Review on the Comparison of the Chemical Reactivity of Cyanex 272, Cyanex 301 and Cyanex 302 for Their Application to Metal Separation from Acid Media
Abstract
:1. Introduction
2. Synthesis Method
2.1. Synthesis Methods of Phosphinic Acids and Their Derivatives
2.2. Syntheis of Dialkylphosphinic Acids and Thio Derivatives
2.2.1. Cyanex 272
2.2.2. Cyanex 301
2.2.3. Cyanex 302
3. Chemical Reactivity Analysis of Cyanex Extractants Based on Chemical Structure
3.1. Solubility and Self-Association Form

3.2. Acidity and Extraction Potential
3.3. Diluent Interaction
3.4. Interaction in Binary Mixtures
4. Extraction and Stripping Characteristics of Cyanex 272 and Its Derivatives
4.1. Extraction Behavior of Cyanex for Some Metal Ions from Inorganic Acid Solutions
4.1.1. From Hydrochloric Acid Solutions
4.1.2. From Sulfuric Acid Solutions
4.1.3. From Nitric Acid Solutions
4.1.4. Stripping Characteristics
4.2. Stability/Degradation of Cyanex 272 and Derivatives in a Solvent Extraction Process
4.2.1. Effect of Acidity
4.2.2. Effect of Other Factors
4.3. Recycling of the Extractants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Svara, J.; Weferling, N.; Hofmann, T. Phosphorus Compounds, Organic. In Ullman’s Encyclopedia of Industial Chemistry; Wiley VCH: Weinheim, Germany, 2006. [Google Scholar]
- Rickelton, W.A.; Boyle, R.J. Solvent extraction with organophosphines-commercial and potential applications. Sep. Sci. Technol. 1988, 23, 1227–1250. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. A Process for the Separation of Noble Metals from HCl Liquor Containing Gold(III), Palladium(II), Platinum(IV), Rhodium(III), and Iridium(IV) by Solvent Extraction. Processes 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Frieser, H. Extraction of Lanthanide metals with bis(2,4,4-trimethylpentyl) phosphinic acid. Solvent Extr. Ion Exch. 1986, 4, 739–755. [Google Scholar] [CrossRef]
- Xun, F.; Yahong, X.; Shuyun, X.; Shaona, Z.; Zhengshui, H. Study on the thiophosphinic extractants. i. the basic properties of the extractants and the phase behavior in their saponified systems. Solvent Extr. Ion Exch. 2002, 20, 331–344. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B. Solvent extraction characteristics of thiosubstituted organophosphinic acid extractants. Hydrometallurgy 1992, 30, 345–365. [Google Scholar] [CrossRef]
- Torkaman, R.; Asadollahzadeh, M.; Torab-Mostaedi, M.; Ghanadi Maragheh, M. Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process. Sep. Purif. Technol. 2017, 186, 318–325. [Google Scholar] [CrossRef]
- Robertson, A.J.; Ozog, T. Preparation of Dialkyldithiophosphinates. U.S. Patent 4,308,214, 29 December 1981. [Google Scholar]
- Sole, K.C.; Hiskey, J.B.; Ferguson, T.L. An assessment of the long-term stabilities of cyanex 302 and cyanex 301 in sulfuric and nitric acids. Solvent Extr. Ion Exch. 1993, 11, 783–796. [Google Scholar] [CrossRef]
