Removal of Antimony from Industrial Crude Arsenic by Vacuum Sublimation: Combination of Thermodynamics and Ab Initio Molecular Dynamics
Abstract
:1. Introduction
2. Computational and Experimental Methods
2.1. Thermodynamic Calculation
2.1.1. Saturated Vapour Pressure
2.1.2. Gibbs Free Energy
2.2. Dynamic Calculations
2.2.1. Mean Free Range
2.2.2. Ab Initio Molecular Dynamics Calculations
2.3. Materials and Methods
2.3.1. Materials and Reagents
2.3.2. Experimental Equipment and Process
2.3.3. Characterization Analysis
3. Results and Discussion
3.1. Results of the Thermodynamic Calculations
3.1.1. Saturated Vapour Pressure
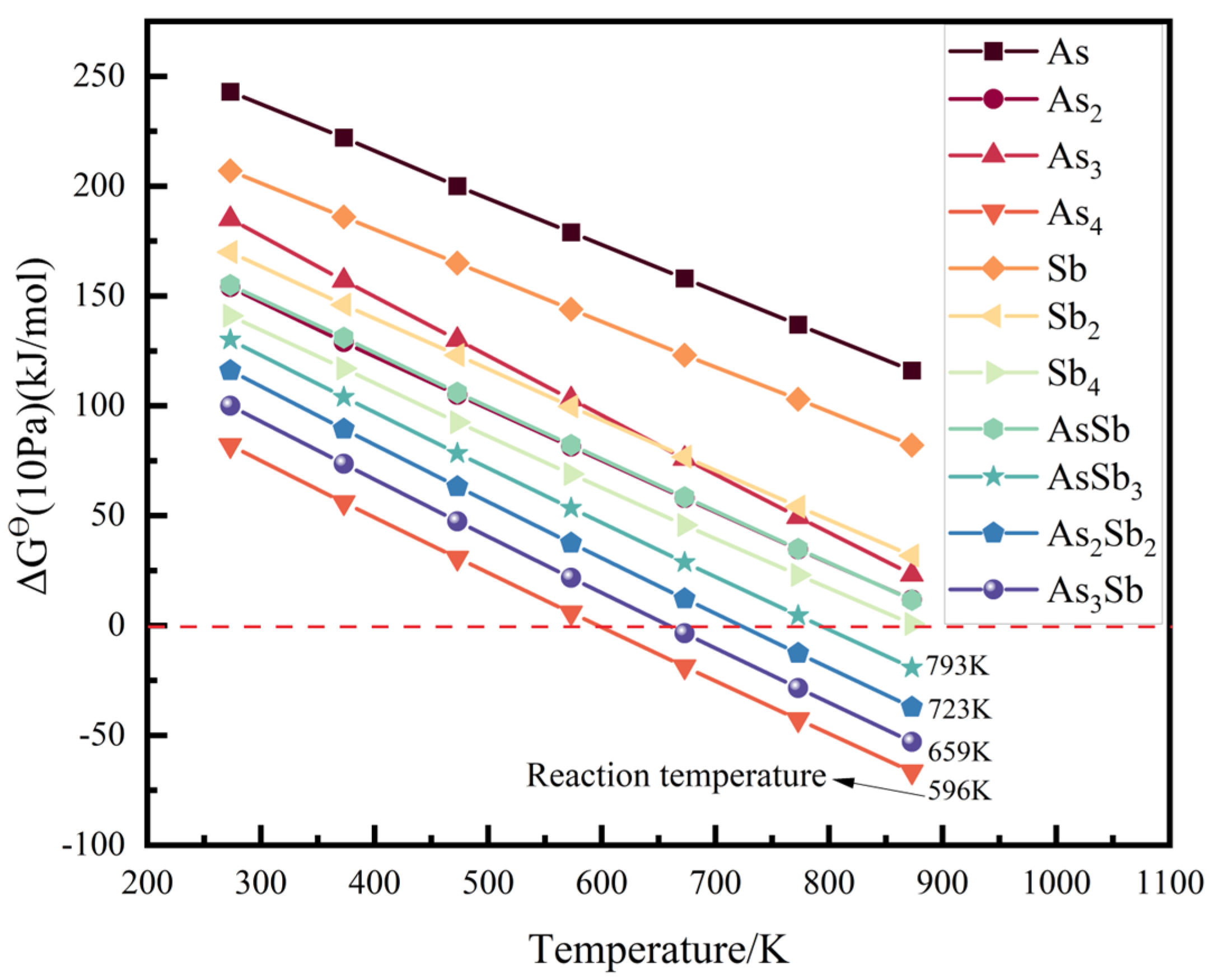
3.1.2. Gibbs Free Energy
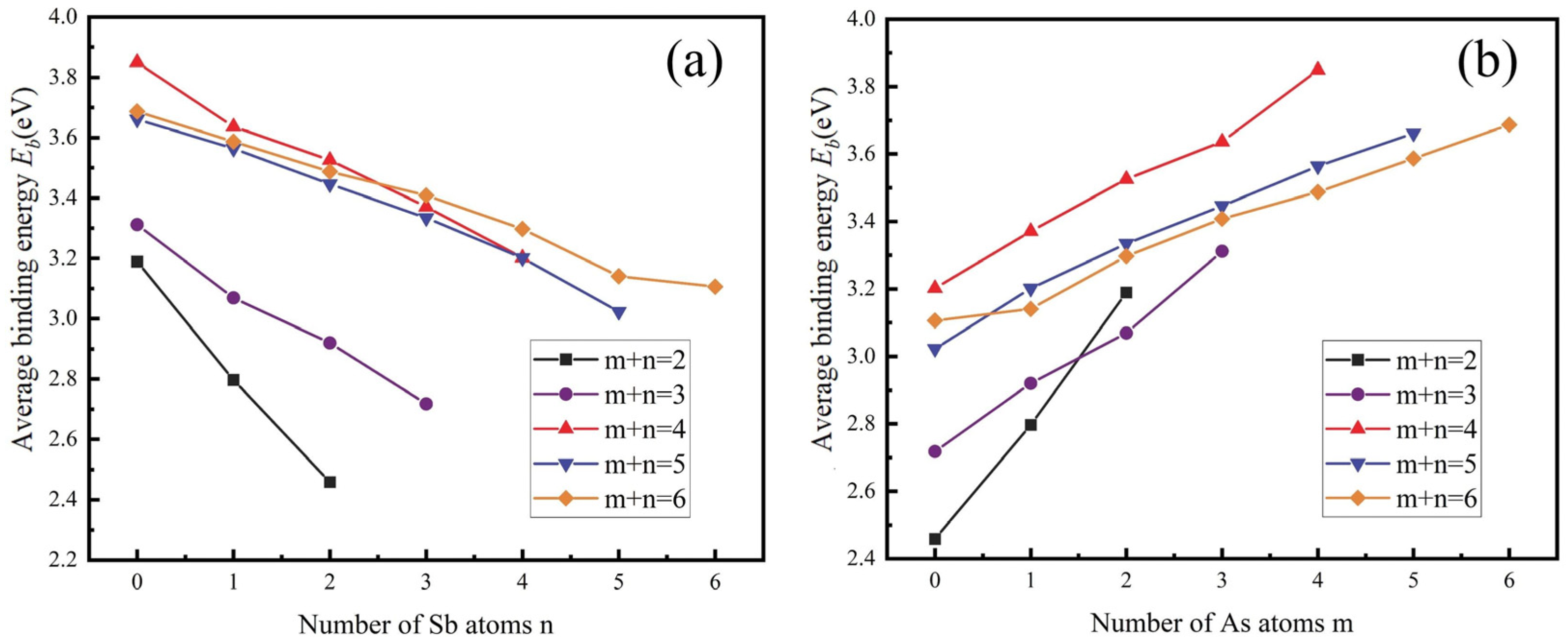
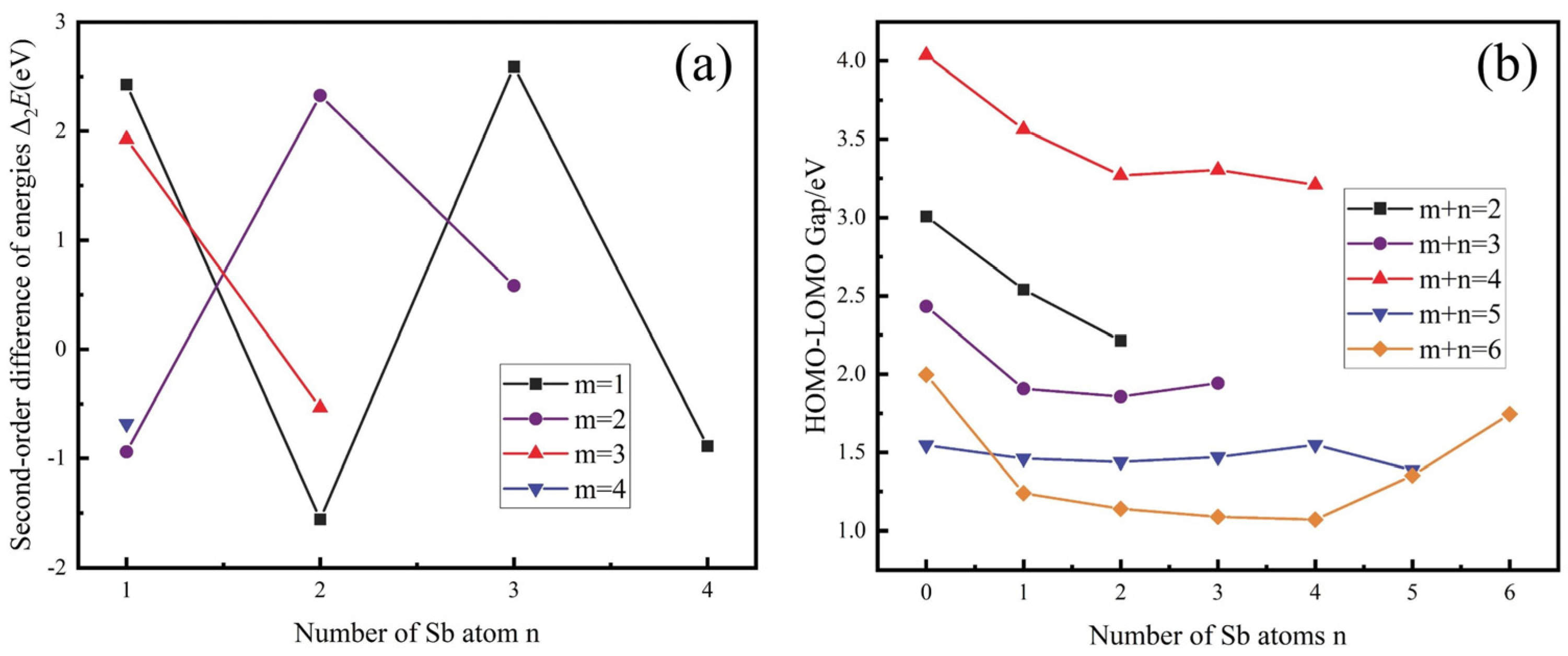
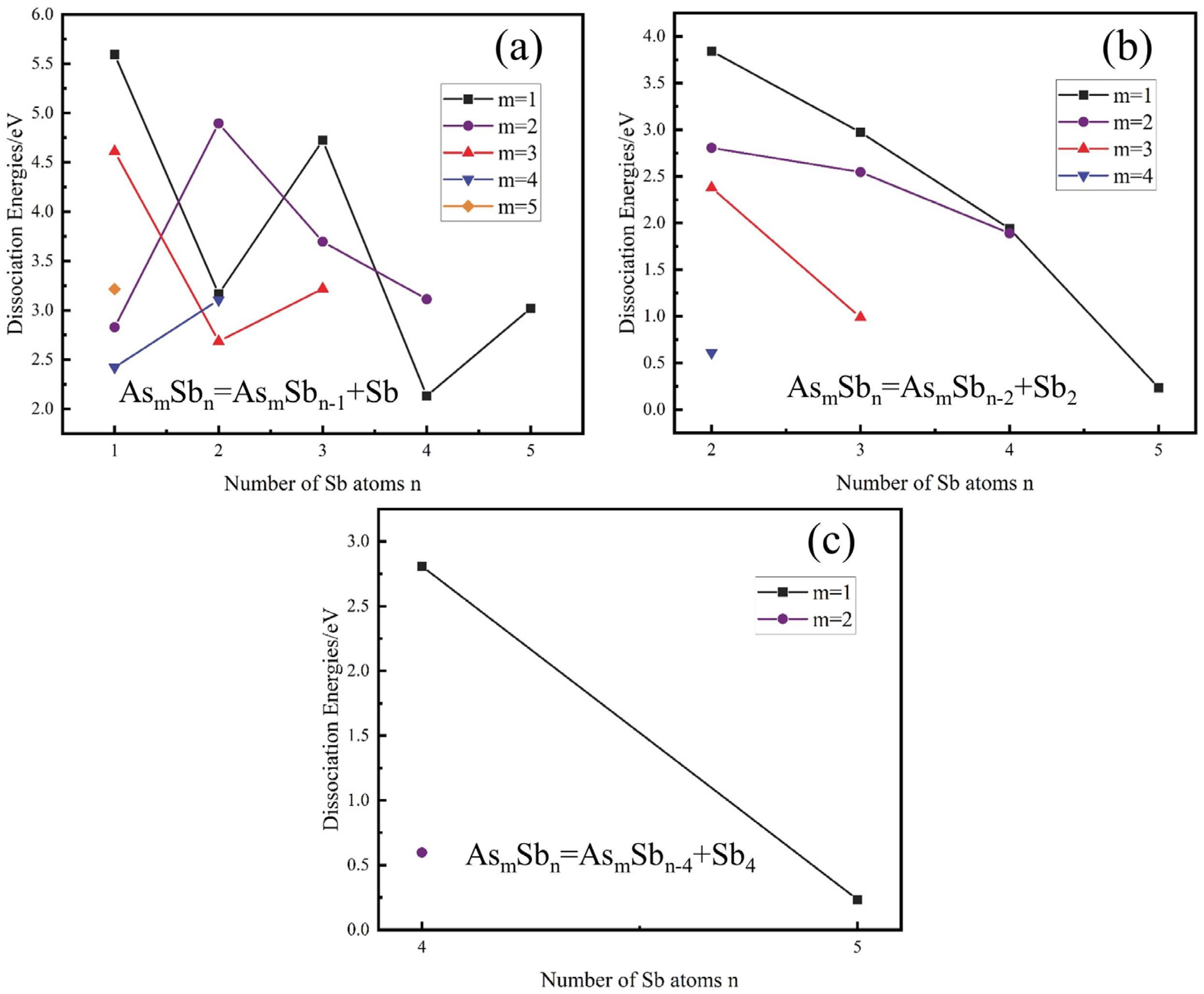
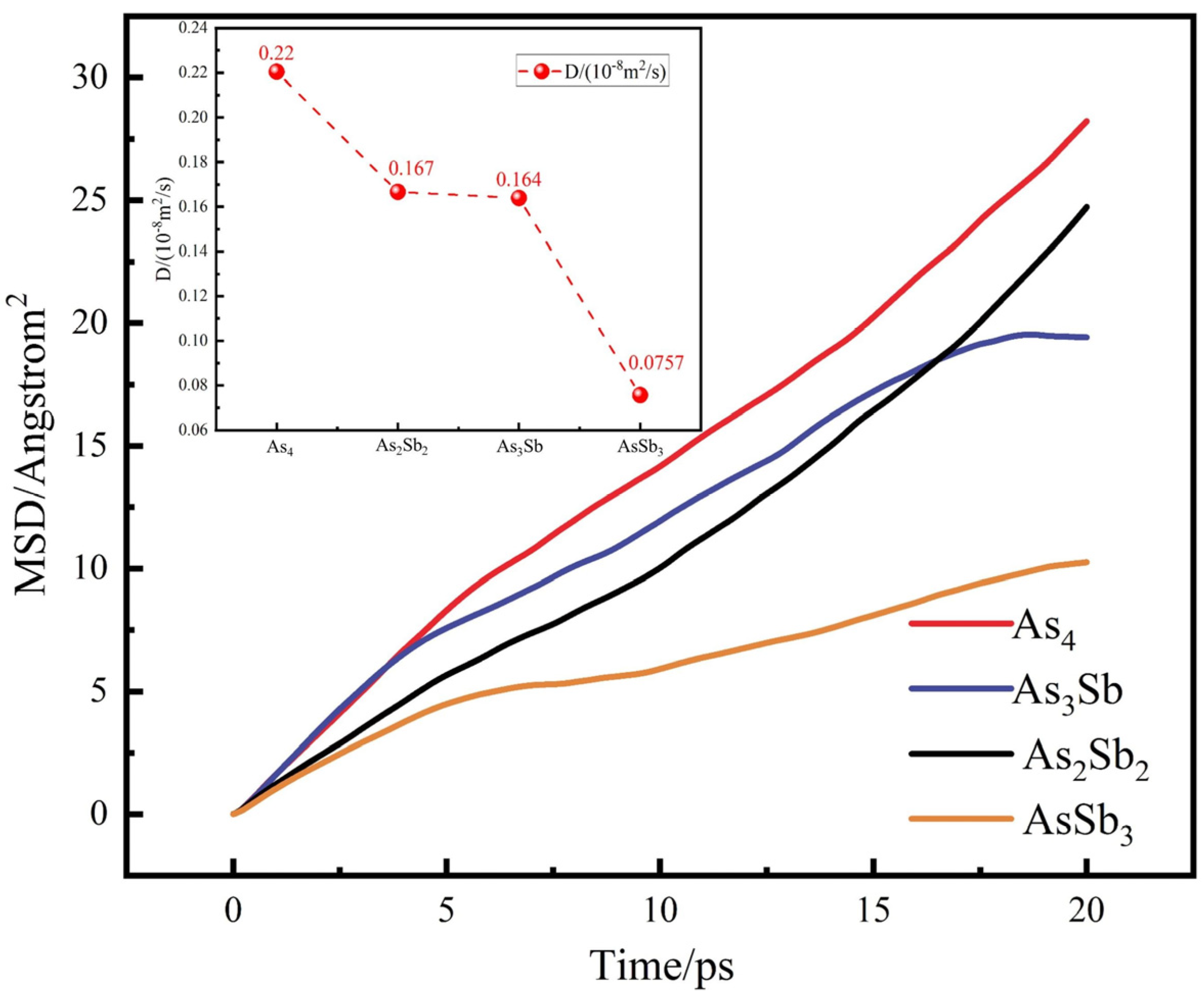
