The Interfacial Microstructure and Mechanical Properties of Diffusion-Bonded Joints of 316L Stainless Steel and the 4J29 Kovar Alloy Using Nickel as an Interlayer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing Parameters
2.2. Interface Microstructure Characterization
2.3. Evaluation of Mechanical Properties
3. Result and Discussion
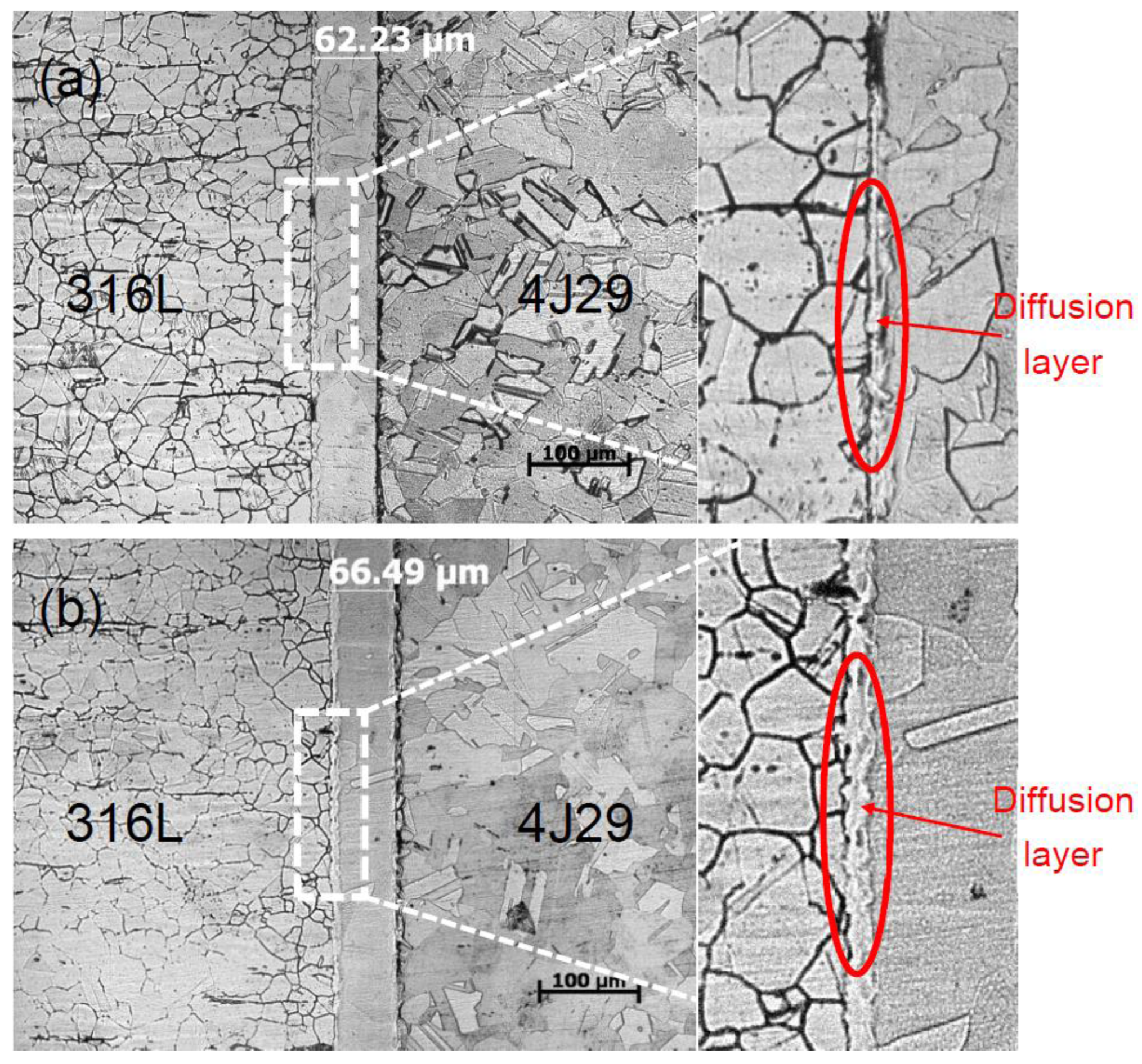
3.1. Optical Microstructure
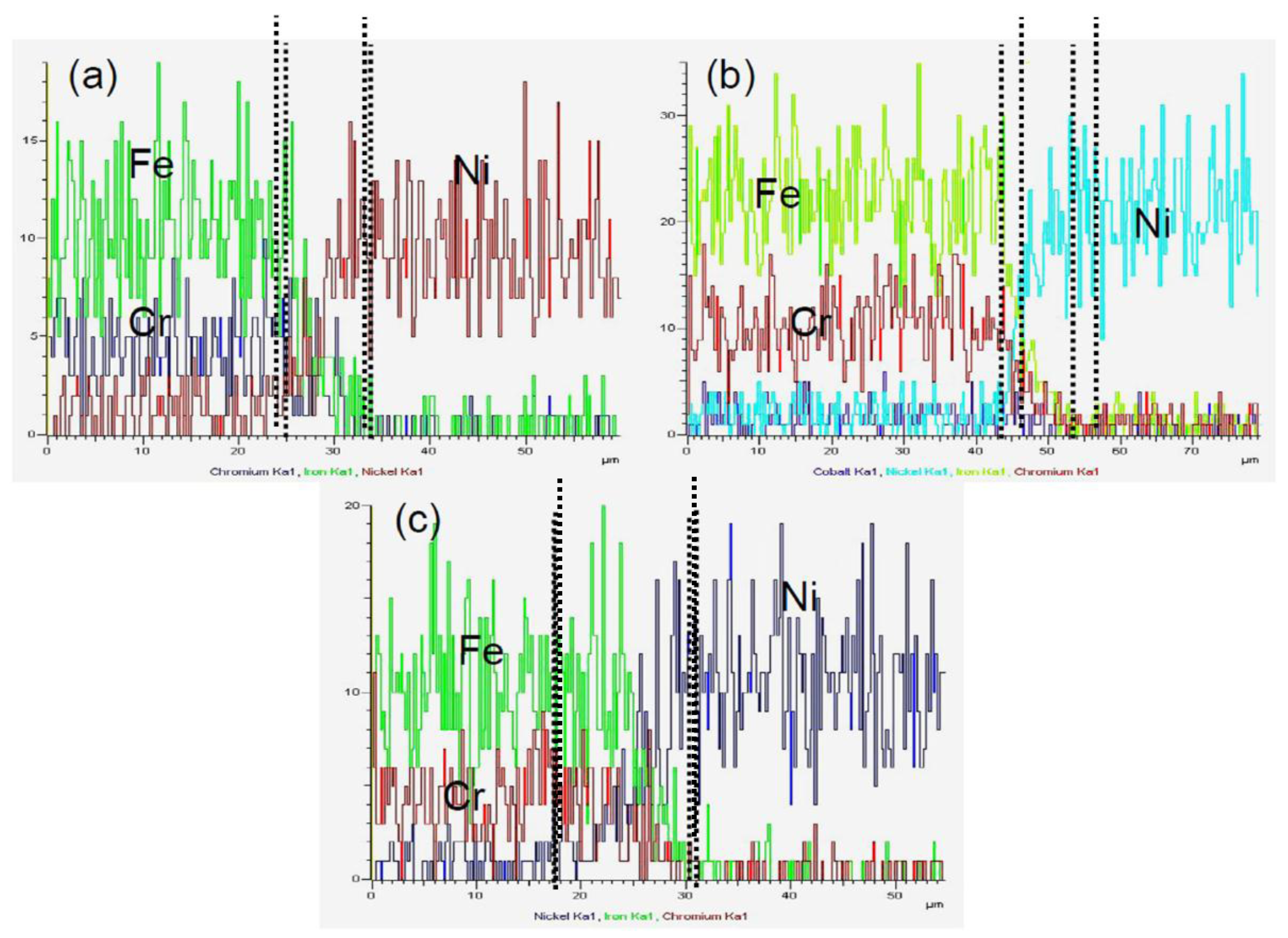
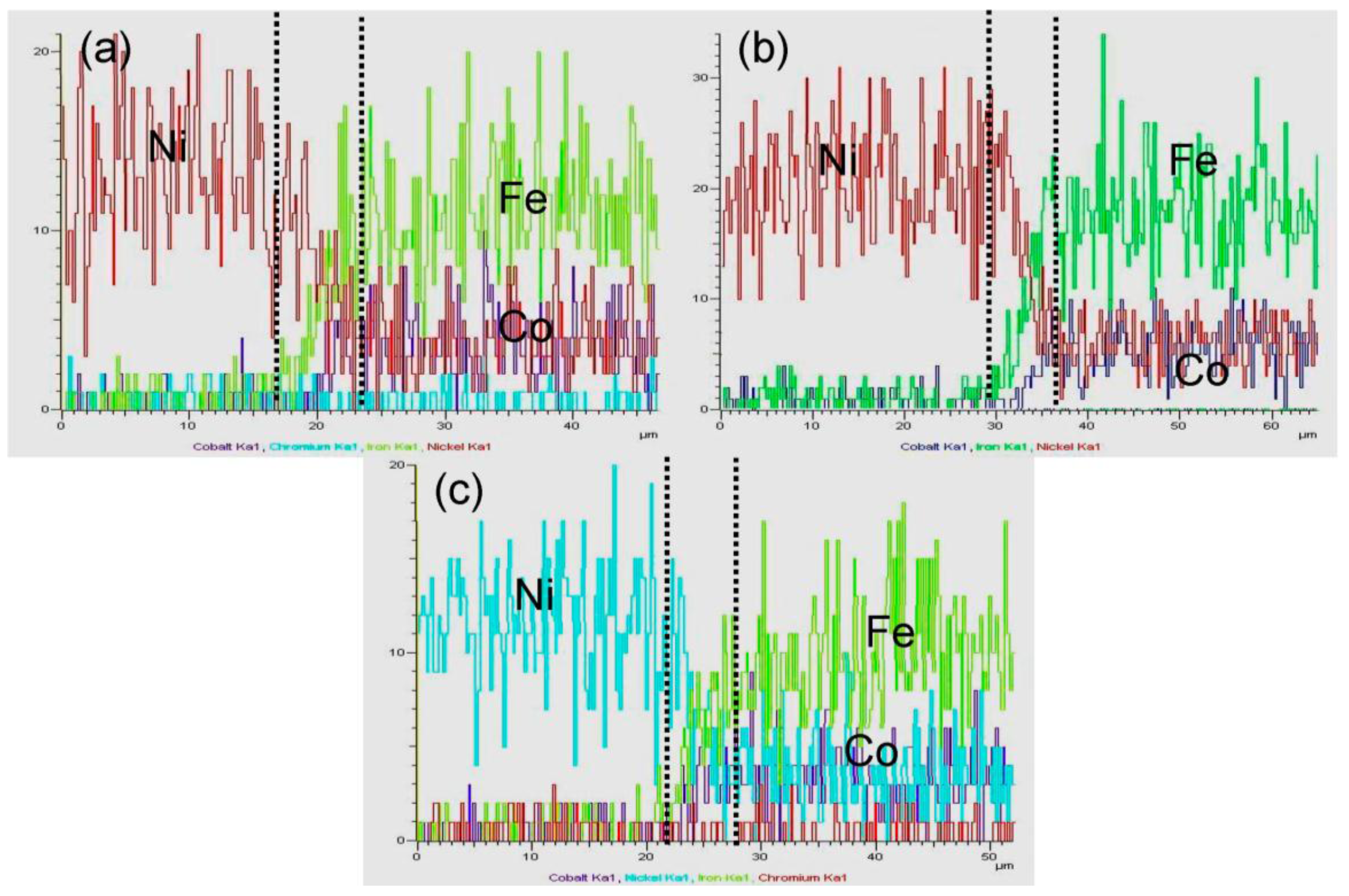
3.2. Element Distribution
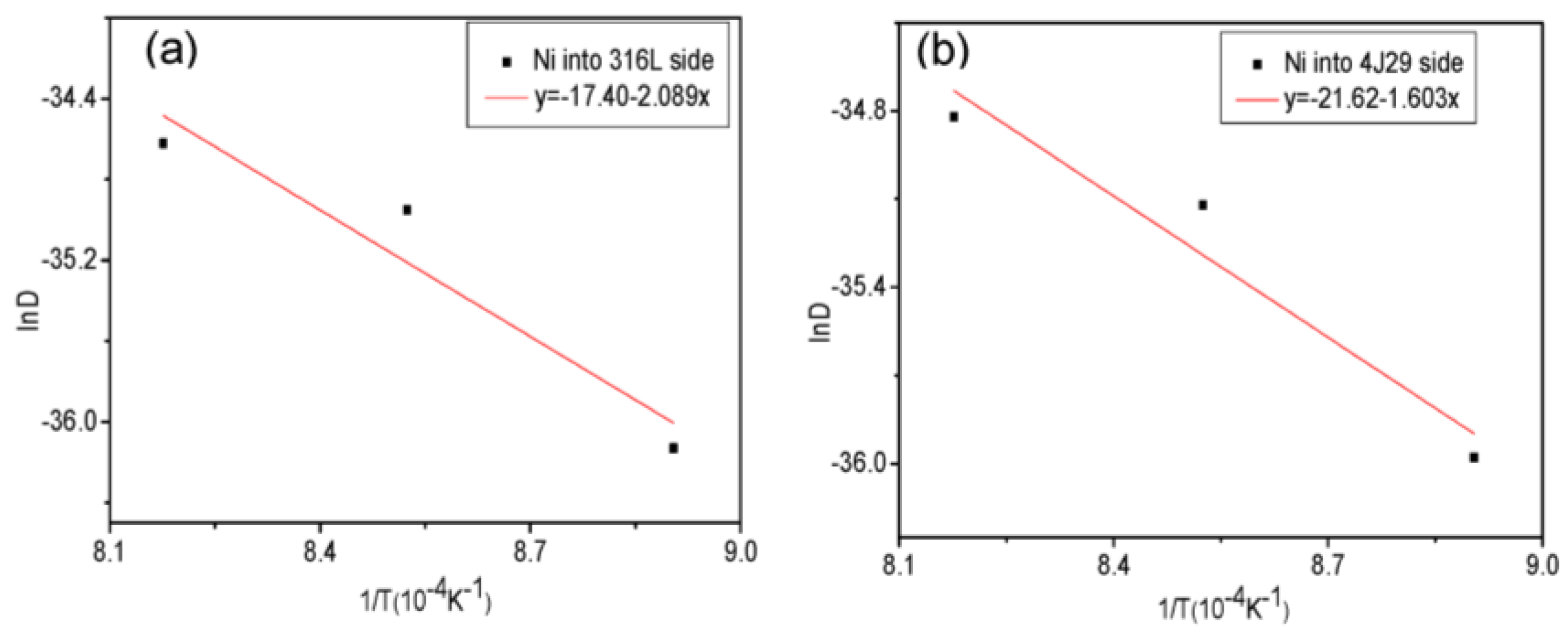
3.3. Diffusion Coefficient
3.4. XRD Analysis
3.5. Hardness
3.6. Tensile Strength and Fracture Analysis
4. Conclusions
- The Ni interlayer can serve as an effective diffusion barrier for the bonding of stainless steel (316L) and the Kovar alloy (4J29). The composition of the joints was 316L/Ni s.s (Fe–Cr–Ni)/remnant Ni/Ni s.s (Fe–Co–Ni)/4J29.
- Growth of the diffusion layer was determined with the diffusion coefficient and activation energy, and the activation energy for the diffusion of Ni into 316L and 4J29 is 173.68 kJ/mol and 133.27 kJ/mol, respectively.