- Rickelton, W.A.; Boyle, R.J. The selective recovery of zinc with new thiophosphinic acids. Solvent Extr. Ion Exch. 1990, 8, 783–797. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Li, L.; Li, J. Synthesis of organic phosphinic acids and studies on the relationship between their structure and extraction–separation performance of heavy rare earths from HNO3 solutions. Hydrometallurgy 2013, 137, 108–114. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Ju, Z.; Zhang, Z.; Liao, F.; Li, G. Dialkyl phosphinic acids: Synthesis and applications as extractant for nickel and cobalt separation. Trans. Nonferr. Met. Soc. China 2010, 20, 205–210. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B. Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy 1995, 37, 129–147. [Google Scholar] [CrossRef]
- Tait, B.K. The extraction of some base metal ions by cyanex 30l cyanex 302 and their binary extractant mixtures with aliquat 336. Solvent Extr. Ion Exch. 1992, 10, 799–809. [Google Scholar] [CrossRef]
- Handley, T. A review of organic compounds containing P==S and p(s)sh groups as separatory and analytical reagents. Talanta 1965, 12, 893–901. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, M.S. Analysis of the Interaction between Organophosphorus Acid and Tertiary Amine Extractants in the Binary Mixtures by Fourier Transform Infrared Spectroscopy (FT-IR). Solvent Extr. Ion Exch. 2015, 34, 74–85. [Google Scholar] [CrossRef]
- Rezaei, K.; Nedjate, H. Diluent effect on the distribution ratio and separation factor of Ni(II) in the liquid–liquid extraction from aqueous acidic solutions using dibutyldithiophosphoric acid. Hydrometallurgy 2003, 68, 11–21. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, M.S. Effect of the diluents on the interaction between components in the binary mixtures of organophosphorus acid and tertiary amine. J. Mol. Liq. 2016, 220, 41–48. [Google Scholar] [CrossRef]
- Fleitlikh, I.Y.; Pashkov, G.L.; Grigorieva, N.A.; Nikiforova, L.K.; Pleshkov, M.A.; Shneerson, Y.M. Cobalt and Nickel Recovery from Sulfate Media Containing Calcium, Manganese, and Magnesium with a Mixture of Cyanex 301 and a Trialkylamine. Solvent Extr. Ion Exch. 2011, 29, 782–799. [Google Scholar] [CrossRef]
- Kumar, B.N.; Reddy, B.R.; Kantam, M.L.; Kumar, J.R.; Lee, J.Y. Synergistic Solvent Extraction of Neodymium(III) from Chloride Solutions using a Mixture of Triisooctylamine and bis(2,4,4-Trimethylpentyl) Monothiophosphinic Acid. Sep. Sci. Technol. 2013, 49, 130–136. [Google Scholar] [CrossRef]
- Grigorieva, N.A.; Pavlenko, N.I.; Pashkov, G.L.; Fleitlikh, I.Y.; Nikiforova, L.K. Investigation of the State of Bis(2,4,4-trimethylpentyl)dithiophosphinic Acid in Nonane in the Presence of Electron-Donor Additives. Solvent Extr. Ion Exch. 2010, 28, 510–525. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Lee, M.S. Application of the data on dielectric constant and viscosity of binary mixtures to the selection of synergistic solvent extraction-binary mixtures of Cyanex and tertiary amine (TEHA). J. Mol. Liq. 2019, 111112. [Google Scholar] [CrossRef]
