Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia)
Abstract
:1. Introduction
2. Materials and Methods
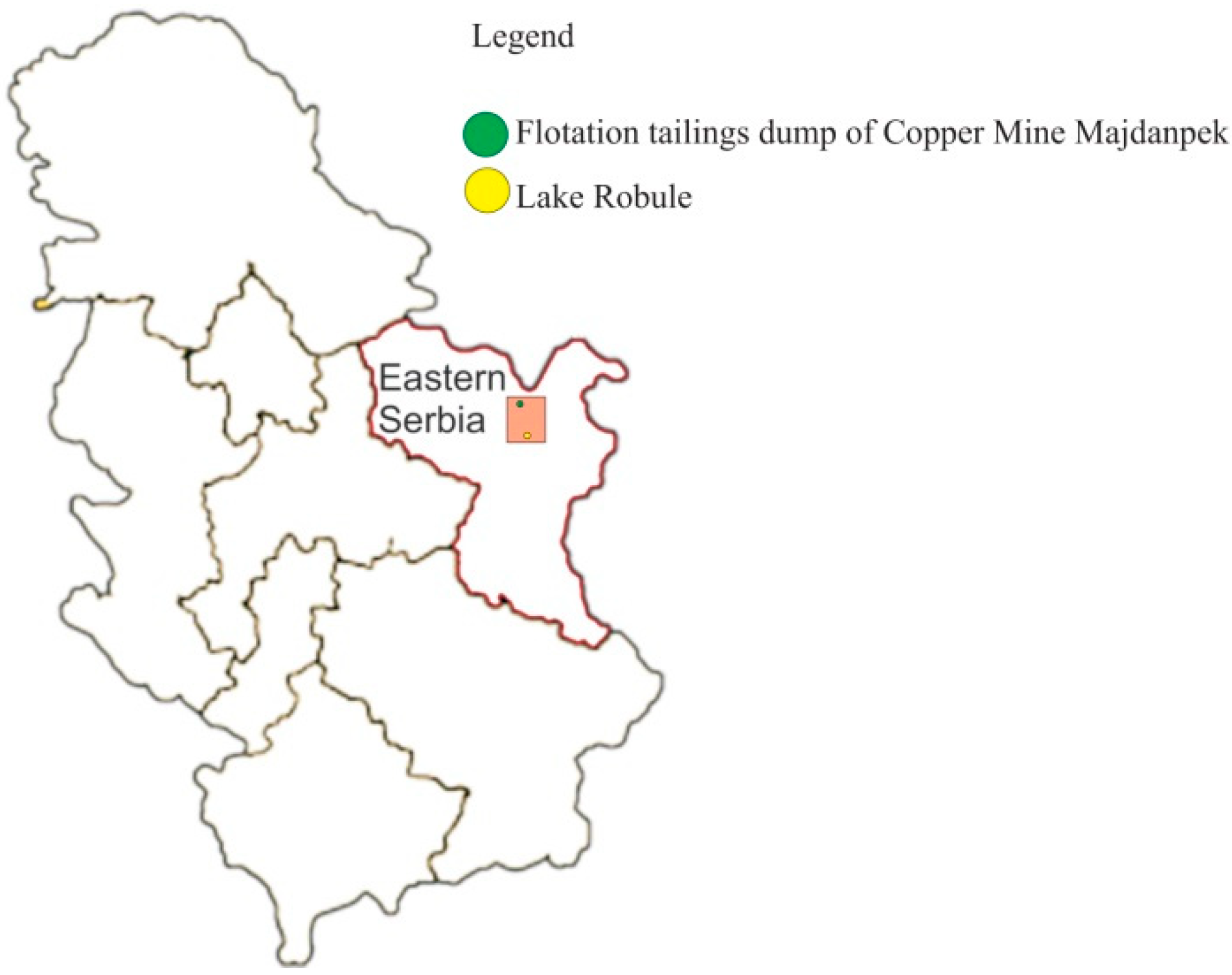
2.1. Sampling
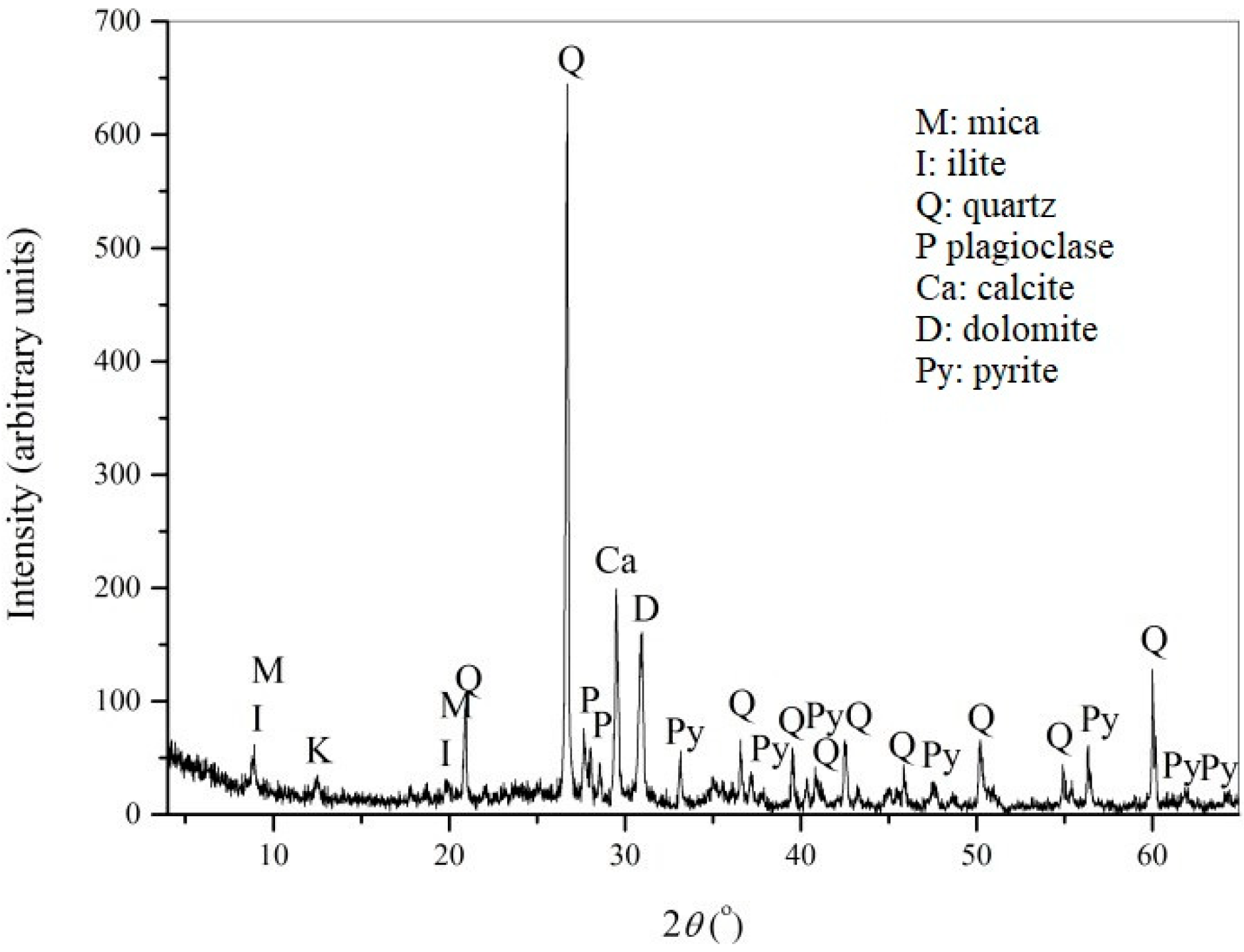
2.2. Characterization of the Flotation Tailings
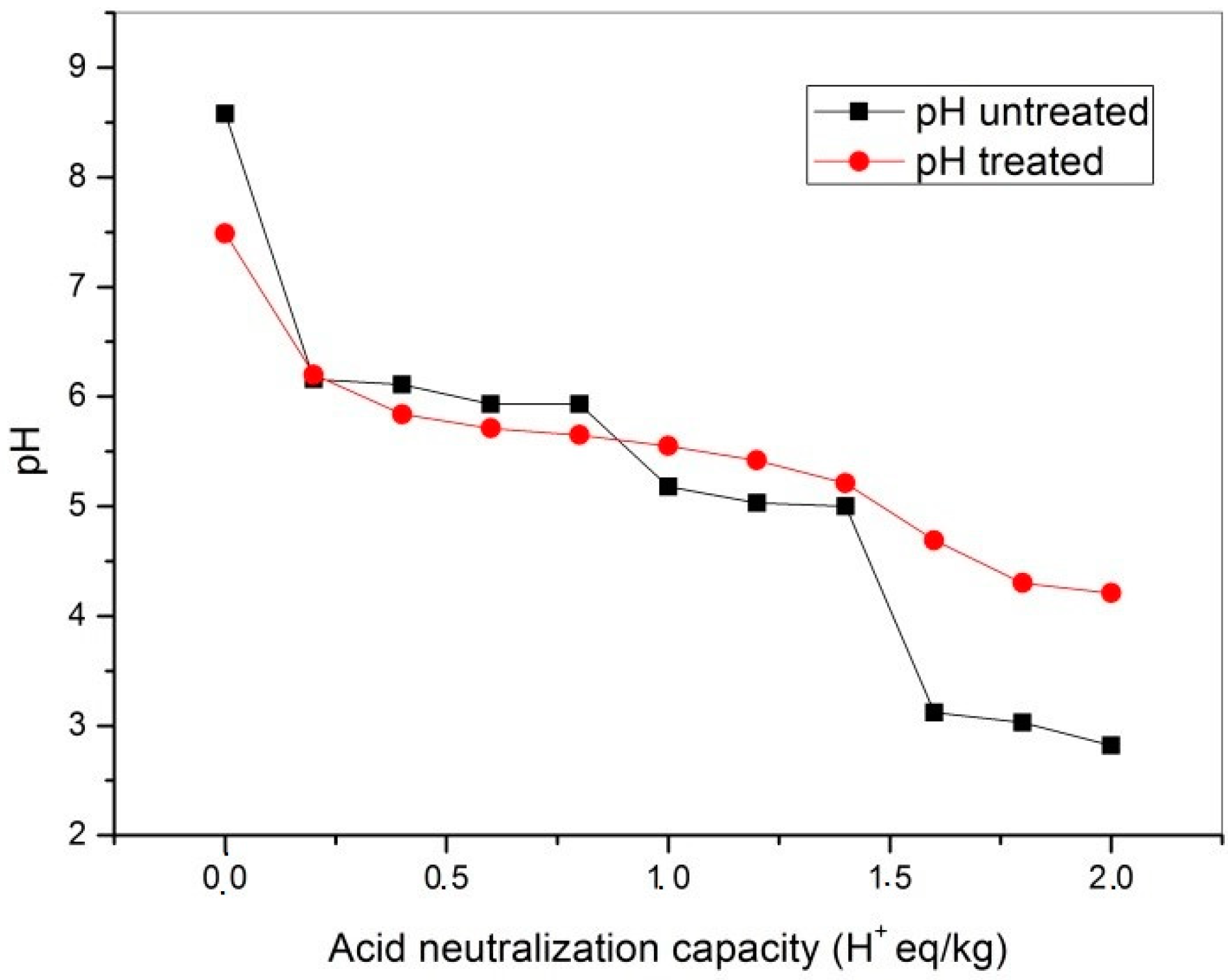
2.3. Acid Neutralization Capacity
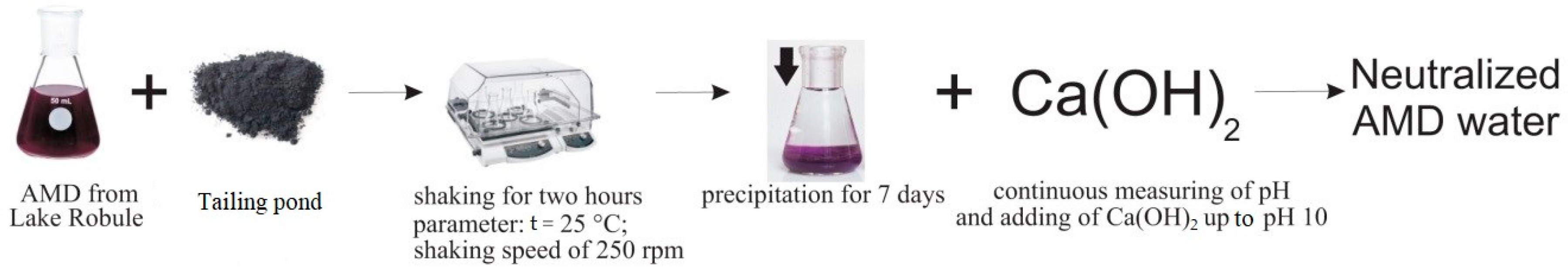
2.4. Determination of the Optimal Quantity of Flotation Tailings Required for Neutralization of the Lake Water Samples and Metal Precipitation
2.5. Treatment of the Lake Water Samples with Hydrated Lime and NaOH
2.6. Simulation of Metal Precipitation Using PHREEQC Software