3.2. Results of Dynamic Calculations
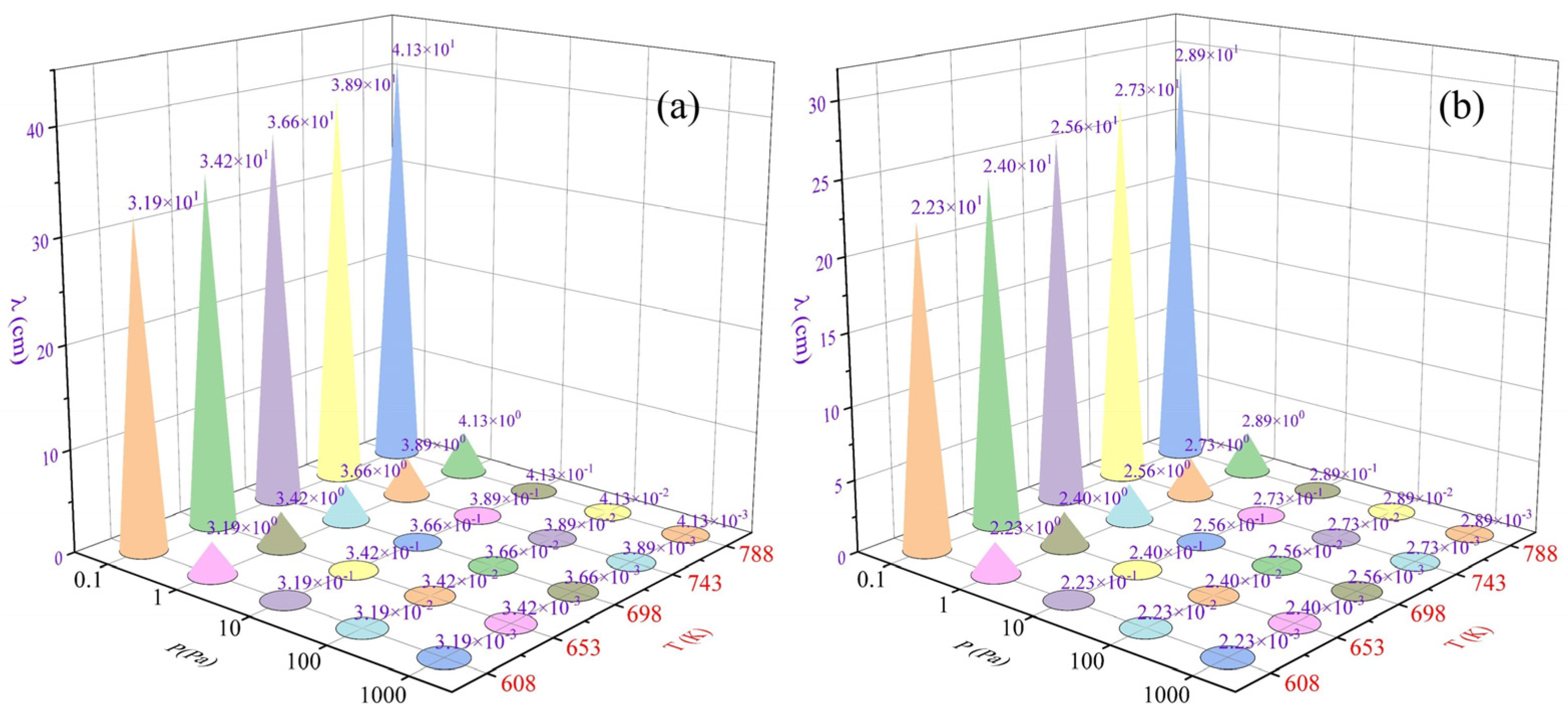
3.2.1. Mean Free Range of As4 and Sb4
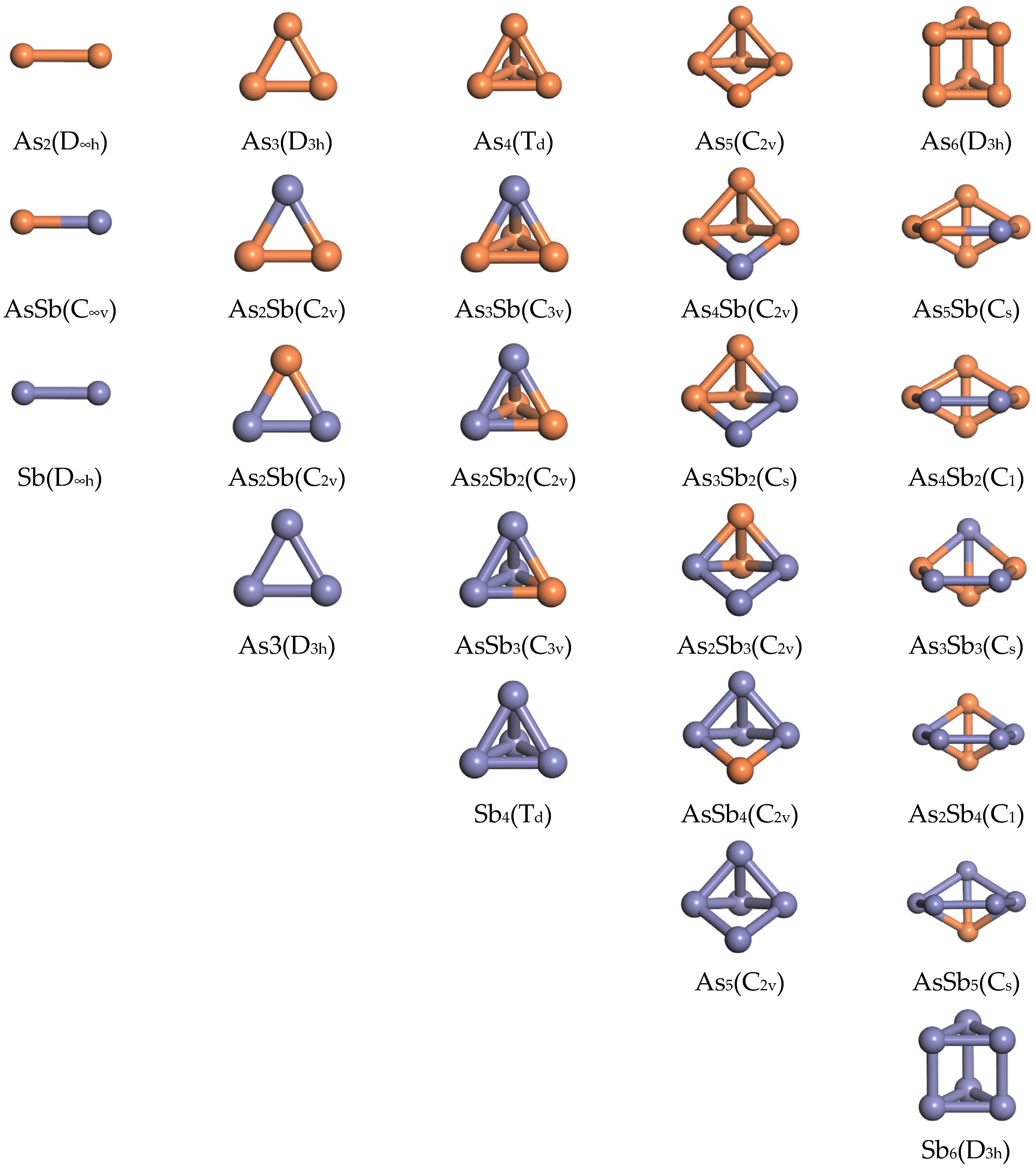
3.2.2. Results of Molecular Dynamics Calculations
| This Work | Theoretical Values | Experimental Values | ||||
|---|---|---|---|---|---|---|
| As2 | Bond length (Å) | 2.142 | 2.164 [16] | 2.12 [17] | 2.10 [18] | 2.103 [19] |
| Average binding energies (eV) | 3.96 | 4.12 [6] | 4.21 [20] | 4.32 [21] | 3.96 [22] | |
