Solvent Extraction of Ni and Co from the Phosphoric Acid Leaching Solution of Laterite Ore by P204 and P507
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Laterite Ore
2.1.2. Chemical Reagents
2.2. Methods
2.2.1. Experimental Procedures
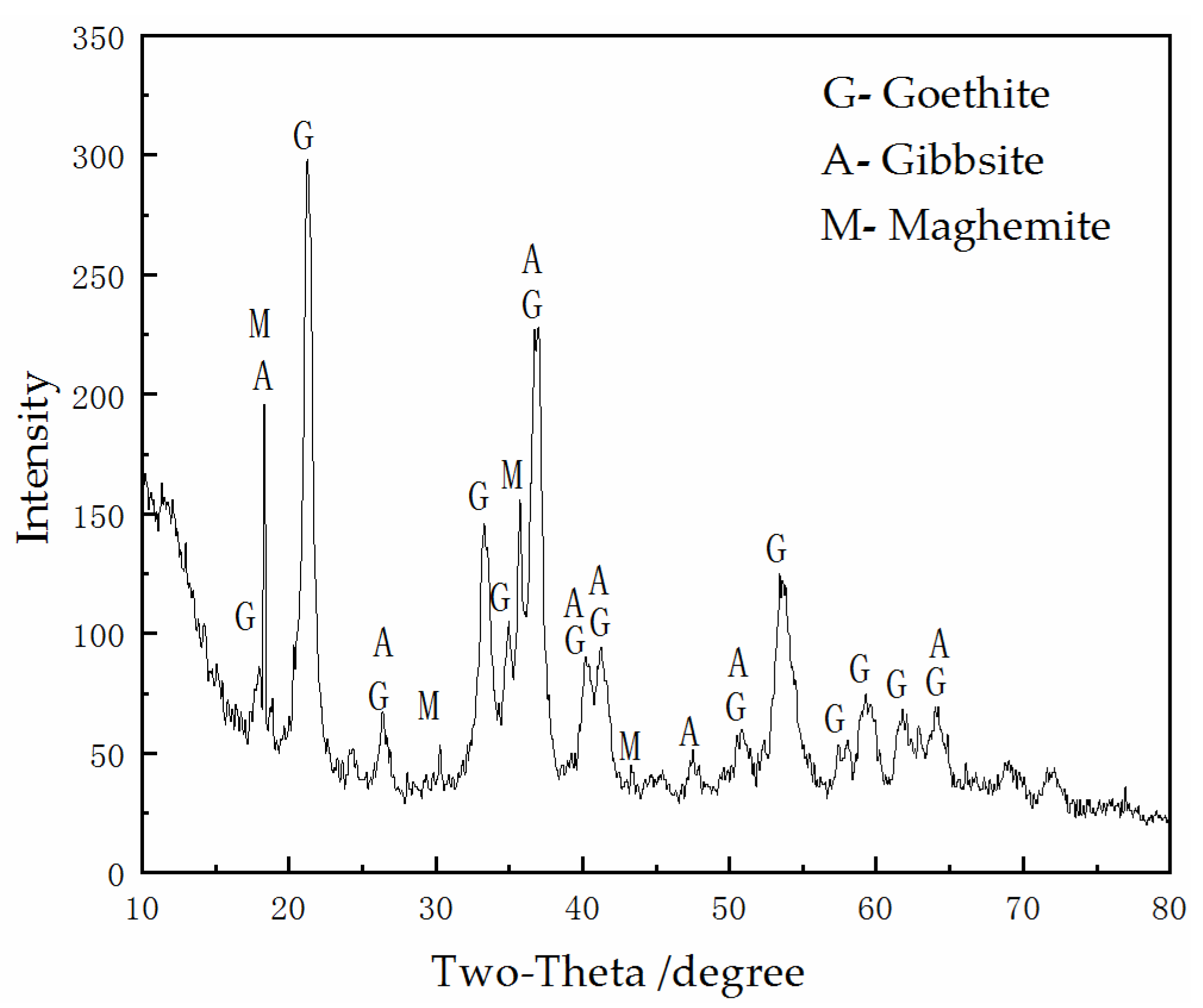
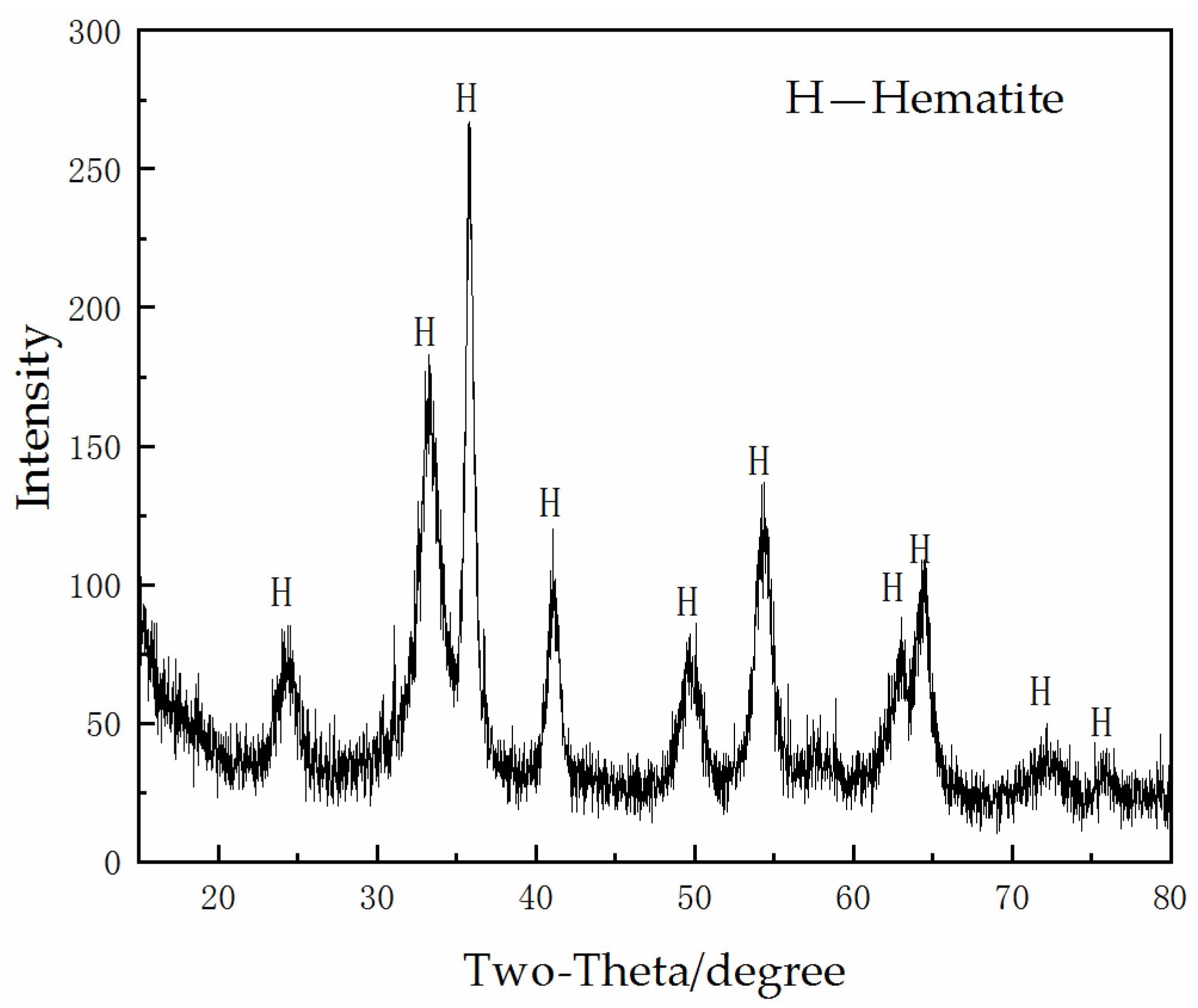
2.2.2. Pre-Roasting
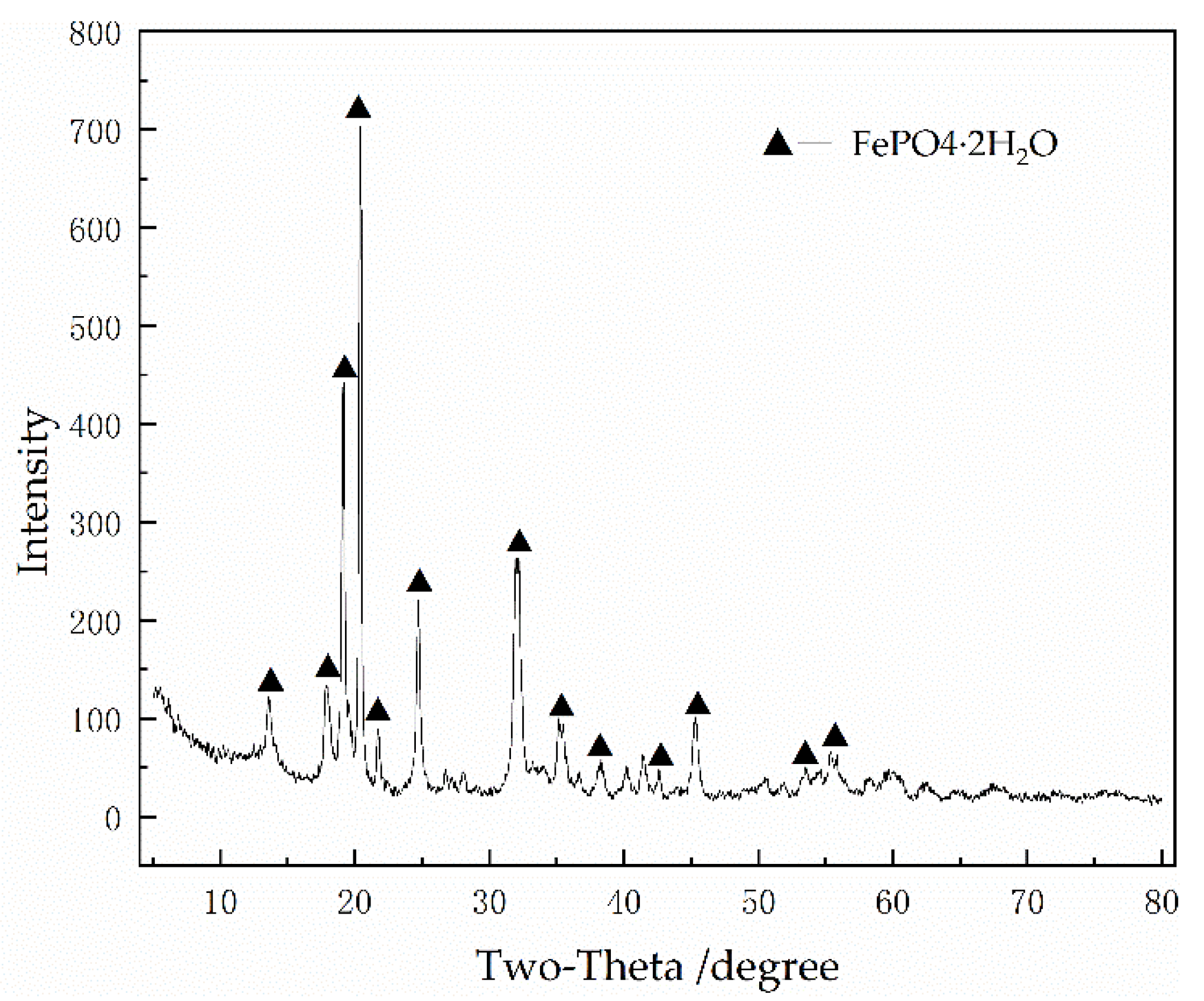
2.2.3. Phosphoric Acid Leaching
2.2.4. Solvent Extraction
2.2.5. Characterization
3. Results and Discussion
3.1. Pre-Roasting and Phosphoric Acid Leaching
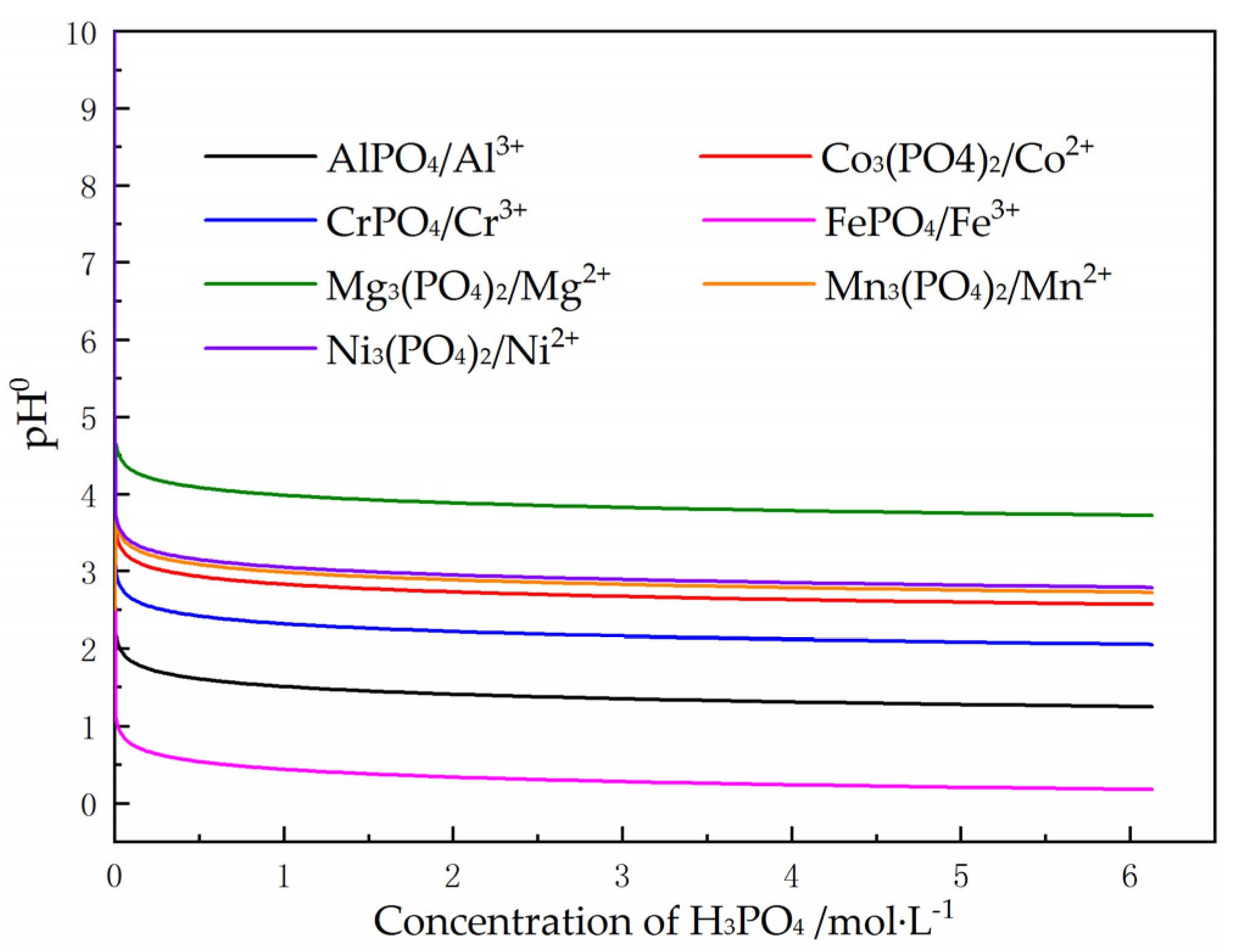
3.2. Effect of pH Change on the Composition of Leaching Solution
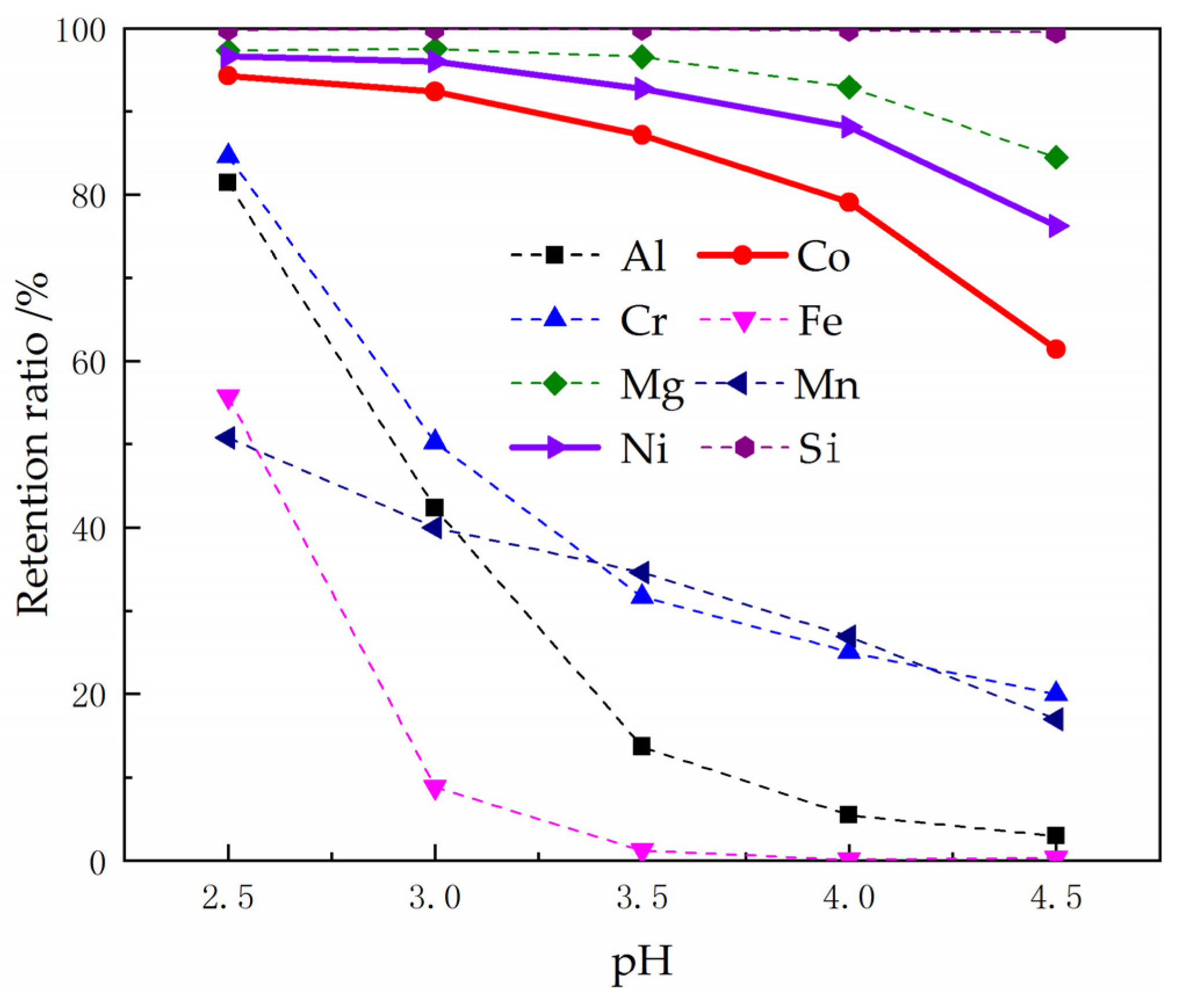
3.2.1. Preliminary Separation of Impurity Metal Ions by Adjusting Solution pH to Precipitate
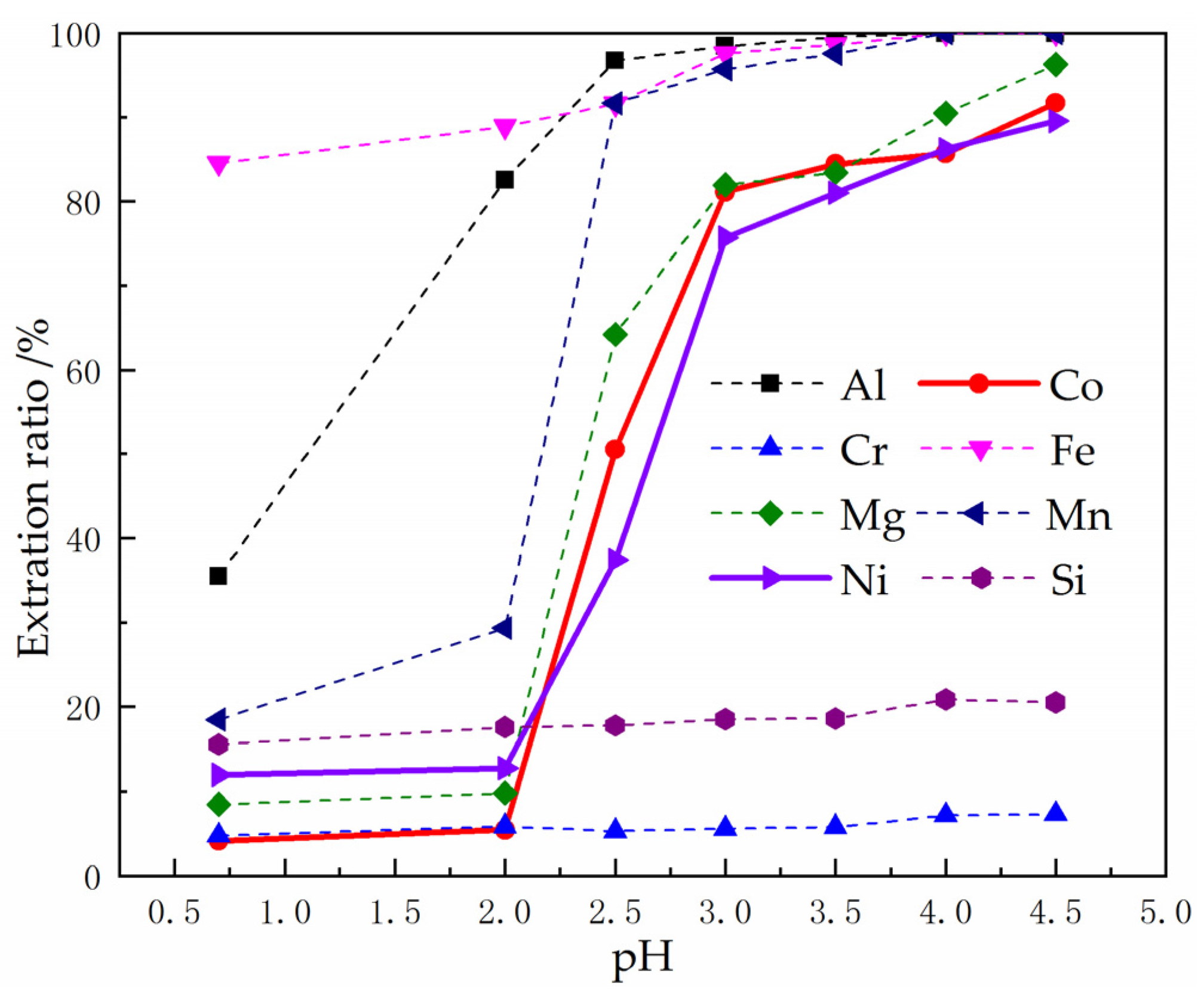
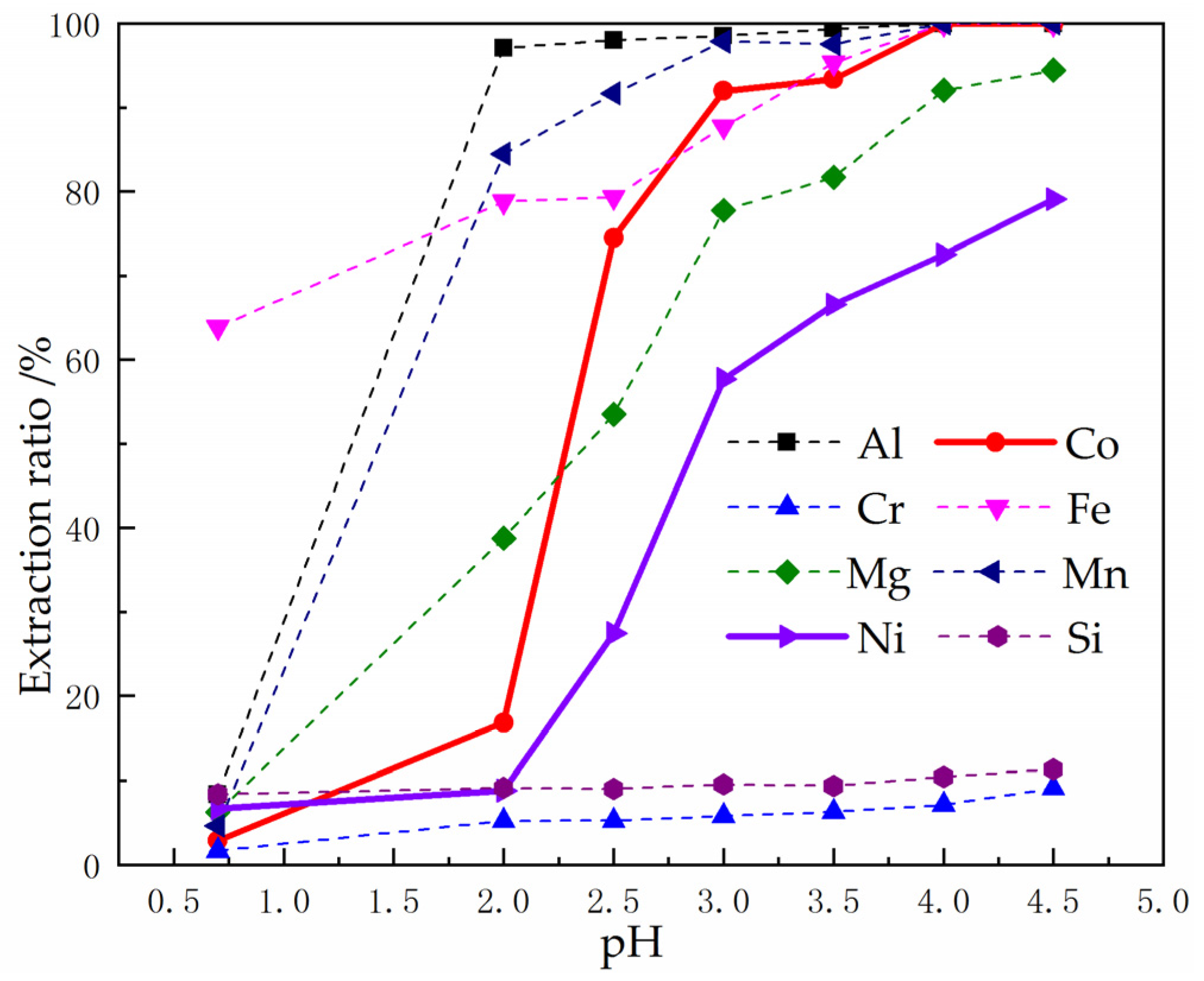
3.2.2. Influence of pH on the Extraction
Using P204 as Extractant
Using P507 as Extractant
3.3. Using P204 to Remove Al3+, Fe3+ and Mn2+
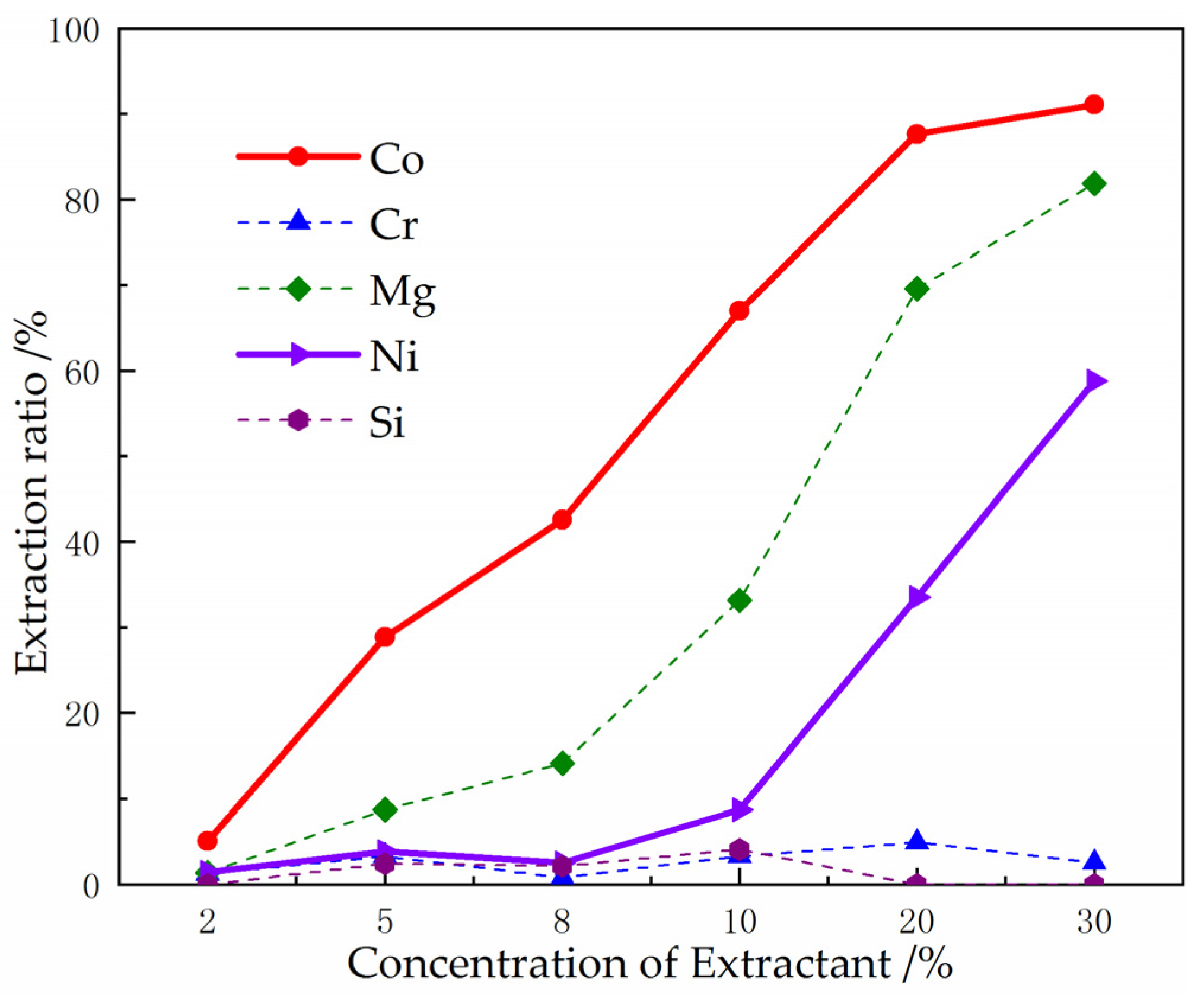