3. Results and Discussion
3.1. Physical and Chemical Properties of the Water Collected from Lake Robule
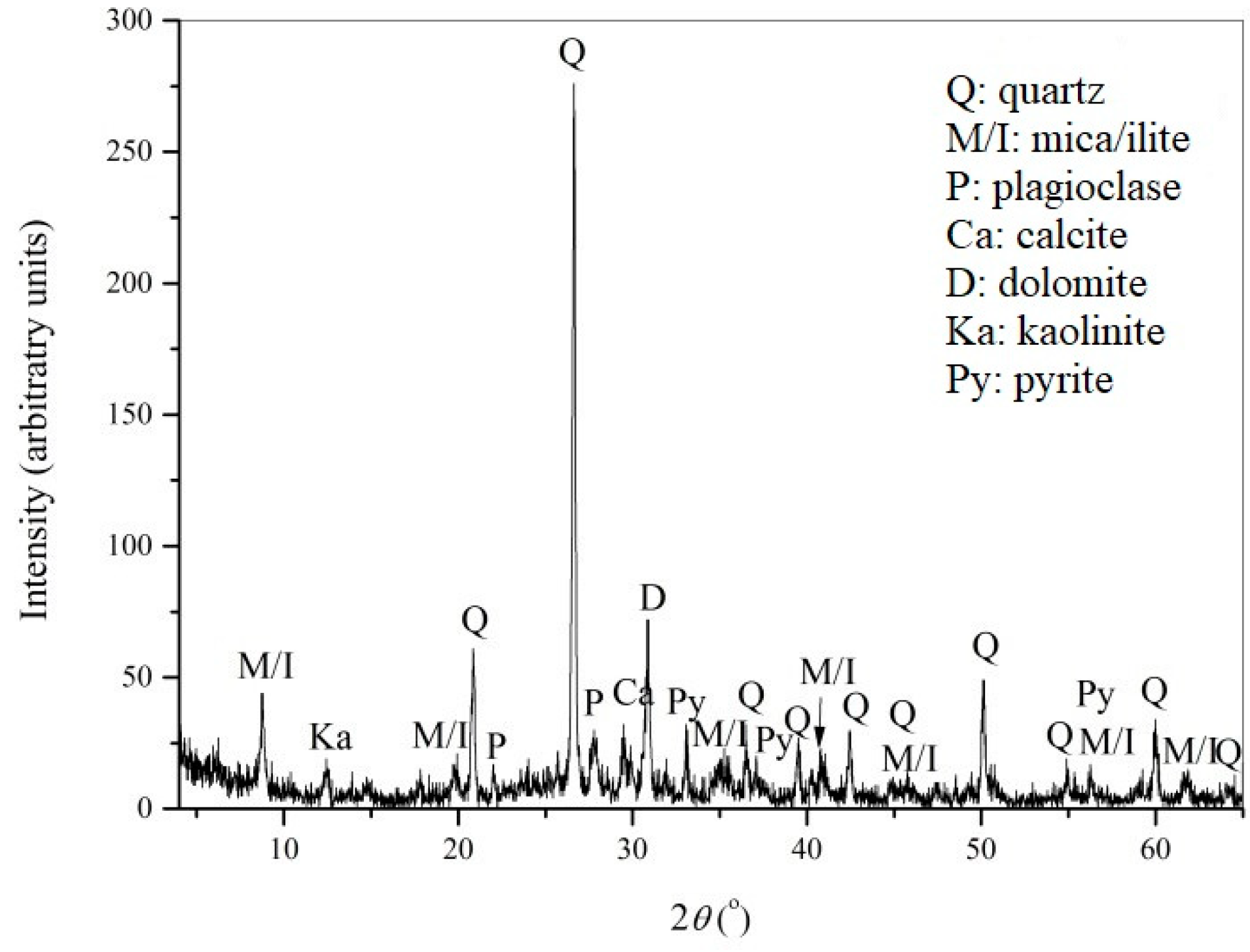
3.2. Characterization of the Flotation Tailings’ Sample from Copper Mine Majdanpek
3.3. Results of the Acid Neutralization Capacity Test
3.4. Treatment of Water from Lake Robule by Using Flotation Tailings
3.4.1. Determination of the Optimal Quantity of Flotation Tailings Required for Neutralization of the Lake Water Samples
3.4.2. Experimental Results of the Treatment
3.5. Results of the PHREEQC Software Simulation of the Water Treatment
3.6. Mechanism of Acid Neutralization and Precipitation of Metals during the Treatment
| Fe2+ |  | Fe3+ |  | α−tFeO(OH) |  | α−tFe2O3 |
| Oxidation | Precipitation | Dehydration |
3.7. Comparison of the Lake Water Neutralization with Flotation Tailings and NaOH
3.8. Post-Treatment of the Lake Water Samples with Hydrated Lime
3.9. Characterization of the Solid Residue after the Treatment of Water from Lake Robule with Flotation Tailings
4. Conclusions
- Flotation tailings’ samples collected from the flotation tailings dump of Copper Mine Majdanpek were rich in carbonate minerals (calcite and dolomite) and possessed significant acid neutralization capacity.