| Vibrational frequencies (cm−1) | 429.3 | 430 [23] | 429.5 [24] | 428 [24] | 429.55 [20] | |
| Sb2 | Bond length (Å) | 2.549 | 2.550 [25] | 2.490 [13] | 2.476 [13] | 2.340 [25] |
| Average binding energies (eV) | 3.01 | 3.18 [25] | 1.99 [13] | 3.10 [8] | 3.09 [8] | |
| Vibrational frequencies (cm−1) | 276.0 | 276.0 [25] | 280.0 [8] | 270.0 [26] | 270.0 [25] | |
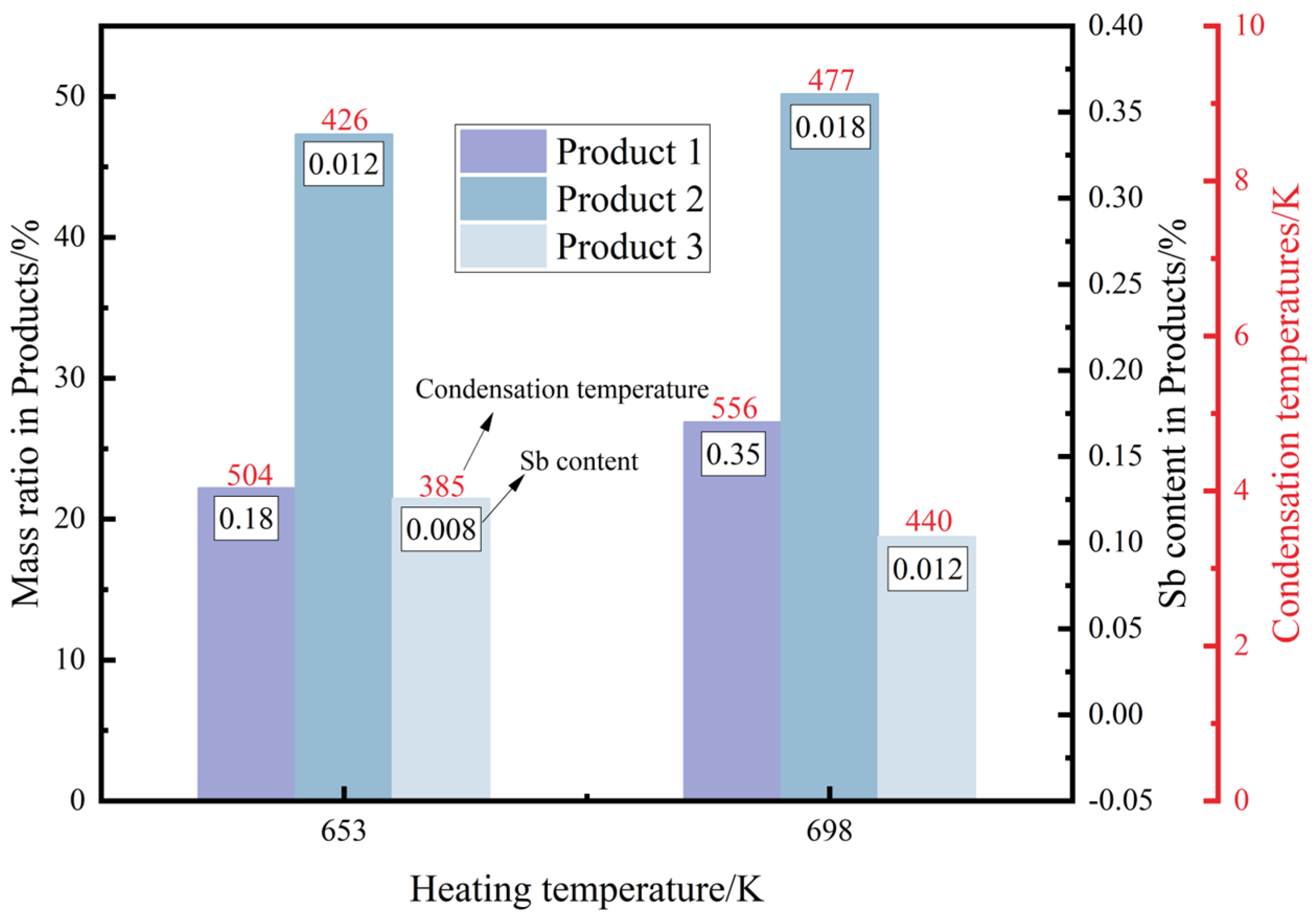