3.4. Using P507 to Extract Co2+ from Raffinate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Supriyatna, Y.I.; Sihotang, I.H.; Sudibyo, A. Preliminary Study of Smelting of Indonesian Nickel Laterite Ore using an Electric Arc Furnace. Mater. Today Proc. 2019, 13, 127–131. [Google Scholar] [CrossRef]
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- Kim, J.; Dodbiba, G.; Tanno, H.; Okaya, K.; Matsuo, S.; Fujita, T. Calcination of low-grade laterite for concentration of Ni by magnetic separation. Min. Eng. 2010, 23, 282–288. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Fornasiero, D.; Filippov, L. Upgrading nickel in laterite ores by flotation. Min. Eng. 2018, 121, 100–106. [Google Scholar] [CrossRef]
- Mu, W.; Lu, X.; Cui, F.; Luo, S.; Zhai, Y. Transformation and leaching kinetics of silicon from low-grade nickel laterite ore by pre-roasting and alkaline leaching process. Trans. Nonferr. Met. Soc. China 2018, 28, 169–176. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H.; Ma, B.; Wang, C.; Luo, X. Mineralogical characterization and design of a treatment process for Yunnan nickel laterite ore, China. Int. J. Min. Process. 2017, 159, 51–59. [Google Scholar] [CrossRef]
