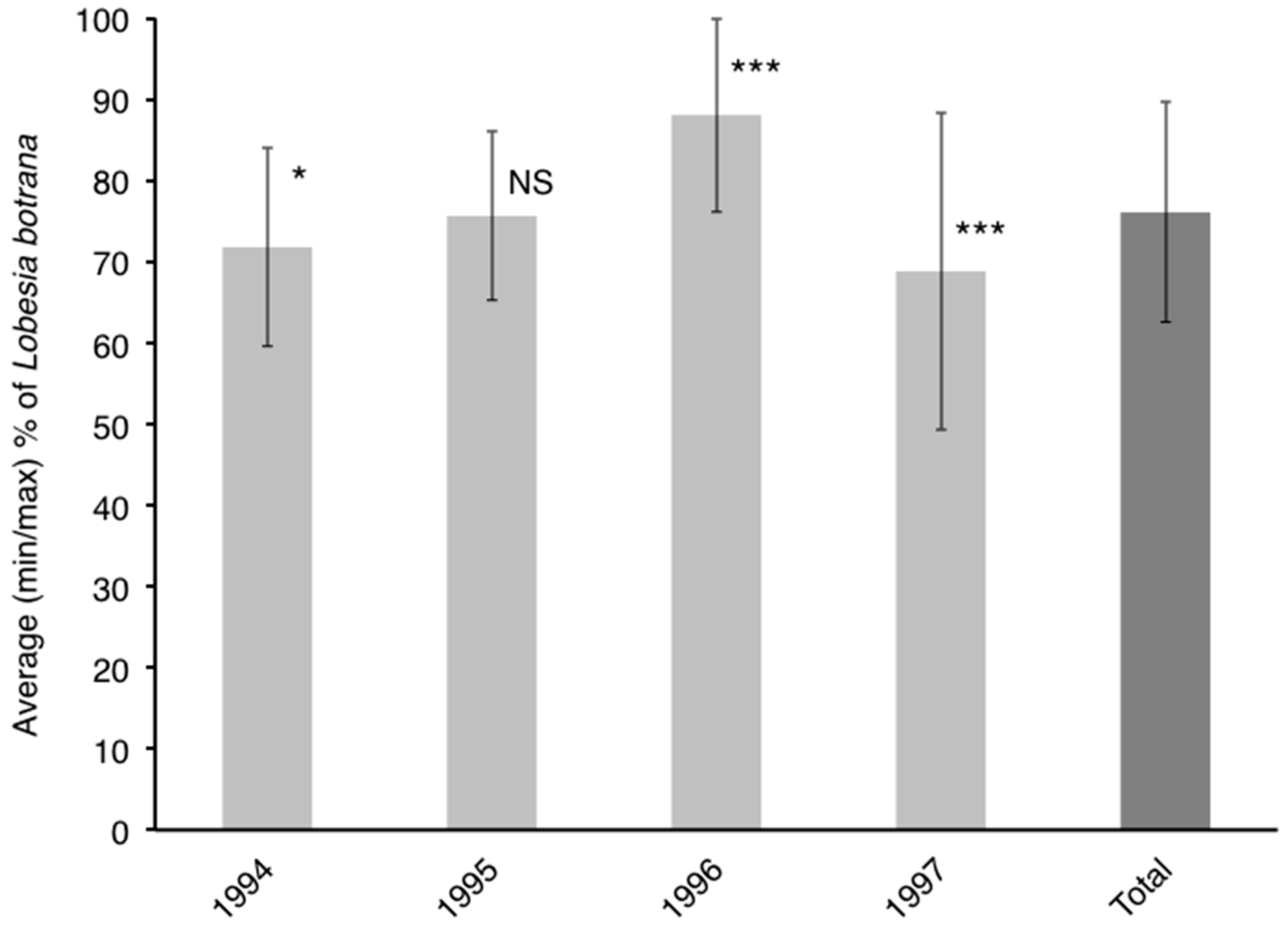
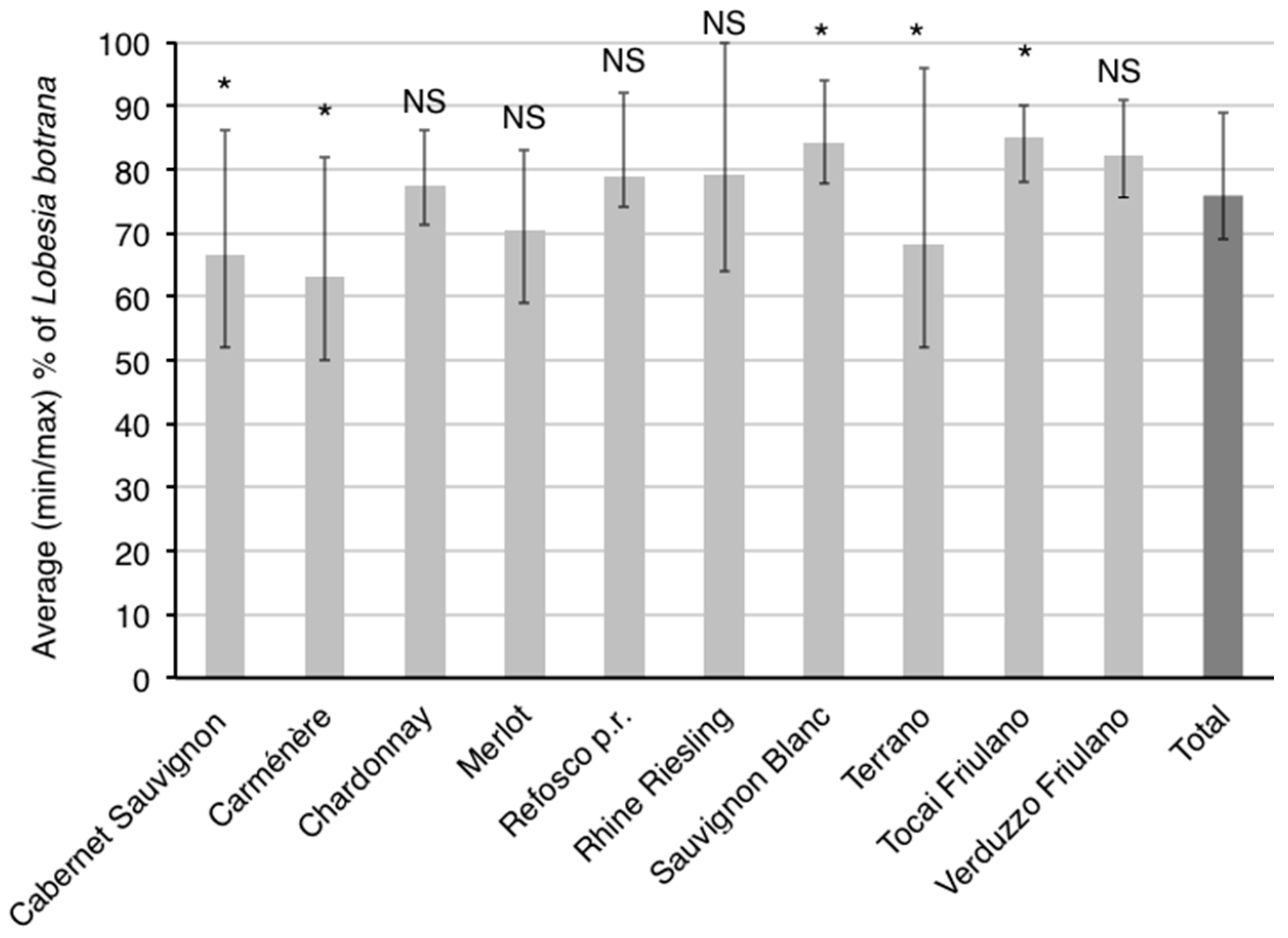
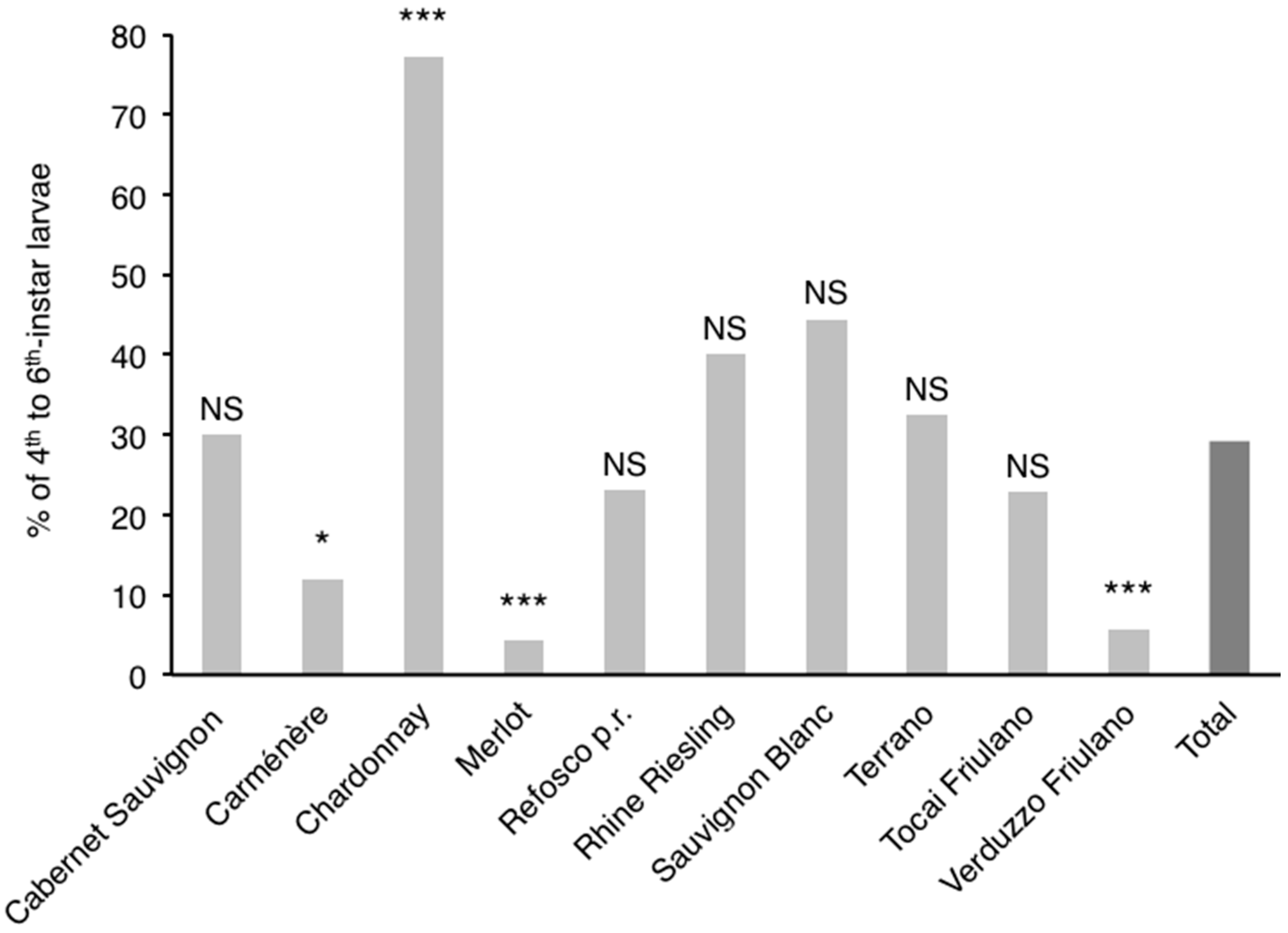
3.3. Influence of Cultivar on Phenology of L. botrana Larvae
3.4. Cultivar Bunch-Traits and L. botrana Demographic Parameters
3.5. Correlation between First- and Second-Generation Demographic Parameters
4. Discussion
4.1. Influence of Cultivar on Second-Generation Population Levels of the Two Vine Moths
4.2. Influence of Cultivar on Phenology of L. botrana
4.3. Influence of Bunch Traits on L. botrana Infestation and Phenology
4.4. Relationship between First- and Second-Generation Infestations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pavan, F.; Floreani, C.; Barro, P.; Zandigiacomo, P.; Dalla Montà, L. Occurrence of two different development patterns in Lobesia botrana (Lepidoptera: Tortricidae) larvae during the second generation. Agric. For. Entomol. 2013, 15, 398–406. [Google Scholar] [CrossRef]
- Galet, P. Les Maladies et les Parasites de la Vigne. Tome II. les Parasites Animaux; Paysan du Midi: Montpellier, France, 1982. [Google Scholar]
- Coscollà, R. La Polilla del Racimo de la vid (Lobesia Botrana Den. y Schiff.); Generalitat Valenciana: Valencia, Spain, 1997. [Google Scholar]
- Sharon, R.; Zahavi, T.; Soroker, V.; Harari, A.R. The effect of grape vine cultivars on Lobesia botrana (Lepidoptera: Tortricidae) population levels. J. Pest Sci. 2009, 82, 187–193. [Google Scholar] [CrossRef]
- Geisler, G. Untersuchungen zur Resistenzzüchtung gegen “Heuwurm”-Befall bei Reben. Vitis 1959, 2, 84–100. [Google Scholar]