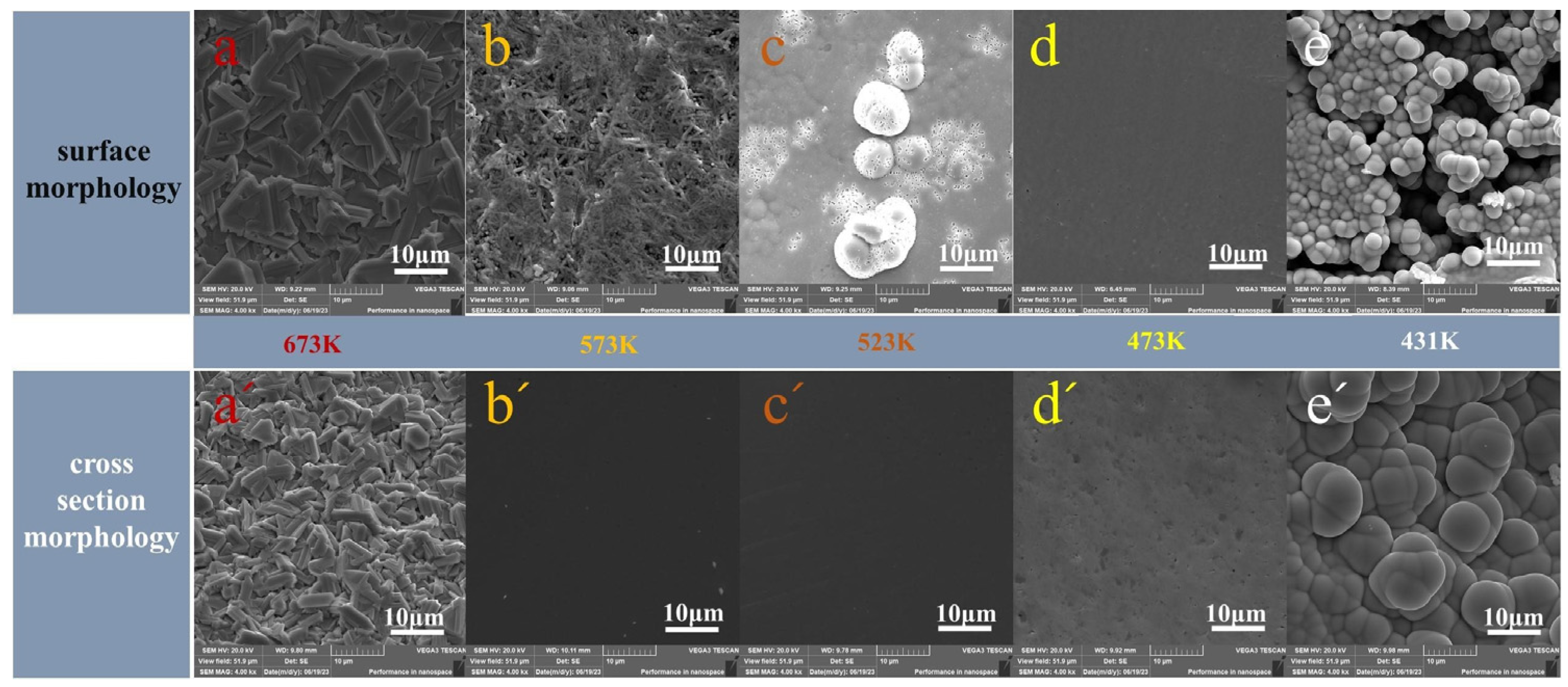
3.3. Results of the Experiment
4. Conclusions
- From a thermodynamic perspective, the saturation vapour pressure of arsenic and antimony is quite different, and controlling the temperature can achieve their effective separation. Meanwhile, it may be accompanied by arsenic–antimony compounds such as As3Sb, As2Sb2, and AsSb3 in the vacuum distillation process.
- Dynamics calculations show differences in the mean free ranges of As4 and Sb4 that can be used to guide the design of condenser structures. The ab initio molecular dynamics simulations of AsmSbn (m + n ≤ 6) clusters show that As4, As3Sb, As2Sb2, and AsSb3 are well stabilised. The maximum diffusion coefficient and migration distance of As4 make it possible to separate it from Sb-containing clusters during gas phase diffusion and condensation.