- Chen, S.; Guo, S.; Jiang, L.; Xu, Y.; Ding, W. Thermodynamic of selective reduction of laterite ore by reducing gases. Trans. Nonferr. Met. Soc. China 2015, 25, 3133–3138. [Google Scholar] [CrossRef]
- Garces-Granda, A.; Lapidus, G.T.; Restrepo-Baena, O.J. The effect of calcination as pre treatment to enhance the nickel extraction from low-grade laterites. Min. Eng. 2018, 120, 127–131. [Google Scholar] [CrossRef]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of ferronickel slag-based geopolymers. Min. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Warner, A.; Diaz, C.M.; Dalvi, A.D.; Mackey, P.J.; Tarasov, A.V. JOM World Nonferrous Smelter Survey, part III: Nickel: Laterite. JOM 2006, 58, 11–20. [Google Scholar] [CrossRef]
- Rao, M.; Li, G.; Jiang, T.; Luo, J.; Zhang, Y.; Fan, X. Carbothermic Reduction of Nickeliferous Laterite Ores for Nickel Pig Iron Production in China: A Review. Jom-Us 2013, 65, 1573–1583. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Q.; Zhu, Z.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Selective leaching of nickel and cobalt from limonitic laterite using phosphoric acid: An alternative for value-added processing of laterite. J. Clean. Prod. 2018, 189, 620–626. [Google Scholar] [CrossRef]