- Snjezana, H. Susceptibility of some grapevine cultivars in area of vineyards of Podgorica on the attack of European grape berry moth—Lobesia botrana Den. & Schiff. (Lepidoptera, Tortricidae). Acta Hortic. 2004, 652, 355–358. [Google Scholar]
- Pavan, F.; Stefanelli, G.; Cargnus, E.; Villani, A. Assessing the influence of inflorescence traits on the susceptibility of grape to vine moths. J. Appl. Entomol. 2009, 133, 394–401. [Google Scholar] [CrossRef]
- Pavan, F.; Girolami, V.; Cecchini, A.; Turbian, E. Evoluzione dei danni delle tignole della vite, Lobesia botrana (Den. e Schiff.) ed Eupoecilia ambiguella (Hb.), nell’Italia nord orientale e lotta insetticida. Redia 1993, 76, 417–431. [Google Scholar]
- Moleas, T. Biologia ed etologia della Lobesia botrana in Puglia. In Atti del 3° Incontro Sulla Difesa Integrata Della Vite; Regione Lazio: Rome, Italy, 1984; pp. 91–97. [Google Scholar]
- Baldacchino, F.; Moleas, T. Suscettibilità di alcune cultivar di vite a Lobesia botrana (Denis et Schiffermüller) (Lepidoptera: Tortricidae). Atti Giorn. Fitopatol. 2000, 1, 441–444. [Google Scholar]
- Gennuso, E.; Ragusa, E.; Tsolakis, H. Evaluation of infestation by Lobesia botrana (Dennis et Schiffermüller) (Lepidoptera, Tortricidae) and its relation to territorial differences and cultivar susceptibility. IOBC/WPRS Bull. 2013, 85, 203–210. [Google Scholar]