- At lower bonding temperatures and times, fracture takes place at the interface of the Ni–4J29 side due to insufficient bonding. After the width of the nickel solid solution (Fe–Co–Ni) increased, failure located at the 4J29 side and the fracture surface indicated a ductile nature. The highest tensile strength of 504.91 MPa with an elongation of 38.75% was obtained at 900 °C for 240 min.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alejandro, H.; Jorge, M.; Ashley, R.; Pedro, F.; Peter, H.; Lawrence, E.M.; Ryan, B.W. Joining of Inconel 718 and 316 Stainless Steel using electron beam melting additive manufacturing technology. Mater. Des. 2016, 94, 17–27. [Google Scholar]
- Casalino, G.; Campanelli, S.L.; Ludovico, A.D. Laser-arc hybrid welding of wrought to selective laser molten stainless steel. Int. J. Adv. Manuf. Technol. 2013, 68, 209–216. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tseng, K.H.; Wang, H.C. Small-scale projection lap-joint welding of Kovar alloy and SPCC steel. J. Chin. Inst. Eng. 2012, 35, 211–218. [Google Scholar] [CrossRef]
- Zhu, W.W.; Chen, J.C.; Jiang, C.H. Effects of Ti thickness on microstructure and mechanical properties of alumina-Kovar joints brazed with Ag-Pd/Ti filler. Ceram. Int. 2014, 40, 5699–5705. [Google Scholar] [CrossRef]
- Wei, J.H.; Deng, B.H.; Gao, X.Q. Interface structure characterization of Fe36Ni alloy with ultrasonic soldering. J. Alloy. Compd. 2013, 576, 386–392. [Google Scholar] [CrossRef]
- Baghjari, S.H.; Akbari, M.S. Experimental investigation on dissimilar pulsed Nd:YAG laser welding of AISI 420 stainless steel to Kovar alloy. Mater. Des. 2014, 57, 128–134. [Google Scholar] [CrossRef]
- Akbari, M.S.; Sufizadeh, A.R. Metallurgical investigations of pulsed Nd:YAG laser welding of AISI 321 and AISI 630 stainless steels. Mater. Des. 2009, 30, 3150–3157. [Google Scholar] [CrossRef]
- Rossini, M.; Spena, P.R.; Cortese, L.; Matteis, P.; Firrao, D. Investigation on dissimilar laser welding of advanced high strength steel sheets for the automotive industry. Mater. Sci. Eng. A 2015, 628, 288–296. [Google Scholar] [CrossRef]
- Casalino, G.; Mortello, M.; Peyre, P. Yb–YAG laser offset welding of AA5754 and T40 butt joint. J. Mater. Process. Technol. 2015, 223, 139–149. [Google Scholar] [CrossRef]
- Mai, T.A.; Spowage, A.C. Characterization of dissimilar joints in laser welding of steel-Kovar, copper-steel and copper-aluminum. Mater. Sci. Eng. A 2004, 374, 224–233. [Google Scholar] [CrossRef]
- Nekouie, E.M.; Coupland, J.; Marimuthu, S. Microstructure and mechanical properties of a laser welded low carbon-stainless steel joint. J. Mater. Process. Technol. 2014, 214, 2941–2948. [Google Scholar]
- Wu, W.Y.; Hu, S.S.; Shen, J.Q. Microstructure, mechanical properties and corrosion behavior of laser welded dissimilar joints between ferritic stainless steel and carbon steel. Mater. Des. 2015, 65, 855–861. [Google Scholar] [CrossRef]
- Verena, W.; Bernhard, D.; Silvia, H.; Michael, R. Investigations of dissimilar welds of the high temperature steels P91 and PM2000. Fus. Eng. Des. 2013, 88, 2539–2542. [Google Scholar]
- Wang, T.; Zhang, B.G.; Chen, G.Q.; Feng, J.C.; Tang, Q. Electron beam welding of Ti-15-3 titanium alloy to 304 stainless steel with copper interlayer sheet. Trans. Nonferr. Met. Soc. China 2010, 20, 1829–1834. [Google Scholar] [CrossRef]
- Yuan, X.J.; Tang, K.L.; Deng, Y.Q.; Luo, J.; Sheng, G.M. Impulse pressuring diffusion bonding of a copper alloy to a stainless steel with/without a pure nickel interlayer. Mater. Des. 2013, 52, 359–366. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Sheng, G.M.; Xu, C. Evaluation of the microstructure and mechanical properties of diffusion bonded joints of titanium to stainless steel with a pure silver interlayer. Mater. Des. 2013, 46, 84–87. [Google Scholar] [CrossRef]
- Wang, J.D.; He, X.Q.; Li, X.P.; En, Y.F. Hermetic Packaging of Kovar Alloy and Low-carbon Steel Structure in Hybrid Integrated Circuit (HIC) System Using Parallel Seam Welding Process. In Proceedings of the 15th International Conference on Electronic Packaging Technology, Chengdu, China, 12–15 August 2014.