- Li, G.; Rao, M.; Jiang, T.; Huang, Q.; Peng, Z. Leaching of limonitic laterite ore by acidic thiosulfate solution. Min. Eng. 2011, 24, 859–863. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Guo, X.; Shi, W.; Li, D.; Tian, Q. Leaching behavior of metals from limonitic laterite ore by high pressure acid leaching. Trans. Nonferr. Met. Soc. China 2011, 21, 191–195. [Google Scholar] [CrossRef]
- Landers, M.; Gilkes, R. Dehydroxylation and dissolution of nickeliferous goethite in New Caledonian lateritic Ni ore. Appl. Clay Sci. 2007, 35, 162–172. [Google Scholar] [CrossRef]
- Landers, M.; Gilkes, R.; Wells, M. Dissolution kinetics of dehydroxylated nickeliferous goethite from limonitic lateritic nickel ore. Appl. Clay Sci. 2009, 42, 615–624. [Google Scholar] [CrossRef]
- Li, J.; Bunney, K.; Watling, H.R.; Robinson, D.J. Thermal pre-treatment of refractory limonite ores to enhance the extraction of nickel and cobalt under heap leaching conditions. Min. Eng. 2013, 41, 71–78. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R.L. Thermal treatment of natural goethite: Thermal transformation and physical properties. Thermochim. Acta 2013, 568, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Löffler, L.; Mader, W. Anisotropic X-ray peak broadening and twin formation in hematite derived from natural and synthetic goethite. J. Eur. Ceram. Soc. 2006, 26, 131–139. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Hu, Q.; Wang, Z.; Zhou, Y.; Zheng, J.; Liu, W.; Li, L. Effect of pre-roasting on leaching of laterite. Hydrometallurgy 2009, 99, 84–88. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, G.; Wen, J. Separation of divalent cobalt and nickel ions using a synergistic solvent extraction system with P507 and Cyanex272. Chin. J. Process Eng. 2012, 12, 415–419. [Google Scholar]
- Sun, Q.; Yang, L.; Huang, S.; Xu, Z.; Li, Y.; Wang, W. Synergistic solvent extraction of nickel by 2-hydroxy-5-nonylacetophenone oxime mixed with neodecanoic acid and bis(2-ethylhexyl) phosphoric acid: Stoichiometry and structure investigation. Min. Eng. 2019, 132, 284–292. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Y.; Yu, J. Removal of ferric ions from aluminum solutions by solvent extraction, part I: Iron removal. Sep. Purif. Technol. 