- Laboratory experiments confirmed that the flotation tailings could be applied in order to neutralize water from the extremely acidic Lake Robule located near the town of Bor (Serbia).
- After neutralization with flotation tailings, over 99% of Al, Fe, and Cu precipitated, 98% of Pb, and 92% of Zn. The concentrations of Cd and Mn increased due to leaching of these metals from flotation tailings.
- In order to remove Mn below discharge limits, application of hydrated lime was required in order to increase the pH up to 10.
- The flotation tailings could be applied in the active treatment of AMD in combination with hydrated lime. The results of this research were a step further in the implementation of the principles of the sustainable development including minimization, treatment, and reuse of waste within the same industry.
- Our future activities shall be performed in order to propose a continuous neutralization process for the application of the flotation tailings as an alternative material for acid mine drainage remediation.
- Furthermore, this methodology will be studied in scale-up conditions, in order to obtain more realistic data on the consumption of flotation tailing amount and percent of acid mine water
Author Contributions
Funding
Conflicts of Interest
References
- Dimitrijević, M.D. Pyrite Oxidation and Acid Mine Drainage; University of Belgrade: Belgrade, Serbia, 2013; pp. 75–95. (In Serbian) [Google Scholar]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, G.; Trumić, M.; Trumić, M.; Antić, D.V. Mining waste management: Genesis and possibility of processing. Recycl. Sustain. Dev. 2011, 4, 37–43. (In Serbian) [Google Scholar]
- Korać, M.; Kamberović, Ž. Characterization of wastewater streams from Bor site. Metall. Mater. Eng. 2007, 13, 41–51. [Google Scholar]
- Stevanović, Z.; Obradović, L.; Marković, R.; Jonović, R.; Avramović, L.; Bugarin, M.; Stevanović, J. Mine waste water management in the Bor municipality in order to protect the Bor River water. In Waste Water—Treatment Technologies and Recent Analytical Developments; Einschlag, F.S.G., Carlos, L., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Stanković, S.; Morić, I.; Pavić, A.; Vasiljević, B.; Johnson, D.B.; Cvetković, V. Investigation of the microbial diversity of an extremely acidic metal-rich water body (Lake Robule, Bor, Serbia). J. Serb. Chem. Soc. 2014, 79, 729–741. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Papić, P.; Dragišić, V.; Matić, V.; Vrvić, M.M. Long term studies on the impact of thionic bacteria on the global pollution of waters with toxic ions. Adv. Mater. Res. 2009, 71, 105–108. [Google Scholar] [CrossRef]
- Pavlović, J.; Stopić, S.; Friedrich, B.; Kamberović, Z. Selective removal of heavy metals from metal-bearing wastewater in a cascade line reactors. Environ. Sci. Pollut. Res. Int. 2007, 14, 518–522. [Google Scholar] [CrossRef]
- Masuda, N.; Marković, R.; Bozić, D.; Bessho, M.; Inoue, T.; Hoshino, K.; Ishiyama, D.; Stevanović, Z. Experimental results of metal recovery by a two-step neutralization process from AMD from a copper mine in Serbia. In Proceedings of the IMWA 2019, Conference “Mine Water: Technological and Ecological Challenges”, Perm, Russia, 15–19 July 2019; Wolkersdorfer, C., Khayrulina, E., Polyakova, S., Bogush, A., Eds.; Perm State University: Perm, Russia, 2019; pp. 232–237. [Google Scholar]