- Delrio, G.; Luciano, P.; Prota, R. Researches on grape-vine moths in Sardinia. In Integrated Pest Control in Viticulture; Cavalloro, R., Ed.; Balkema: Rotterdam, The Netherlands, 1987; pp. 57–67. [Google Scholar]
- Birgücü, A.K.; Turanlı, F.; Gümüş, E.; Güzel, B.; Karsavuran, Y. The effect of grape cultivars on oviposition preference and larval survival of Lobesia botrana Den. & Schiff. (Lepidoptera: Tortricidae). Fresenius Environ. Bull. 2015, 24, 33–38. [Google Scholar]
- Moreau, J.; Rahme, J.; Benrey, B.; Thiéry, D. Larval host plant origin modifies the adult oviposition preference of the female European grapevine moth Lobesia botrana. Naturwissenschaften 2008, 95, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Schmitz, V.; Stockel, J. Effect of the phenological stage and of grape cultivar on the establishment and survival of the first generation of larvae of the grape vine moth Lobesia botrana Den. and Schiff. (Lepidoptera; Tortricidae). Bol. Sanid. Veg. Plagas 1992, 18, 755–764. [Google Scholar]
- Gabel, B.; Roehrich, R. Sensitivity of grapevine phenological stages to larvae of European grapevine moth, Lobesia botrana Den. et Schiff. (Lep., Tortricidae). J. Appl. Entomol. 1995, 119, 127–130. [Google Scholar] [CrossRef]
- Fermaud, M. Cultivar susceptibility of grape berry clusters to larvae of Lobesia botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 1998, 91, 974–980. [Google Scholar] [CrossRef]
- Moreau, J.; Villemant, C.; Benrey, B.; Thiéry, D. Species diversity of larval parasitoids of the European grapevine moth (Lobesia botrana, Lepidoptera: Tortricidae): The influence of region and cultivar. Biol. Control 2010, 54, 300–306. [Google Scholar] [CrossRef]