- Barnard, K.R.; Shiers, D.W. Mechanism and rate of butyl phosphinate formation from reaction of phosphinic acid (Cyanex 272) and tributyl phosphate. Hydrometallurgy 2011, 106, 76–83. [Google Scholar] [CrossRef]
- Barnard, K.R.; Shiers, D.W.; Kelly, N.J. Chemical Reactivity of Tributyl Phosphate with Selected Solvent Extraction Reagents. Solvent Extr. Ion Exch. 2012, 30, 651–667. [Google Scholar] [CrossRef]
- Baba, A.A.; Sosanya1, D.G.; Adekola, F.A.; Alabi, A.G.F.; Aremu, A.S.; Adeboye, S.E. Extraction of copper from leach liquor of metallic component in discarded cell phone by Cyanex 272. Int. J. Eng. Sci. Technol. 2016, 11, 861–871. [Google Scholar]
- El-Hefny, N.E.; Daoud, J.A. Extraction of Copper(II) by CYANEX 302 in Kerosene from Different Aqueous Media. Solvent Extr. Ion Exch. 2007, 25, 831–843. [Google Scholar] [CrossRef]
- Adekola, F.A.; Baba, A.A.; Ayanda, O.S. Solvent Extraction of Cobalt and Nickel From Nigerian Lateritic Soil. J. Chem. Soc. Niger. 2009, 35, 123–128. [Google Scholar]
- Wang, L.; Lee, M.S. Solvent extraction of cobalt and nickel from chloride solution by mixtures of acidic organophosphorus extractants and amines. Geosystem Eng. 2016, 19, 261–265. [Google Scholar] [CrossRef]
- Ali, M.; Biswas, R.; Salam, S.; Akhter, A.; Karmakar, A.; Ullah, M. Cyanex 302: An extractant for Fe(III) from Chloride Medium. Bangladesh J. Sci. Ind. Res. 2011, 46, 407–414. [Google Scholar] [CrossRef]
- Alam, M.S.; Inoue, K.; Yoshizuka, K.; Dong, Y.; Zhang, P. Solvent extraction of silver from chloride media with some commercial sulfur-containing extractants. Hydrometallurgy 1997, 44, 245–254. [Google Scholar] [CrossRef]
- Kakoi, T.; Goto, M.; Nakashio, F. Solvent Extraction Palladium with bis(2,4,4,-trimetylpentyl)dithiophosphinic acid and bis(2,4,4,-trimetylpentyl)monothiophosphinic acid. Solvent Extr. Ion Exch. 1994, 12, 541–555. [Google Scholar] [CrossRef]
- Zhidkova, T.I.; Belova, V.V.; Brenno, Y.Y.; Zhidkov, L.L.; Khol’kin, A.I. Palladium extraction by a cyanex 301-based binary extractant from chloride solutions. Russ. J. Inorg. Chem. 2009, 54, 1502–1506. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S.; Son, S.H. Extraction of Palladium(II) from hydrochloride acid solution by solvent extraction with mixtures containing either Cyanex 301 or LIX63. Metals 2017, 7, 541. [Google Scholar] [CrossRef] [Green Version]