- Stopic, S.; Dertmann, C.; Xakalashe, B.; Lucas, H.; Alkan, G.; Aygmurlu, B.; Friedrich, B. A near zero waste valorization vision for bauxite residue through experimental results. In Proceedings of the XXI YuCorr International Conference, Tara Mountain, Serbia, 17–20 September 2019; Pavlović, M., Pavlović, M., Eds.; Serbian Society of Corrosion and Materials Protection (UISKoZaM): Belgrade, Serbia, 2019; pp. 120–125. [Google Scholar]
- Mwewa, B.; Stopic, S.; Ndlovu, S.; Simate, G.; Xakalashe, B.; Friedrich, B. Synthesis of poly-alumino-ferric sulphate coagulant from acid mine drainage by precipitation. Metals 2019, 9, 1166. [Google Scholar] [CrossRef] [Green Version]
- Masindi, V.; Ndiritu, J.G.; Maree, J.P. Fractional and step-wise recovery of chemical species from acid mine drainage using calcined cryptocrystalline magnesite nano-sheets: An experimental and geochemical modelling approach. J. Environ. Chem. Eng. 2018, 6, 1634–1650. [Google Scholar] [CrossRef]
- Pérez-López, R.; Castillo, J.; Quispe, D.; Nieto, J.M. Neutralization of acid mine drainage using the final product from CO2 emissions capture with alkaline paper mill waste. J. Hazard. Mater. 2010, 177, 762–772. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total. Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Aube, B.; Zinck, J. Lime treatment of acid mine drainage in Canada. In Proceedings of the Brazil-Canada Seminar on Mine Rehabilization: Techological innovations, Florianopolis, Brazil, 1–3 December 2003; Barbosa, J.P., Ed.; CETEM/MCT: Rio de Janiero, Brazil, 2003; pp. 26–30. [Google Scholar]
- Ivšić-Bajčeta, D.; Kamberović, Ž.; Korać, M.; Gavrilovski, M. A solidification/stabilization process for wastewater treatment of sludge from primary copper smelter. J. Serb. Chem. Soc. 2013, 78, 725–739. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Johnes, B.W.; Millar, G.J. Alternative neutralisation materials for acid mine drainage treatment. J. Water Process. Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Dold, B. Sustainability in metal mining: From exploration, over processing to mine waste management. Rev. Environ. Sci. Bio/Technol. 2008, 7, 275–285. [Google Scholar] [CrossRef]
- Kraus, W.; Noize, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Wahlström, M.; Laine-Ylijoki, J.; Kaartinen, T.; Hjelmar, O.; Bendz, D. Acid Neutralization Capacity of Waste—Specification of Requirement Stated in Landfill Regulations; Nordic Council of Ministers: Copenhagen, Denmark, 2009; p. 51. [Google Scholar]
- Stegemann, J.A.; Cote, P.L. Summary of an investigation of test methods for solidified waste evaluation. Waste Manag. 1990, 10, 41–52. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appel, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2014; p. 497. [Google Scholar]
- Štulović, M.; Radovanović, D.; Kamberović, Ž.; Korać, M.; Anđić, Z. Assessment of leaching characteristics of solidified products containing secondary alkaline lead slag. Int. J. Environ. Res. Publi. Health 2019, 16, 2005. [Google Scholar] [CrossRef] [Green Version]