- Moreau, J.; Benrey, B.; Thiéry, D. Grape variety affects larval performance and also female reproductive performance of the European grapevine moth Lobesia botrana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2006, 96, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Thiéry, D.; Troussard, J.P.; Benrey, B. Grape variety affects female but also male reproductive success in wild European grapevine moths. Ecol. Entomol. 2007, 32, 747–753. [Google Scholar] [CrossRef]
- Thiéry, D.; Monceau, K.; Moreau, J. Different emergence phenology of European grapevine moth (Lobesia botrana, Lepidoptera: Tortricidae) on six varieties of grapes. Bull. Entomol. Res. 2014, 104, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Masante-Roca, I.; Anton, S.; Delbac, L.; Dufour, M.C.; Gadenne, C. Attraction of the grapevine moth to host and non-host plant parts in the wind tunnel: Effects of plant phenology, sex, and mating status. Entomol. Exp. Appl. 2007, 122, 239–245. [Google Scholar] [CrossRef]
- Tasin, M.; Lucchi, A.; Ioriatti, A.; Mraihi, M.; De Cristofaro, A.; Boger, Z.; Anfora, G. Oviposition response of the moth Lobesia botrana to sensory cues from a host plant. Chem. Sens. 2011, 36, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, S.; Dalla Montà, L.; Duso, C. Observations on the biology and control of grape moths in Venetia. In Integrated Pest Control in Viticulture; Cavalloro, R., Ed.; Balkema: Rotterdam, The Netherlands, 1987; pp. 27–37. [Google Scholar]