- Kolarik, Z.; Pankova, H. Acidic organophosphorous extractants–I extraction of lanthanides by means of dialkyl phosphoric acids-effect of structure and size of alkyl group. Inorg. Nucl. Chem. 1966, 28, 2325–2333. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.S.; Lee, M.S. Solvent extraction separation of La from chloride solution containing Pr and Nd with Cyanex 272. Hydrometallurgy 2012, 121–124, 74–80. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- El-Hefny, N.E.; El-Nadi, Y.A.; Daoud, J.A. Equilibrium and mechanism of samarium extraction from chloride medium using sodium salt of CYANEX 272. Sep. Purif. Technol. 2010, 75, 310–315. [Google Scholar] [CrossRef]
- Olushola, S.A.; Folahan, A.; Alafara, A.B.; Bhekumusa, J.X.; Olalekan, S.F. Application of Cyanex extractant in Cobalt/Nickel separation process by solvent extraction. Int. J. Phys. Sci. 2013, 8, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Van de Voorde, I.; Pinoy, L.; Courtijn, E.; Verpoort, F. Equilibrium Studies of Nickel(II), Copper(II), and Cobalt(II) Extraction with Aloxime 800, D2EHPA, and Cyanex Reagents. Solvent Extr. Ion Exch. 2006, 24, 893–914. [Google Scholar] [CrossRef]
- Fleitlikh, I.Y.; Grigorieva, N.A.; Logutenko, O.A. Extraction of Non-Ferrous Metals and Iron with Systems based on Bis(2,4,4-Trimethylpentyl)Dithiophosphinic Acid (CYANEX 301), A Review. Solvent Extr. Ion Exch. 2018, 36, 1–21. [Google Scholar] [CrossRef]
- Tait, B.K. Cobalt-nickel separation: The extraction of cobalt(II) and nickel(II) by Cyanex 301, Cyanex 302 and Cyanex 272. Hydrometallurgy 1993, 32, 365–372. [Google Scholar] [CrossRef]
- Lenhard, Z. Extraction and separation of Cobalt and Nickel with extractants Cyanex 302, Cyanex 272 and their mixture. Kem. Ind. 2008, 57, 417–423. [Google Scholar]
- Tsakiridis, P.E.; Agatzini-Leonardou, S. Process for the recovery of cobalt and nickel in the presence of magnesium from sulphate solutions by Cyanex 272 and Cyanex 302. Miner. Eng. 2004, 17, 913–923. [Google Scholar] [CrossRef]
- Deep, A.; Correia, P.F.M.; Carvalho, J.M.R. Separation and Recovery of Fe(III) and Cr(III) from a Tannery Filtrate using Cyanex 272. Ind. Eng. Chem. Res. 2006, 45, 3200–3206. [Google Scholar] [CrossRef]
- Senapati, D.; Chaudhury, G.R.; Sarma, P.V.R.B. Purification of nickel sulphate solutions containing iron, copper, cobalt, zinc and manganese. J. Chem. Technol. Biotechnol. 1994, 59, 335–339. [Google Scholar] [CrossRef]
- Sole, K.C.; Ferguson, T.L.; Hiskey, J.B. Solvent extraction of silver by Cyanex 272, Cyanex 302 and Cyanex 301. Solvent Extr. Ion Exch. 1994, 12, 1033–1050. [Google Scholar] [CrossRef]
- Mansingh, P.S.; Chakravortty, V.; Dash, K.C. Solvent Extraction of Thorium(IV) by Cyanex 272/Cyanex 302/Cyanex 301/PC-88A and their Binary Mixtures with TBP/DOSO from Aq. HNO3 and H2SO4 Media. Radiochim. Acta 1996, 73. [Google Scholar] [CrossRef]
- Daoud, J.A.; Ibrahim, O.E.; Aly, H.F. Solvent extraction of Zr(IV) from nitric acid solutions using CYANEX 272, CYANEX 301 and CYANEX 302. Sharm El-Sheikh Egypt 2008, 9, 11–14. [Google Scholar]