- Madzivire, G.; Gitari, W.M.; Vadapalli, V.R.K.; Ojumu, T.V.; Petrik, L.F. Fate of sulphate removed during the treatment of circumneutral mine water and acid mine drainage with coal fly ash: Modelling and experimental approach. Miner. Eng. 2011, 24, 1467–1477. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Schweda, M.; Stopić, S.; Friedrich, B. Techno-economical comparison between chemical precipitation and electrocoagulation for heavy metal removal in industrial wastewater. Met. Berl. 2007, 61, 208–214. [Google Scholar]
- Dimitrijević, M.D. Acid mine drainage. Bakar 2012, 37, 33–44. (In Serbian) [Google Scholar]
- Dimitrijević, M.D.; Alagić, S.Č. Passive treatment of acid mine drainage. Bakar 2012, 37, 57–68. (In Serbian) [Google Scholar]
- Dimitrijević, M.D.; Nujkić, M.M.; Milić, S.M. The lime Treatment of acid mine drainage. Bakar 2012, 37, 45–56. (In Serbian) [Google Scholar]
- Sverdrup, H.U. The Kinetics of Base Cation Release Due to Chemical Weathering; Lund University Press: Lund, Sweden, 1990; p. 245. [Google Scholar]
- Lawrence, R.W.; Scheske, M. A method to calculate the neutralization potential of mining waste. Environ. Geol. 1997, 32, 100–106. [Google Scholar] [CrossRef]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Benson, C.H.; Aydilek, A.H.; Edil, T.B. Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manag. 2015, 38, 174–184. [Google Scholar] [CrossRef]
- Masuda, N.; Marković, R.; Bessho, M.; Božić, D.; Obradović, L.; Marinković, V.; Ishiyama, D.; Stevanović, Z. A new approach to recover dissolved metals in AMD by two-step pH control on the neutralization method. In Proceedings of the 13th International Mine Water Association Congress “Mine Water and Circular Economy” (IMWA 2017), Lappeenranta, Finland, 25–30 June 2017; Wolkersdrofer, C., Sartz, L., Silanpaa, M., Hakkinen, A., Eds.; Lappeenranta University of Technology: Lappeenranta, Finland, 2017; pp. 1111–1118. [Google Scholar]
- Park, S.M.; Yoo, J.C.; Ji, S.W.; Yang, J.S.; Baek, K. Selective recovery of dissolved Fe, Al, Cu and Zn in acid mine drainage based on modeling to predict precipitation pH. Environ. Sci. Pollut. Res. Int. 2015, 22, 3013–3022. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Giannopoulou, I.; Panias, D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions. Hydrometallurgy 2008, 90, 137–146. [Google Scholar] [CrossRef]
- Park, S.M.; Yoo, J.C.; Ji, S.W.; Yang, J.S.; Baek, K. Selective recovery of Cu, Zn, and Ni from acid mine drainage. Environ. Geochem. Health 2013, 35, 735–743. [Google Scholar] [CrossRef]
- Patterson, J.W.; Allen, H.E.; Scala, J.J. Carbonate precipitation for heavy metals pollutants. J. Water Pollut. Control. Fed. 1977, 49, 2397–2410. [Google Scholar]

| Characteristic | Unit | Value | Characteristic | Unit | Value |
|---|---|---|---|---|---|
| Temperature | °C | 7 | Fe3+ | mg/L | 286.9 |
| Color | - | Yes | Fe2+ | mg/L | 0.013 |
| Odor | - | None | Boron (B) | mg/L | 0.0201 |
| pH | - | 2.47 | Vanadium (V) | mg/L | <0.002 |
| Eh | - | 615.1 | Cadmium (Cd) | mg/L | 0.012 |
| Iron (Fe) | mg/L | 287 | Selenium (Se) | mg/L | <0.001 |
| Chromium (Cr) | mg/L | 0.002 | Silver (Ag) | mg/L | 0.013 |