2016, 159, 18–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, C. Recovery of copper and cobalt from alloy scraps under high temperature. Copp. Eng. 2000, 2, 34–36. (In Chinese) [Google Scholar]
- Zhang, S.; Qian, X. Analysis and Control of Influencing Factors of P507 De-impurity Process. Chem. Enterp. Manag. 2019, 7, 203–204. (In Chinese) [Google Scholar]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Separation of Nickel and Cobalt by Solvent Extraction. In Extractive Metallurgy of Nickel, Cobalt, and Platinum Group Metals; Elsevier: New York, NY, USA, 2011; pp. 315–326. [Google Scholar]
- Ye, Q.; Li, G.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Solvent extraction behavior of metal ions and selective separation Sc3+ in phosphoric acid medium using P204. Sep. Purif. Technol. 2019, 209, 175–181. [Google Scholar] [CrossRef]
- El-Khaiary, M.I. Extraction of Al(iii) from phosphoric acid by HDDNSA. Sep. Purif. Technol. 1997, 12, 13–16. [Google Scholar] [CrossRef]
- Wu, W.; Li, D.; Zhao, Z.; Chen, J.; Zhang, F.; Yin, S.; Qian, M.; Bian, X. Formation mechanism of micro emulsion on aluminum and lanthanum extraction in P507-HCl system. J. Rare Earths 2010, 28, 174–178. [Google Scholar] [CrossRef]



| Ni | Co | TFe | MgO | Al2O3 | Cr2O3 | MnO2 | SiO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| 1.01 | 0.13 | 43.9 | 0.99 | 10.44 | 3.25 | 1.25 | 3.90 | 15.2 |
| Reagents Name | Chemical Formula | Purity |
|---|---|---|
| 85% Phosphoric acid solution | H3PO4 | Analytical grade |
| Ammonia | NH3·H2O | Analytical grade |
| P204 | C16H35O4P | Analytical grade |
| P507 | (C8H17)2HPO3 | Analytical grade |
| Sulfonated kerosene | Analytical grade |
| Composition | Al | Co | Cr | Fe | Mg | Mn | Ni | Si |
|---|---|---|---|---|---|---|---|---|
| Leaching ratio | 55.42 | 90.88 | 87.15 | 3.29 | 99.78 | 40.69 | 99.28 | 46.66 |
| Previous results | 89.8 | 1.3 | 98.7 |
| Composition | Al | Co | Cr | Fe | Mg | Mn | Ni | Si |
|---|---|---|---|---|---|---|---|---|
| Content | 2.47 | 0.10 | 0.687 | 1.66 | 0.74 | 0.65 | 1.05 | 0.51 |
| Extraction Stage | Extraction Ratios | ||||
|---|---|---|---|---|---|
| Co | Cr | Mg | Ni | Si | |
| 1 | 54.17 | 1.81 | 24.21 | 2.51 | 1.51 |
| 2 | 84.97 | 5.31 | 54.14 | 8.91 | 4.11 |