- Singh, R.; Kliwaja, A.R.; Gupta, B.; Tandon, S.N. Extraction and separation of nickel(H) using bis (2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex 301) and its recovery from spent catalyst and electroplating bath residue. Solvent Extr. Ion Exch. 1999, 17, 367–390. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, D. Extraction and stripping of rare earths using mixtures of acidic phosphorus-based reagents. J. Rare Earths 2011, 29, 413–415. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slimes by solvent extraction. Part II: Recovery of silver and copper with LIX 63 and Alamine 336. Hydrometallurgy 2018, 180, 49–57. [Google Scholar] [CrossRef]
- Othman, N.; Mat, H.; Goto, M. Separation of silver from photographic wastes by emulsion liquid membrane system. J. Membr. Sci. 2006, 282, 171–177. [Google Scholar] [CrossRef]
- El-Hefny, N.E. Kinetics and mechanism of extraction of Cu(II) by Cyanex 302 from nitrate medium and oxidative stripping of Cu(I) using Lewis cell technique. Chem. Eng. Process. 2010, 49, 84–90. [Google Scholar] [CrossRef]
- Gajda, B.; Apostoluk, W. Equilibria of Cobalt (II) and Nickel (II) Extraction from Sulphate Solutions with Cyanex 301. Ars Sep. Acta 2002, 1, 55–60. [Google Scholar]
- Grigorieva, N.A.; Fleitlikh, I.Y. Redox Processes in the Organic Phase during Cobalt Extraction with the bis(2,4,4-trimethylpentyl)dithiophosphinic Acid and Trioctyl Phosphine Oxide Mixtures. Solvent Extr. Ion Exch. 2015, 33, 278–294. [Google Scholar] [CrossRef]
- Tian, M.; Jia, Q.; Liao, W. Studies on Synergistic solvent extraction of rare earth elements from nitrate medium by mixtures of 8-hydroxyquinoline with Cyanex 301 or Cyanex 302. J. Rare Earth 2013, 31, 604–608. [Google Scholar] [CrossRef]
- Principe, F.; Demopoulos, G.P. The solubility and stability of organophosphoric acid extractants in H2SO4and HCl media. Hydrometallurgy 2003, 68, 115–124. [Google Scholar] [CrossRef]
- Su, W.; Chen, J.; Jing, Y. Aqueous Partition Mechanism of Organophosphorus Extractants in Rare Earths Extraction. Ind. Eng. Chem. Res. 2016, 55, 8424–8431. [Google Scholar] [CrossRef]
- Groenewold, G.S.; Peterman, D.R.; Klaehn, J.R.; Delmau, L.H.; Marc, P.; Custelcean, R. Oxidative degradation of bis(2,4,4-trimethylpentyl)dithiophosphinic acid in nitric acid studied by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2195–2203. [Google Scholar] [CrossRef]
- Menoyo, B.; Elizalde, M.P.; Almela, A. Determination of the degradation compounds formed by the oxidation of thiophosphinic acids and phosphine sulfides with nitric acid. Anal. Sci. 2002, 18, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Wieszczycka, K.; Tomczyk, W. Degradation of organothiophosphorous extractant Cyanex 301. J. Hazard. Mater. 2011, 192, 530–537. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Zembrzuska, J. Photodegradation and by-products identification of commercial extractant Cyanex 302′. J. Radioanal. Nucl. Chem. 2014, 299, 709–720. [Google Scholar] [CrossRef]
- Chauhan, S.; Patel, T. A Review on Solvent Extraction of Nickel. Int. J. Adv. Res. Technol. 2014, 3, 1315–1322. [Google Scholar]