- Varner, M.; Mattedi, L. Le tignole nella Piana Rotaliana. Inf. Agrar. 2004, 60, 63–69. [Google Scholar]
- Roehrich, R. Recherches sur la nuisibilité de Eupoecilia ambiguella Hb. et Lobesia botrana Den. et Schiff. Déf. Vég. 1978, 191, 106–124. [Google Scholar]
- Dalla Montà, L.; Marchesini, E.; Pavan, F. Relazione fra tignole della vite e attacchi di Botrytis cinerea. Inf. Fitopatol. 2007, 57, 28–35. [Google Scholar]
- Pavan, F.; Sbrissa, F. Dannosità delle tignole della vite, Lobesia botrana (Den. e Schiff.) ed Eupoecilia ambiguella (Hb.), su cultivar a maturazione tardiva nell’Italia nord-orientale. Frustula Entomol. 1994, 17, 43–53. [Google Scholar]
- Fornasiero, D.; Pavan, F.; Pozzebon, A.; Picotti, P.; Duso, C. Relative infestation level and sensitivity of grapevine cultivars to the leafhopper Empoasca vitis (Hemiptera: Cicadellidae). J. Econ. Entomol. 2016, 109, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.; Picotti, P. Influence of grapevine cultivars on the leafhopper Empoasca vitis and its egg parasitoids. BioControl 2009, 54, 55–63. [Google Scholar] [CrossRef]
- Milek, T.M.; Bjeliš, M.; Šimala, M. Intensity of scale insects infestation in relation to grapevine variety and soli type in Croatia. Cereal Res. Commun. 2008, 36, 1735–1738. [Google Scholar]
- Pavan, F.; Girolami, V.; Sacilotto, G. Second generation of grape berry moths, Lobesia botrana (Den. & Schiff.) (Lep., Tortricidae) and Eupoecilia ambiguella (Hb.) (Lep., Cochylidae): Spatial and frequency distributions of larvae, weight loss and economic injury level. J. Appl. Entomol. 1998, 122, 361–368. [Google Scholar]