| Copper (Cu) | mg/L | 66.39 | Carbonates (CO32−) | mg/L | 1.2 |
| Nickel (Ni) | mg/L | 0.6 | Hydrogen carbonates (HCO3−) | mg/L | 2.44 |
| Arsenic (As) | mg/L | <0.007 | Antimony (Sb) | mg/L | <0.001 |
| Zinc (Zn) | mg/L | 17.6 | Barium (Ba) | mg/L | <0.001 |
| Lead (Pb) | mg/L | 0.188 | Beryllium (Be) | mg/L | 0.013 |
| Manganese (Mn) | mg/L | 66 | Cobalt (Co) | mg/L | <0.001 |
| Aluminum (Al) | mg/L | 1017.62 | Sulfate (SO42−) | g/L | 7.5 |
| Element | Cu (%) | Zn (%) | Fe (%) | S (%) | Ag (ppm) | Au (ppm) | Mn (%) | Al (%) | Cd (%) | Pb (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.072 | 0.086 | 10.7 | 7.01 | 1.667 | 0.405 | 0.138 | 5.07 | 0.0006 | 0.0079 |
| Pulp Density (%) | Mass (g) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 120 | 240 | 1440 | 4320 | 10,080 | ||
| 1 | 0.5 | 2.53 | 2.93 | 3.06 | 3.08 | 3.1 | 3.8 | 4.23 | 4.24 |
| 3 | 1.5 | 2.89 | 3.23 | 3.97 | 4.12 | 4.21 | 4.34 | 4.71 | 5.3 |
| 5 | 2.5 | 2.67 | 3.85 | 4.25 | 4.33 | 4.43 | 4.77 | 5.56 | 6.46 |
| 10 | 5 | 2.85 | 4.44 | 4.81 | 5.19 | 5.43 | 5.92 | 6.67 | 6.76 |
| 15 | 7.5 | 2.88 | 4.76 | 5.47 | 5.81 | 5.92 | 6.23 | 6.8 | 7 |
| 20 | 10 | 3.14 | 5.03 | 5.71 | 6.02 | 6.16 | 6.32 | 6.86 | 7.06 |
| 25 | 12.5 | 3.16 | 5.29 | 5.86 | 6.1 | 6.35 | 6.51 | 6.86 | 7.04 |
| 30 | 15 | 4.11 | 5.44 | 5.93 | 6.15 | 6.26 | 6.51 | 6.88 | 7.07 |
| 40 | 20 | 4.44 | 5.62 | 6.01 | 6.28 | 6.41 | 6.57 | 6.95 | 7.2 |
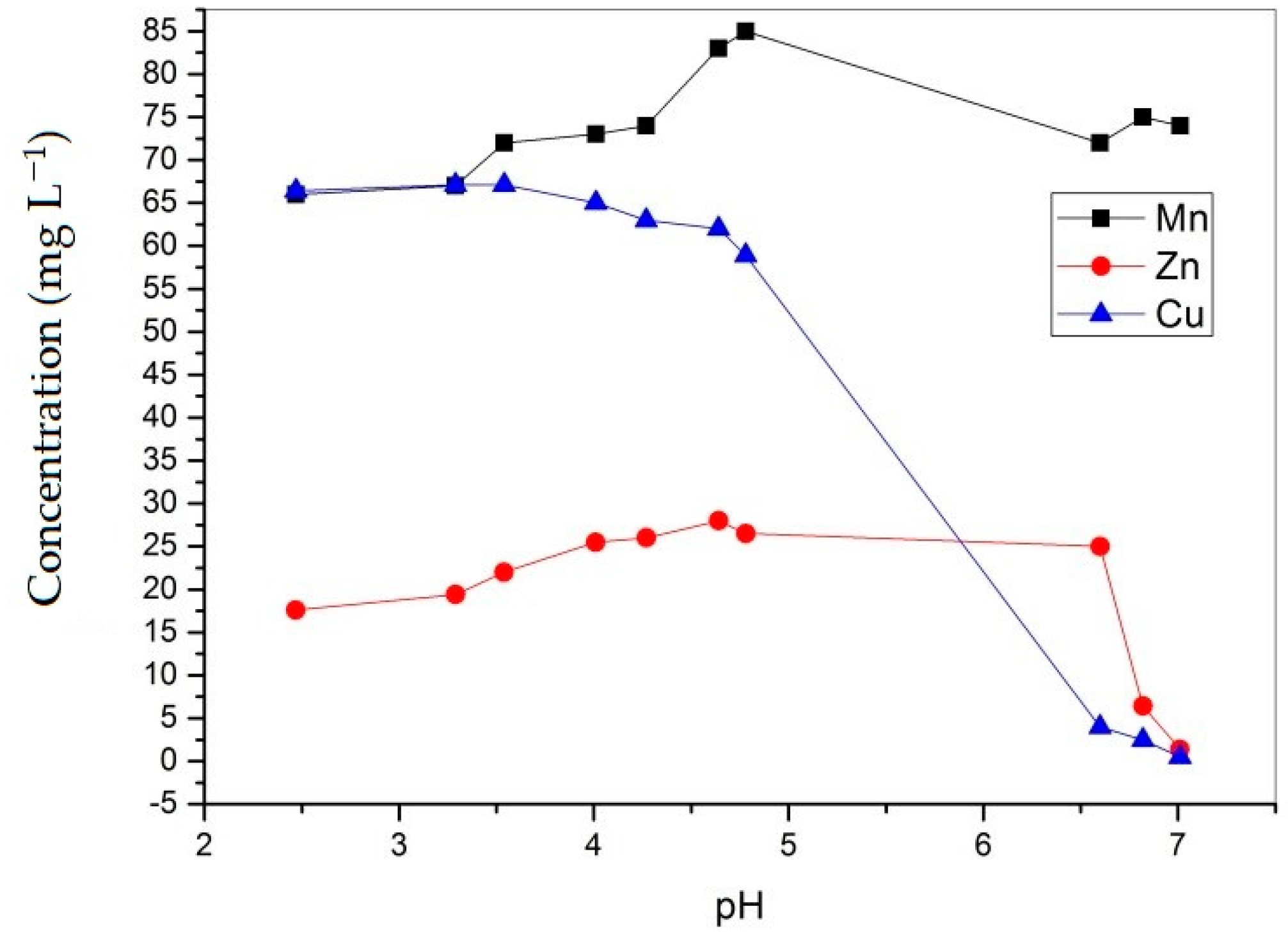
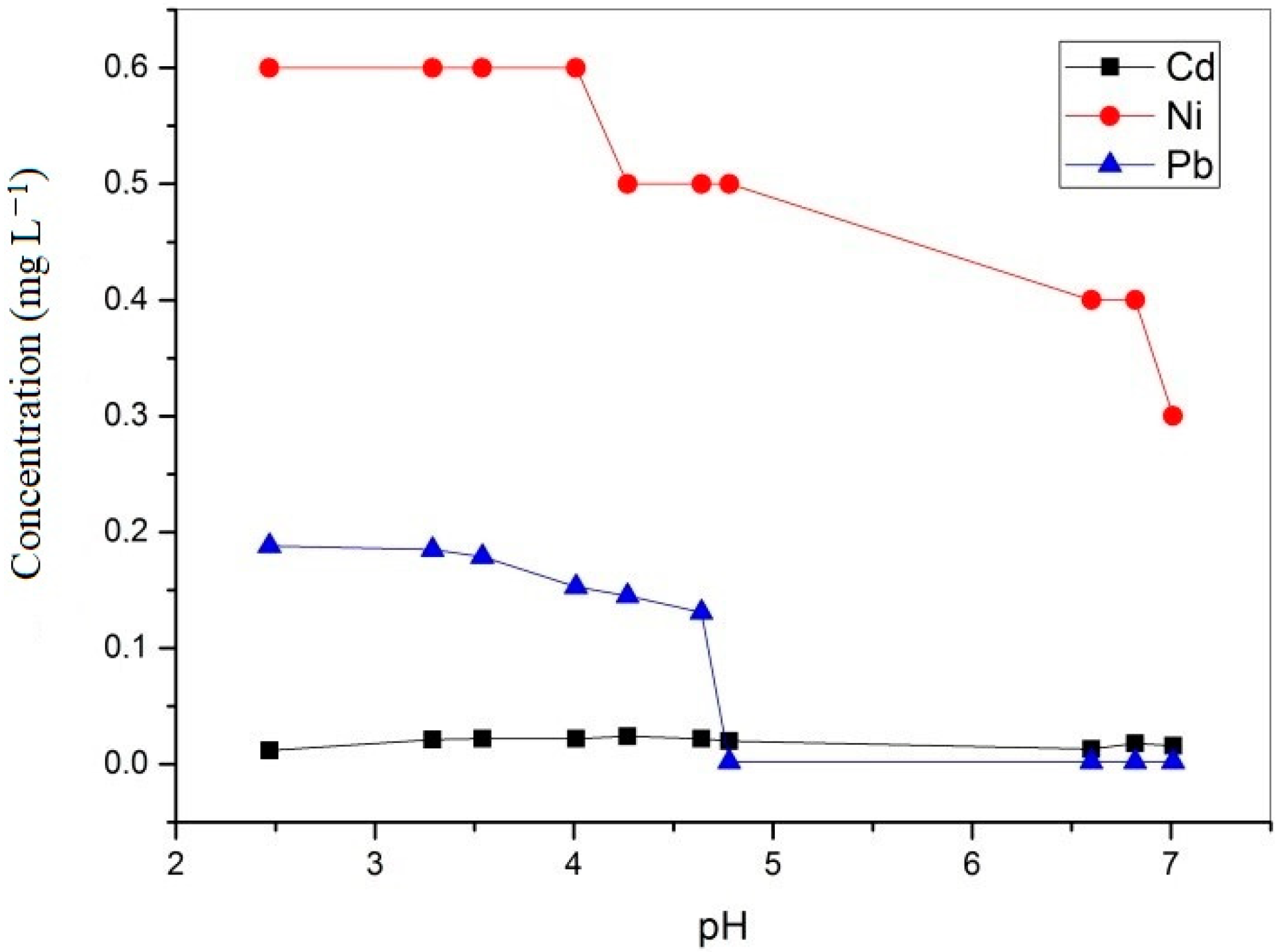
| Time (min) | pH | Cu mg/L | Fe mg/L | Al mg/L | As mg/L | Cd mg/L | Mn mg/L | Ni mg/L | Zn mg/L | Pb mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.47 | 66.39 | 287 | 1017.62 | <0.007 | 0.012 | 66 | 0.6 | 17.6 | 0.188 |
| 5 | 3.29 | 67.1 | 205 | 981 | <0.007 | 0.021 | 67 | 0.6 | 19.4 | 0.185 |
| 10 | 3.54 | 67.1 | 151 | 916 | <0.007 | 0.022 | 72.75 | 0.6 | 22 | 0.179 |
| 15 | 4.01 | 65 | 26 | 832 | <0.007 | 0.022 | 73.75 | 0.6 | 25.5 | 0.153 |
| 30 | 4.27 | 63 | 5.18 | 471 | <0.007 | 0.024 | 73.75 | 0.5 | 26 | 0.145 |
| 60 | 4.64 | 62 | 8.5 | 213 | <0.007 | 0.022 | 83 | 0.5 | 28 | 0.131 |
| 120 | 4.78 | 58.9 | 0.268 | 21 | <0.007 | 0.02 | 85.5 | 0.5 | 26.5 | 0.002 |
| 1440 | 6.60 | 3.98 | 0.16 | 0.288 | <0.007 | 0.013 | 72 | 0.4 | 25 | 0.002 |
| 4320 | 6.82 | 2.49 | 0.09 | 0.1 | <0.007 | 0.018 | 75.5 | 0.4 | 6.4 | 0.002 |
| 10080 | 7.01 | 0.49 | 0.059 | 0.1 | <0.007 | 0.016 | 74 | 0.3 | 1.4 | 0.002 |
| Precipitation (%) | - | >99% | >99% | >99% | - | +33% | +12% | 50% | 92% | 98% |
| Element | Mineral | Time (min) | 0 | 5 | 30 | 60 | 120 | 1440 | Last |
|---|---|---|---|---|---|---|---|---|---|
| pH | 2.5 | 4.3 | 4.7 | 5.2 | 5.7 | 6.6 | 7.0 | ||
| Formula | - | - | - | - | - | - | - | ||
| Al | Al(OH)3(a) | Al(OH)3 | −6.60 | −4.12 | −1.62 | −1.59 | −2.30 | −0.21 | −0.21 |
| Gibbsite | Al(OH)3 | −3.84 | −1.37 | 1.14 | 1.16 | 0.46 | 2.54 | 2.54 | |
| Fe | Fe(OH)3(a) | - | −0.80 | 0.72 | 1.61 | 1.62 | 1.85 | 2.12 | 2.09 |
| Goethite | FeOOH | 4.83 | 6.35 | 7.24 | 7.25 | 7.49 | 7.75 | 7.73 | |
| Hematite | Fe2O3 | 11.64 | 14.68 | 16.46 | 16.48 | 16.95 | 17.48 | 17.42 | |
| Melanterite | FeSO4⋅7H2O | −10.03 | −10.83 | −10.37 | −8.36 | −8.15 | −9.00 | −10.00 | |