- The experimental results show that low-temperature sublimation purification is beneficial to inhibit the formation and diffusion of Sb-containing clusters. As vapour is transformed from crystalline to amorphous with increasing subcooling, and Sb-containing clusters entering the gas phase are mainly condensed and deposited at the proximal end of the heating zone. At a pressure of 10 Pa and a vacuum sublimation temperature of 653 K, the removal rate of Sb in the product by increasing the condensation disc and extending the condensation zone was 99.35% without considering the volatility.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-Bandgap Semiconductors: Research Opportunities and Challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, T.; Ye, B.; Liang, X.; Chen, H.; Nie, Q.; Tian, D.; Yang, D.; Ma, X. Arsenic Precipitation in Heavily Arsenic-Doped Czochralski Silicon. Phys. Status Solidi—Rapid Res. Lett. 2023, 17, 2200403. [Google Scholar] [CrossRef]
- Potolokov, V.N.; Efremov, V.A.; Zhukov, E.G.; Pashinkin, A.S.; Potolokov, N.A.; Fedorov, V.A. Preparation of high-purity arsenic via hydrogen reduction of arsenic trichloride. Inorg. Mater. 2003, 39, 15–21. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Liu, D.; Jiang, W.; Zha, G.; Huang, D.; Deng, P.; Li, B. Waste-free separation and recovery of copper telluride slag by directional sulfidation-vacuum distillation. J. Clean. Prod. 2022, 335, 130356. [Google Scholar] [CrossRef]
- Liang, G.; Wu, Q.; Yang, J. Probing the electronic structure and property of neutral and charged arsenic clusters (Asn (+ 1, 0,–1), n ≤ 8) using Gaussian-3 theory. J. Phys. Chem. A 2011, 115, 8302–8309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, W.; Li, Q.; Xie, Y.; Schaefer, H. The arsenic clusters Asn (n = 1–5) and their anions: Structures, thermochemistry, and electron affinities. J. Comput. Chem. 2004, 25, 907–920. [Google Scholar] [CrossRef]
- Morino, Y.; Ukaji, T.; Ito, T. Molecular structure determination by gas electron diffraction at high temperatures. I. Arsenic. Bull. Chem. Soc. Jpn. 1966, 39, 64–71. [Google Scholar] [CrossRef]
- Igel-Mann, G.; Stoll, H.; Preuss, H. Structure and ionization potentials of clusters containing heavy elements: I. homonuclear group v clusters up to hexamers. Mol. Phys. 1993, 80, 325–339. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Chen, X.; Lu, W. Structural and electronic properties of Sbn (n = 2–10) clusters using density-functional theory. Phys. Rev. A 2005, 72, 053203. [Google Scholar] [CrossRef]
- Kordis, J.; Gingerich, K.A. Mass spectroscopic investigation of the equilibrium dissociation of gaseous Sb2, Sb3, Sb4, SbP, SbP3, and P2. J. Chem. Phys. 1973, 58, 5141–5149. [Google Scholar] [CrossRef]
- Yang, B.; Xu, B. Vacuum Metallurgy; Central South University Press: Changsha, China, 2022; p. 519. (In Chinese) [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Yang, J. Theoretical Study on the Growth Sequence and Electronic Properties of Arsenic and Antimony Clusters; Nanjing Normal University: Nanjing, China, 2018. (In Chinese) [Google Scholar]
- Song, B.; Xu, B.; Yang, B. Ab Initio Molecular Dynamics Studies of PbmSbn (m + n ≤ 9) Alloy Clusters. Metall. Mater. Trans. A 2017, 48, 4905–4913. [Google Scholar] [CrossRef]
- Kumar, V. Electronic and atomic stuctures of Sb4 and Sb8 clusters. Phys. Rev. B 1993, 48, 8470–8473. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Bonding and energetics in small clusters of gallium and arsenic. Heteroat. Chem. Int. J. Main Group Elem. 2003, 14, 189–196. [Google Scholar] [CrossRef]
- Kim, K.; Jordan, K.D. Comparison of density functional and MP2 calculations on the water monomer and dimer. J. Phys. Chem. 1994, 98, 10089–10094. [Google Scholar] [CrossRef]