- Sathiskumar, J.; Torsten, S.; Davies, H.M.; Eggert, D.R.; Brown, S.G.R. Localized microstructural characterization of a dissimilar metal electron beam weld joint from an aerospace component. Mater. Des. 2016, 90, 101–114. [Google Scholar]
- Madhusudhan, R.G.; Venkata, R.P. Role of nickel as an interlayer in dissimilar metal friction welding of maraging steel to low alloy steel. J. Mater. Process. Technol. 2012, 212, 66–77. [Google Scholar] [CrossRef]
- Sam, S.; Kundu, S.; Chatterjee, S. Diffusion bonding of titanium alloy to micro-duplex stainless steel using a nickel alloy interlayer: Interface microstructure and strength properties. Mater. Des. 2012, 40, 237–244. [Google Scholar] [CrossRef]
- Kundu, S.; Sam, S.; Mishra, B.; Chatterjee, S. Diffusion bonding of microduplex stainless steel and Ti alloy with and without interlayer: Interface microstructure and strength properties. Metall. Mater. Trans. A 2014, 45, 371–383. [Google Scholar] [CrossRef]
- Kundu, S.; Sam, S.; Chatterjee, S. Interface microstructure and strength properties of Ti-6Al-4V and microduplex stainless steel diffusion bonded joints. Mater. Des. 2011, 32, 2997–3003. [Google Scholar] [CrossRef]
- Sun, J.C. Investigation of Surface Nanocrystallinzation and Alloying of Commercial Iron and Diffusion Behavior. Ph.D. Thesis, Chongqing University, Chongqing, China, March 2012. [Google Scholar]
- Vigraman, T.; Ravindran, D.; Narayanasamy, R. Effect of phase transformation and intermetallic compounds on the microstructure and tensile strength properties of diffusion-bonded joints between Ti-6Al-4V and AISI304L. Mater. Des. 2012, 36, 714–727. [Google Scholar] [CrossRef]






| Mn | Si | C | Fe | Co | Ni | Cr | S | P | Mo | Alloy |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.00 | 1.00 | 0.03 | Bal. | - | 11.05 | 18.17 | 0.03 | 0.04 | 2.00 | 316L |
| 0.4 | 0.2 | 0.02 | Bal. | 17.17 | 28.67 | - | 0.02 | 0.02 | - | 4J29 |
| Alloy | Density (g/cm3) | Melting Point (°C) | Expansion Coefficient (10−6 K−1) | Ultimate Tensile Strength (MPa) |
|---|---|---|---|---|
| 316L | 8.9 | 1375 | 16 | 573 |
| 4J29 | 8.1 | 1460 | 4.7 | 580 |
| Sample (°C, min) | Diffusion of Ni into 316L | Diffusion of Ni into 4J29 |
|---|---|---|
| (×10−16 m2/s) | (×10−16 m2/s) | |
| 850, 120 | 2.03 | 2.37 |
| 900, 120 | 6.62 | 5.60 |
| 950, 120 | 9.23 | 7.58 |
| Sample (°C, min) | Ultimate Tensile Strength (MPa) | Elongation (%) | Failure Location |
|---|---|---|---|
| 850, 120 | 215.86 | 6 | Interface |
| 900, 120 | 451.99 | 25.55 | Interface/4J29 side |
| 950, 120 | 490.62 | 31.25 | 4J29 side |
| 900, 180 | 501.84 | 38.75 | 4J29 side |
| 900, 240 | 504.91 | 38.75 | 4J29 side |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Jiang, X.; Shao, Z.; Mo, D.; Zhu, D.; Zhu, M. The Interfacial Microstructure and Mechanical Properties of Diffusion-Bonded Joints of 316L Stainless Steel and the 4J29 Kovar Alloy Using Nickel as an Interlayer. Metals 2016, 6, 263. https://doi.org/10.3390/met6110263
Song T, Jiang X, Shao Z, Mo D, Zhu D, Zhu M. The Interfacial Microstructure and Mechanical Properties of Diffusion-Bonded Joints of 316L Stainless Steel and the 4J29 Kovar Alloy Using Nickel as an Interlayer. Metals. 2016; 6(11):263. https://doi.org/10.3390/met6110263
Chicago/Turabian StyleSong, Tingfeng, Xiaosong Jiang, Zhenyi Shao, Defeng Mo, Degui Zhu, and Minhao Zhu. 2016. "The Interfacial Microstructure and Mechanical Properties of Diffusion-Bonded Joints of 316L Stainless Steel and the 4J29 Kovar Alloy Using Nickel as an Interlayer" Metals 6, no. 11: 263. https://doi.org/10.3390/met6110263