- Quintero-Almanza, D.; Gamiño-Arroyo, Z.; Sánchez-Cadena, L.E.; Gómez-Castro, F.I.; Uribe-Ramírez, A.R.; Aguilera-Alvarado, A.F.; Ocampo Carmona, L.M. Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction. Batteries 2019, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Ahn, N.K.; Shim, H.W.; Kim, D.W.; Swain, B. Valorization of waste NiMH battery through recovery of critical rare earth metal: A simple recycling process for the circular economy. J. Waste Manag. 2020, 104, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Kumar, J.R.; Reddy, A.V. Liquid-Liquid Extraction of Tetravalent Zirconium from Acidic Chloride Solutions Using Cyanex 272. Anal. Sci. 2004, 20, 501–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandra Reddy, B.; Rajesh Kumar, J.; Phani Raja, K.; Varada Reddy, A. Solvent extraction of Hf(IV) from acidic chloride solutions using Cyanex 302. Miner. Eng. 2004, 17, 939–942. [Google Scholar] [CrossRef]
- Rajesh Kumar, J.; Ramachandra Reddy, B.; Janardhan Reddy, K.; Varada Reddy, A. Liquid-Liquid Extraction of Tetravalent Hafnium from Acidic Chloride Solutions using Bis(2,4,4-trimethylpentyl) Dithiophosphinic Acid (Cyanex 301). Sep. Sci. Technol. 2007, 42, 865–877. [Google Scholar] [CrossRef]
- Gupta, B.; Mudhar, N.; Tandon, S.N. Extraction and Separation of Gallium Using Cyanex 301: Its Recovery from Bayer’s Liquor. Ind. Eng. Chem. Res. 2005, 44, 1922–1927. [Google Scholar] [CrossRef]
- Gupta, B.; Mudhar, N. Extraction and Separation of Germanium Using Cyanex 301/Cyanex 923. Its Recovery from Transistor Waste. Sep. Sci. Technol. 2006, 41, 549–572. [Google Scholar] [CrossRef]




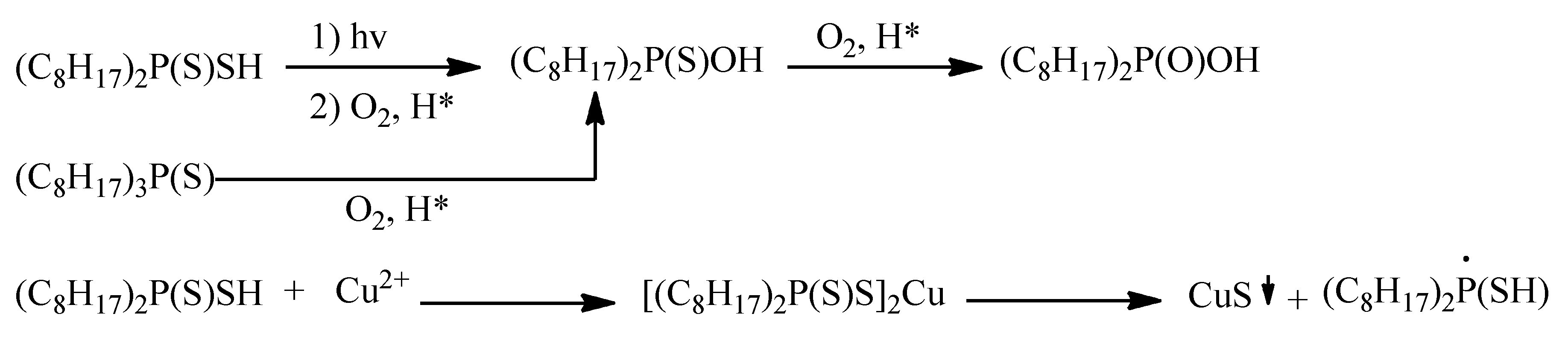
| Extractant | Structure | Density (g cm−3) | Viscosity (cP) | pKa | Molecular Weight |
|---|---|---|---|---|---|
| Cyanex 272 | 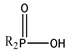 | 0.91 | 142 | 6.37 | 290 |
| Cyanex 302 | 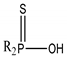 | 0.93 | 195 | 5.63 | 306 |
| Cyanex 301 |  | 0.95 | 78 | 2.61 | 322 |
| Extractant | Species | Concentration (%) |
|---|---|---|
| Cyanex 272 | R2PO2H R3PO Unknown | 87–88 10 ~2 |
| Cyanex 302 | R2PSOH R3PO R2PO2H R2PS2H Unknown | 78–80 10–12 2–3 2 ~8 |
| Cyanex 301 | R2PS2H P3PS R2PSOH Unknown | 75–83 5–8 3–6 ~2 |
| Extractant | Metal Ion | pH | Metal:Ligand Ratio | log K |
|---|---|---|---|---|
| Cyanex 272 | Co(II) | 4.78 | 1:2 | −7.17 |
| Ni(II) | 4.78 | - | - | |
| Cu(II) | 3.00 | 1:2 | −6.39 a | |
| Cyanex 302 | Co(II) | 4.78 | 1:4 | −2.40 |
| Ni(II) | 4.78 | 1:4 | −7.01 a | |
| Cu(II) b | 2.00 | 1:2 | −5.27 | |
| Cyanex 301 | Co(II) | 4.78 | 1:2 | −5.61 |
| Ni(II) | 4.78 | 1:4 | 1.56 | |
| Cu(II) c | 2.00 | 1:2 | 0.92 | |
| Cu(II) d | - | 1:4 | 2.30 |
| Concentration of Extractant | Diluent | Aqueous | Selectivity | Extraction Percentage, % | Stripping Agent, % | Ref. |
|---|---|---|---|---|---|---|