- Silvestri, F. Compendio di Entomologia Applicata; Tipografia Bellavista: Portici, Italy, 1943; Volume 2, pp. 407–416, 462–466. [Google Scholar]
- Pavan, F.; Floreani, C.; Barro, P.; Zandigiacomo, P.; Dalla Montà, L. Influence of generation and photoperiod on larval development of Lobesia botrana (Lepidoptera: Tortricidae). Environ. Entomol. 2010, 39, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.; Sbrissa, F. Soglie economiche di danno per la seconda generazione delle tignole della vite basate sulla perdita in peso. Frustula Entomol. 1999, 20, 18–26. [Google Scholar]
- Kiaeian Moosavi, F.; Cargnus, E.; Pavan, F.; Zandigiacomo, P. Mortality of eggs and newly hatched larvae of Lobesia botrana (Lepidoptera: Tortricidae) exposed to high temperatures in the laboratory. Environ. Entomol. 2017, 46, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Kiaeian Moosavi, F.; Cargnus, E.; Pavan, F.; Zandigiacomo, P. Effect of grapevine bunch exposure to sunlight on berry surface temperature and Lobesia botrana (Lepidoptera: Tortricidae) egg laying, hatching and larval settlement. Agric. For. Entomol. 2017. [Google Scholar] [CrossRef]
- Marchesini, E.; Dalla Montà, L. Nel Veneto quattro generazioni di tignoletta della vite. Inf. Agrar. 2004, 60, 75–78. [Google Scholar]
- Pavan, F. University of Udine: Udine, Italy, Unpublished work; 2007.