| Siderite | FeCO3 | −12.25 | −11.63 | −8.44 | −5.97 | −5.56 | −2.76 | −3.75 | |
| Mn | Hausmannite | Mn3O4 | −32.25 | −29.71 | −26.04 | −23.62 | −23.26 | −8.25 | −8.53 |
| Manganite | MnOOH | −10.87 | −10.49 | −9.73 | −8.92 | −8.88 | −3.35 | −3.52 | |
| Pyrochroite | Mn(OH)2 | −13.57 | −11.79 | −9.65 | −8.87 | −8.58 | −4.62 | −4.57 | |
| Pyrolusite | MnO2⋅H2O | −15.22 | −16.24 | −16.86 | −16.01 | −16.23 | −9.13 | −9.52 | |
| Rhodochrosite | MnCO3 | −8.28 | −6.51 | −3.23 | −2.45 | −2.17 | 1.36 | 1.42 | |
| Zn | Smithsonite | ZnCO3 | −10.13 | −8.20 | −4.99 | −4.22 | −3.94 | −1.42 | −0.37 |
| Zn(OH)2(e) | Zn(OH)2 | −10.55 | −8.61 | −6.53 | −5.75 | −5.47 | −1.48 | −2.53 | |
| Pb | Anglesite | PbSO4 | −3.43 | −2.85 | −2.28 | −2.24 | −2.23 | −2.93 | −3.03 |
| Cerussite | PbCO3 | −8.81 | −6.82 | −3.50 | −3.02 | −2.80 | 0.15 | 0.05 | |
| Pb(OH)2 | Pb(OH)2 | −9.42 | −7.41 | −5.23 | −4.74 | −4.52 | −1.15 | −1.25 | |
| Cd | Cd(OH)2 | Cd(OH)2 | −15.10 | −13.12 | −10.99 | −10.22 | −9.98 | −6.11 | −6.08 |
| CdSO4 | CdSO4 | −11.85 | −11.29 | −10.76 | −10.45 | −10.42 | −10.63 | −10.60 | |
| Otavite | CdCO3 | −10.36 | −8.39 | −5.12 | −4.36 | −4.12 | −0.68 | −0.64 |
| Element | Concentration after FT (mg L−1) | Concentration after NaOH (mg L−1) |
|---|---|---|
| Copper (Cu) | 0.54 | 0.48 |
| Iron (Fe) | 0.13 | 0.18 |
| Aluminum (Al) | 1.53 | 1.47 |
| Arsenic (As) | <0.007 | <0.007 |
| Cadmium (Cd) | 0.10 | 0.10 |
| Manganese (Mn) | 72.00 | 40.75 |
| Nickel (Ni) | 0.10 | 0.13 |
| Zinc (Zn) | 0.61 | 0.36 |
| Lead (Pb) | 0.57 | 0.66 |
| Antimony (Sb) | 0.43 | 0.41 |
| Barium (Ba) | 0.01 | 0.06 |
| Beryllium (Be) | 0.01 | 0.01 |
| Boron (B) | 0.14 | 0.15 |
| Vanadium (V) | <0.002 | <0.002 |
| Cobalt (Co) | 0.15 | 0.16 |
| Chromium (Cr) | 0.01 | 0.02 |
| Selenium (Se) | 0.02 | 0.03 |
| Silver (Ag) | 0.21 | 0.01 |
| Element | Concentration (mg L−1) | National Discharge Limits for Municipal Waste Waters (mg L−1) |
|---|---|---|
| Copper (Cu) | 0.023 | 2 |
| Iron (Fe) | 0.137 | 200 |
| Aluminum (Al) | 0.074 | / |
| Arsenic (As) | 0.007 | 0.2 |
| Cadmium (Cd) | <0.001 | 0.1 |
| Manganese (Mn) | 0.062 | 5 |
| Nickel (Ni) | 0.002 | 1 |
| Zinc (Zn) | 0.012 | 2 |
| Lead (Pb) | <0.002 | 0.2 |
| SO42− | 4340 | / |
| Antimony (Sb) | <0.017 | / |
| Barium (Ba) | 0.025 | / |
| Beryllium (Be) | 0.007 | / |
| Boron (B) | 0.099 | / |
| Vanadium (V) | <0.002 | / |
| Cobalt (Co) | <0.001 | / |
| Chromium (Cr) | <0.001 | 1 |
| Selenium (Se) | <0.001 | / |
| Silver (Ag) | 0.013 | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronijević, N.; Stanković, S.; Radovanović, D.; Sokić, M.; Marković, B.; Stopić, S.R.; Kamberović, Ž. Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia). Metals 2020, 10, 16. https://doi.org/10.3390/met10010016
Petronijević N, Stanković S, Radovanović D, Sokić M, Marković B, Stopić SR, Kamberović Ž. Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia). Metals. 2020; 10(1):16. https://doi.org/10.3390/met10010016
Chicago/Turabian StylePetronijević, Nela, Srđan Stanković, Dragana Radovanović, Miroslav Sokić, Branislav Marković, Srećko R. Stopić, and Željko Kamberović. 2020. "Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia)" Metals 10, no. 1: 16. https://doi.org/10.3390/met10010016