| 3 | 96.61 | 9.67 | 80.60 | 12.32 | 5.81 |
| Co No More Than | Ni9996 Standard | Ni9990 Standard | Average Composition of JinChuan Nickel in 2003 | Thompson Electrolytic Nickel Composition | MCLE(Standard) Electrolytic Nickel Composition |
|---|---|---|---|---|---|
| 0.02 | 0.08 | 0.0112 | 0.06 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, M.; Zhang, T.; Li, G.; Zhou, Q.; Luo, J.; Zhang, X.; Zhu, Z.; Peng, Z.; Jiang, T. Solvent Extraction of Ni and Co from the Phosphoric Acid Leaching Solution of Laterite Ore by P204 and P507. Metals 2020, 10, 545. https://doi.org/10.3390/met10040545
Rao M, Zhang T, Li G, Zhou Q, Luo J, Zhang X, Zhu Z, Peng Z, Jiang T. Solvent Extraction of Ni and Co from the Phosphoric Acid Leaching Solution of Laterite Ore by P204 and P507. Metals. 2020; 10(4):545. https://doi.org/10.3390/met10040545
Chicago/Turabian StyleRao, Mingjun, Tao Zhang, Guanghui Li, Qun Zhou, Jun Luo, Xin Zhang, Zhongping Zhu, Zhiwei Peng, and Tao Jiang. 2020. "Solvent Extraction of Ni and Co from the Phosphoric Acid Leaching Solution of Laterite Ore by P204 and P507" Metals 10, no. 4: 545. https://doi.org/10.3390/met10040545