| Cultivar | Average ± Standard Deviation | ||
|---|---|---|---|
| Both Moth Species | Lobesia botrana | Eupoecilia ambiguella | |
| Cabernet Sauvignon | 21.3 ± 8.1 ab | 13.2 ± 2.2 a | 8.1 ± 6.0 a |
| Carménère | 10.8 ± 8.8 a | 6.1 ± 4.8 a | 4.6 ± 4.1 a |
| Chardonnay | 47.5 ± 27.4 b | 36.0 ± 20.5 bc | 11.5 ± 7.1 a |
| Merlot | 20.8 ± 11.7 ab | 14.4 ± 8.4 ab | 6.4 ± 4.8 a |
| Refosco p.r. | 24.0 ± 9.7 ab | 18.8 ± 7.7 ab | 5.2 ± 2.9 a |
| Rhine Riesling | 21.0 ± 8.1 ab | 16.2 ± 5.3 ab | 4.8 ± 4.0 a |
| Sauvignon Blanc | 23.5 ± 10.8 ab | 19.3 ± 8.2 abc | 4.2 ± 2.7 a |
| Terrano | 20.5 ± 10.0 ab | 11.1 ± 3.7 a | 9.3 ± 6.5 a |
| Tocai Friulano | 44.8 ± 20.4 b | 38.0 ± 17.0 c | 6.8 ± 3.9 a |
| Verduzzo Friulano | 28.8 ± 11.5 ab | 23.5 ± 9.0 abc | 5.2 ± 3.3 a |
| Cultivar | Average ± Standard Deviation | |||
|---|---|---|---|---|
| Compactness Index | Berry Number per Bunch | Total Berry Volume per Bunch (mL) | Average Berry Volume (mL) | |
| Cabernet Sauvignon | 0.31 ± 0.08 ab | 100.9 ± 27.9 b | 49.0 ± 17.6 ab | 0.48 ± 0.06 c |
| Carménère | 0.33 ± 0.08 bc | 65.2 ± 16.7 a | 40.9 ± 9.3 ab | 0.63 ± 0.06 d |
| Chardonnay | 0.41 ± 0.10 c | 82.1 ± 17.2 ab | 45.8 ± 13.7 ab | 0.55 ± 0.06 cd |
| Merlot | 0.20 ± 0.06 a | 64.1 ± 24.7 a | 34.0 ± 10.9 a | 0.54 ± 0.06 cd |
| Refosco p.r. | 0.36 ± 0.12 bc | 106.5 ± 23.4 b | 55.7 ± 10.5 b | 0.54 ± 0.13 cd |
| Rhine Riesling | 0.36 ± 0.07 bc | 69.9 ± 21.5 a | 32.5 ± 13.0 a | 0.46 ± 0.06 bc |
| Sauvignon Blanc | 0.33 ± 0.07 bc | 104.7 ± 12.9 b | 32.2 ± 10.3 a | 0.30 ± 0.08 a |
| Tocai Friulano | 0.27 ± 0.05 ab | 103.2 ± 31.7 b | 38.4 ± 12.1 ab | 0.37 ± 0.06 ab |
| Verduzzo Friulano | 0.26 ± 0.08 ab | 94.7 ± 11.1 b | 33.5 ± 11.0 a | 0.35 ± 0.10 ab |
| Lobesia botrana Demographic Parameters (Y) | Cultivar Bunch Traits (X) | ||
|---|---|---|---|
| Compactness Index | Total Berry Volume per Bunch | Average Berry Volume | |
| Larval nests per 100 bunches (4-year average) | Y = 12.20 + 16.89X; p = 0.77; R2 = 0.01 | Y = 16.9 + 0.015X; p = 0.97; R2 = 0.0002 | Y = 33.0 − 33.0X; p = 0.30; R2 = 0.15 |
| % 4th–6th instar larvae in 1997 | Y = −61.0 +289.1X; p = 0.01; R2 = 0.61 | Y = 13.7 + 0.41X; p = 0.71; R2 = 0.02 | Y = 34.4 − 8.73X; p = 0.92; R2 = 0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, F.; Stefanelli, G.; Villani, A.; Cargnus, E. Influence of Grapevine Cultivar on the Second Generations of Lobesia botrana and Eupoecilia ambiguella. Insects 2018, 9, 8. https://doi.org/10.3390/insects9010008
Pavan F, Stefanelli G, Villani A, Cargnus E. Influence of Grapevine Cultivar on the Second Generations of Lobesia botrana and Eupoecilia ambiguella. Insects. 2018; 9(1):8. https://doi.org/10.3390/insects9010008
Chicago/Turabian StylePavan, Francesco, Giorgio Stefanelli, Alberto Villani, and Elena Cargnus. 2018. "Influence of Grapevine Cultivar on the Second Generations of Lobesia botrana and Eupoecilia ambiguella" Insects 9, no. 1: 8. https://doi.org/10.3390/insects9010008