| 0.2 M Cyanex 272 | Kerosene | 4 M HCl; 1187.0 mg/L Cu(II), 299.0 mg/L Pb, 165.5 mg/L Zn, Mn(II), Fe(III), Co(II), Cd(II) low trace; pH = 5 | Cu(II) over others | 96.3 | 0.1 M HCl: 98 | [25] |
| 0.08 M Cyanex 302 | Kerosene | 0.1 M sodium chloride; 0.063 M Cu(II); pH = 3.8 | Cu(II) | complete | 3–4 M HCl/HNO3: complete 4 M H2SO4: complete | [26] |
| 1 M Cyanex 272 | Kerosene | 4 M HCl; 100 mg/L Co(II) and 100 mg/L Ni(II); pH = 4 | Co(II) over Ni(II) | 93.3 | 1 M HCl: 99.32 | [27] |
| 0.75 M Cyanex 272 | Kerosene | HCl; 350 mg/L Li, 1000 mg/L Co, 400 mg/L Cu, 100 mg/L Al and 100 mg/L Ni; pH = 7.2 | Co(II) over others | 86.15 | 2 M H2SO4: 96.1 | [7] |
| 0.1 M Cyanex 301 | Kerosene | As above | Co(II) over others | 96.65 | 2 M H2SO4: 98.0 | [7] |
| 0.01 M Cyanex 301 | Kerosene | 0.5–9 M HCl, 100 mg/L Pd(II) | Pd(II) | >80 | - | [33] |
| 3 mM Cyanex 301 | n-heptane | 1–8 M HCl, 0.5 mM Pd(II) | Pd(II) | almost 100% | thiourea in 1 M HCl, HClO4, HNO3/LiSCN (0.5 M)-1 M HCl: <5.4 | [31] |
| 3 mM Cyanex 302 | n-heptane | 1–8 M HCl, 0.5 mM Pd(II) | Pd(II) | almost 100% | thiourea in 1 M HCl, HClO4, HNO3/LiSCN (0.5 M)-1 M HCl: > 88–99 | [31] |
| 0.1 M Cyanex 302 | Kerosene | 0.083 M Fe(III); 0.02 M H+ and 1 M Cl− | Fe(III) | 29.41 g Fe(III)/100 g Cyanex 302) | (6 M H2SO4 + 1 M Na2C2O4):95 (three stages) | [29] |
| 0.05 mM Cyanex 302 | Kerosene | 1–10 M HCl, 0.037–0.046 mM Ag(I) | Ag(I) | Almost complete | - | [30] |
| 0.05 mM Cyanex 301 | Kerosene | As above | Ag(I) | Almost complete | - | [30] |
| 0.2 M Na-Cyanex 272 | Kerosene | 1 M sodium chloride solution, 0.0066 M Sm(III); pH = 5.1 | Sm(III) | - | 1 M HCl: 97 | [37] |
| 2 M Na-Cyanex 272 | Escaid 110 | Chloride solution; 781 mg/L La(III), 119 mg/L Pr(III), and 333 mg/L Nd(III); pH = 4.94. | Pr(III) and Nd(III) over La(III) | Pr(III): 96.6; Nd(III): 98.7 La(III): 4.9 | 1 M HCl: complete | [35] |
| 2 M Cyanex 301 | Escaid 110 | As above | Pr(III) and Nd(III) over La(III) | Pr(III): > 85; Nd(III): 85 La(III): > 55 | - | [35] |
| 0.1 M Cyanex 272 | Xylene | H2SO4; 0.001 M Cu(II), 0.5 M Na2SO4; pH = 5–6 | Cu(II) | Almost complete | From 0 to 8 M H2SO4: 100% | [13] |
| 0.1 M Cyanex 302 | Xylene | H2SO4; 0.001 M Cu(II), 0.5 M Na2SO4; pH = 0 | Cu(II) | complete | 13.5 M H2SO4: difficult | [13] |
| 0.1 M Cyanex 301 | Xylene | As above | Cu(II) | complete | 18 M H2SO4: very difficult | [13] |
| 20% Cyanex 272 | Exxsol D-80 with 5% TBP | Sulfate solution; 0.63 g/L of Co(II), 3.8 g/L of Ni(II), 5.75 g/L of Mg(II); pH = 6 | Co(II), Mg(II) over Ni(II) | Co(II): 99.7; Mg(II): 99.3 | 4 M H2SO4: Co(II): 99.6; Mg(II): 99.1 | [43] |
| 20% Cyanex 302 | Exxsol D-80 with 5% TBP | Sulfate solution; 0.63 g/L of Co(II), 5.75 g/L of Mg(II); pH = 5.0 | Co(II) over Mg(II) | 99.6 | cobalt electrowinning: 99.5 | [43] |
| 10% Cyanex 272 | Exxsol D-80 with 5% TBP | Sulfate solution of 3.8 g/L Ni(II) | Ni(II) | 99.5 | nickel electrowinning: 99.4 | [43] |