- Reuse, F.A.; Khanna, S.N. Geometry, electronic structure, and magnetism of small Nin (n = 2–6, 8, 13) clusters. Chem. Phys. Lett. 1995, 234, 77–81. [Google Scholar] [CrossRef]
- Ballone, P.; Jones, R.O. Density functional study of phosphorus and arsenic clusters using local and nonlocal energy functionals. J. Chem. Phys. 1994, 100, 4941–4946. [Google Scholar] [CrossRef]
- Gong, X.; Tosatti, E. Structure of Small Gallium Clusters. Phys. Lett. A 1992, 166, 369–372. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Pang, J.; Kong, L.; Yang, Z.; Chen, L.; Xu, B.; Yang, B. Sustainable safe and environmentally friendly process to recovery valuable materials from hazardous waste InP. Waste Manag. 2023, 155, 153–161. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.D.; Liu, B.; Chandler, G.S. Computed self-consistent field and singles and doubles configuration interaction spectroscopic data and dissociation energies for the diatomics B2, C2, N2, O2, F2, CN, CP, CS, PN, SiC, SiN, SiO, SiP, and their ions. J. Chem. Phys. 1992, 97, 8459–8464. [Google Scholar] [CrossRef]
- Rajesh, C.; Majumder, C. Atomic and electronic structures of neutral and charged Pbn clusters (n = 2–15): Theoretical investigation based on density functional theory. J. Chem. Phys. 2007, 126, 244704. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Niu, B.; Lee, Y.T.; Shirley, D.A.; Balasubramanian, K. Photoelectron spectroscopy and electronic structure of heavy group IV–VI diatomics. J. Chem. Phys. 1990, 92, 899–908. [Google Scholar] [CrossRef]
- Krebs, H.; Holz, W.; Worms, K.H. Über die Struktur und die Eigenschaften der Halbmetalle, X. Eine Neue Rhombische Arsenmodifikation und Ihre Mischkristallbildung mit Schwarzem Phosphor. Chem. Berichte 1957, 90, 1031–1037. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X.; Xu, B.; Yang, B.; Jiang, W.; Tian, Y.; Wang, L.; Wang, W. Study on the behavior of clusters in the physical recovery of GaAs scrap. J. Environ. Manag. 2024, 352, 120049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lai, X.; Zhou, Y.; Deng, F.; Song, J.; Yang, Z.; Peng, C.; Ding, F.; Zhao, F.; Hu, Z.; et al. Enhancing the anti-oxidation stability of vapor-crystallized arsenic crystals via introducing iodine. J. Hazard. Mater. 2022, 439, 129573. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.A. Modern Crystallography III: Crystal Growth; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]


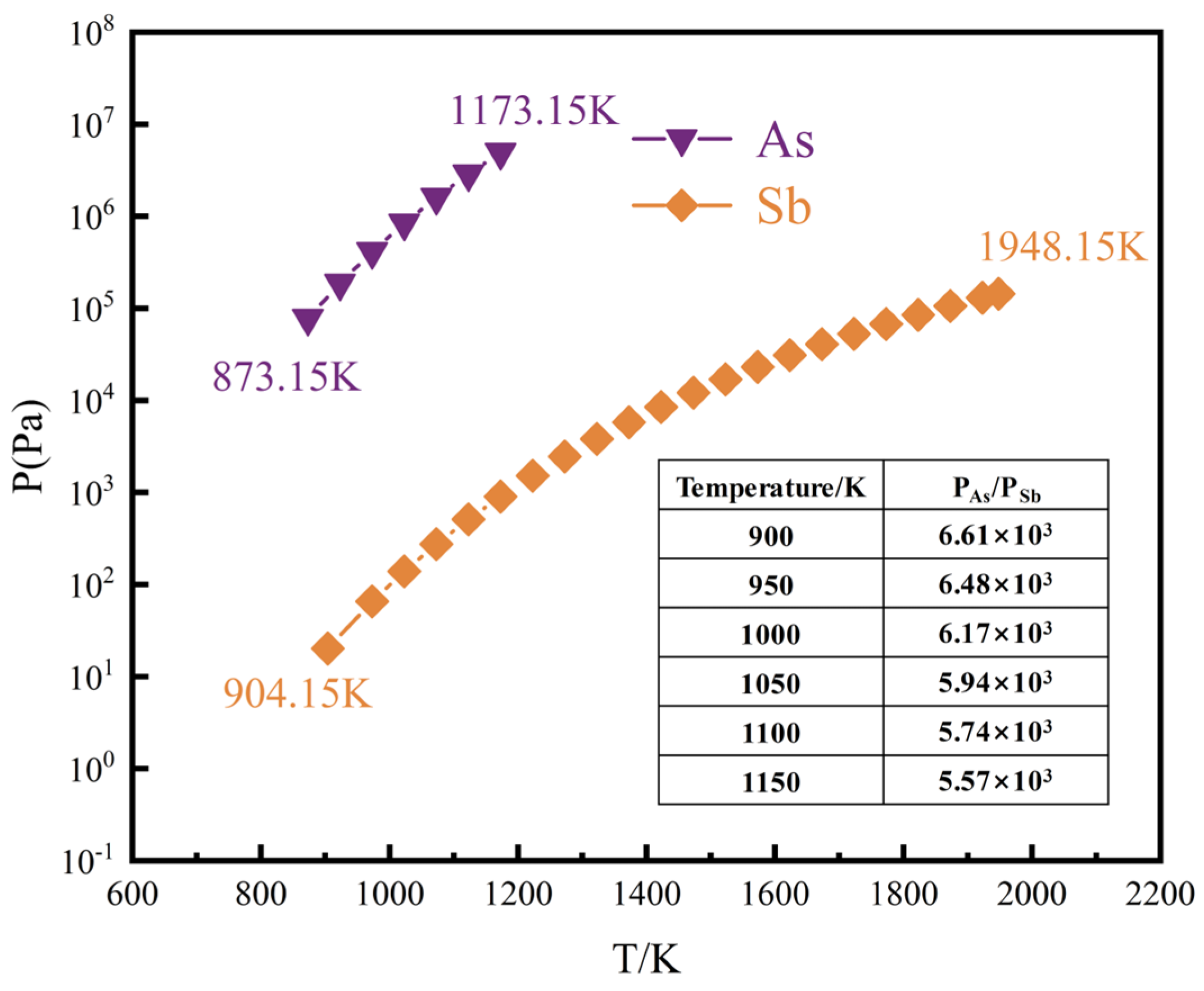


| Materialistic | lgP/Pa | Temperature Range/K | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| As | −6160 | - | - | 11.94 | 873.15–1173.15 |
| Sb | −6500 | - | - | 8.49 | 904.15–1948.15 |
| Element | As | Sb | Others |
|---|---|---|---|
| Wt.% | 96.33 | 1.86 | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Z.; Duan, M.; Liu, X.; Chen, X.; Luo, H.; Shi, T.; Lei, X.; Tian, Y.; Yang, B.; Xu, B. Removal of Antimony from Industrial Crude Arsenic by Vacuum Sublimation: Combination of Thermodynamics and Ab Initio Molecular Dynamics. Metals 2024, 14, 490. https://doi.org/10.3390/met14050490
Zuo Z, Duan M, Liu X, Chen X, Luo H, Shi T, Lei X, Tian Y, Yang B, Xu B. Removal of Antimony from Industrial Crude Arsenic by Vacuum Sublimation: Combination of Thermodynamics and Ab Initio Molecular Dynamics. Metals. 2024; 14(5):490. https://doi.org/10.3390/met14050490
Chicago/Turabian StyleZuo, Zibin, Mengping Duan, Xinyang Liu, Xiumin Chen, Huan Luo, Tengteng Shi, Xianjun Lei, Yang Tian, Bin Yang, and Baoqiang Xu. 2024. "Removal of Antimony from Industrial Crude Arsenic by Vacuum Sublimation: Combination of Thermodynamics and Ab Initio Molecular Dynamics" Metals 14, no. 5: 490. https://doi.org/10.3390/met14050490