| 0.25 M Cyanex 272 | Toluene | Sulfate solution; 100 g/L NiSO4.6H2O and 2 g/L COSO4. 7H2O; pH = 6.5 | Co(II) over Ni(II) | Co(II):>80 Ni(II):<10 | - | [41] |
| 0.25 M Cyanex 302 | Toluene | As above | Co(II) over Ni(II) | Co(II):>90 Ni(II):<10 | - | [41] |
| 0.25 M Cyanex 301 | Toluene | As above, pH = 1.1 | Co(II) over Ni(II) | Co(II): 100 Ni(II):<5 | Difficult stripping | [41] |
| 0.2 M Cyanex 272 | Escaid 110 | Sulfate solution; 300 mg/L Fe(III), 500 mg/L Cr(III), and 6 mg/L Zn(III); pHeq = 1.8 | Fe(III) over Cr(III) and Zn(II) | 90.0 | 0.5 M H2SO4: complete | [44] |
| 0.2 M Cyanex 272 | Kerosene and TBP as a modifier | Sulfate solution; 7.04 g/L Fe(III), 2.82 g/L Ni(II), 0.184 g/L Co(II), 1.26 g/L Mn(II), 0.05 g/L Zn(II), 0.03 g/L Cu(II); pH = 2.5 | Fe(III) over others | 95% for two stage of counter current; A/O =1/5 | 15.0 g/L H2SO4: 78 (one stage)/100 (three counter-current stages at equal phase ratio) | [45] |
| 0.027 M Cyanex 272 | TBP/DOSO | 0.4 M H2SO4; 0.001 M Th(IV) | Th(IV) | 26.1 | - | [47] |
| 0.024 M Cyanex 302 | TBP/DOSO | As above | Th(IV) | 88.7 | - | [47] |
| 0.024 M Cyanex 301 | TBP/DOSO | As above | Th(IV) | 97.5 | - | [47] |
| 0.01 M Cyanex 272 | Xylene | Nitrate solution; 0.001 M Ag(I), 0.5 M NaNO3; pH = 6–8 | Ag(I) | Almost complete | - | [46] |
| 0.01 M Cyanex 302 | Xylene | 1–3 M HNO3; 0.001 M Ag(I), 0.5 M NaNO3 | Ag(I) | complete | - | [46] |
| 0.01 M Cyanex 301 | Xylene | 1–4 M HNO3; 0.001 M Ag(I), 0.5 M NaNO3 | Ag(I) | complete | 16 M HNO3: very difficult | [46] |
| 0.5% Cyanex 272 | Kerosene | 3 M HNO3; 0.0109 M Zr(IV) | Zr(IV) | complete | 2.5 M H2SO4: complete | [48] |
| 0.5% Cyanex 302 | Kerosene | As above | Zr(IV) | complete | 2.5 M H2SO4: 76 | [48] |
| 0.5% Cyanex 301 | Kerosene | As above | Zr(IV) | >90 | 2.0 M H2SO4: complete | [48] |
| 0.027 M Cyanex 272 | TBP/DOSO | 0.2 M HNO3; 0.001 M Th(IV) | Th(IV) | 98.9 | - | [47] |
| 0.024 M Cyanex 302 | TBP/DOSO | As above | Th(IV) | 97.3 | - | [47] |
| 0.024 M Cyanex 301 | TBP/DOSO | As above | Th(IV) | 91.2 | - | [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.N.H.; Nguyen, T.H.; Lee, M.S. Review on the Comparison of the Chemical Reactivity of Cyanex 272, Cyanex 301 and Cyanex 302 for Their Application to Metal Separation from Acid Media. Metals 2020, 10, 1105. https://doi.org/10.3390/met10081105
Nguyen VNH, Nguyen TH, Lee MS. Review on the Comparison of the Chemical Reactivity of Cyanex 272, Cyanex 301 and Cyanex 302 for Their Application to Metal Separation from Acid Media. Metals. 2020; 10(8):1105. https://doi.org/10.3390/met10081105
Chicago/Turabian StyleNguyen, Viet Nhan Hoa, Thi Hong Nguyen, and Man Seung Lee. 2020. "Review on the Comparison of the Chemical Reactivity of Cyanex 272, Cyanex 301 and Cyanex 302 for Their Application to Metal Separation from Acid Media" Metals 10, no. 8: 1105. https://doi.org/10.3390/met10081105





