Leaching Kinetics of Hemimorphite in Ammonium Chloride Solution
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Leaching Methods
3. Results
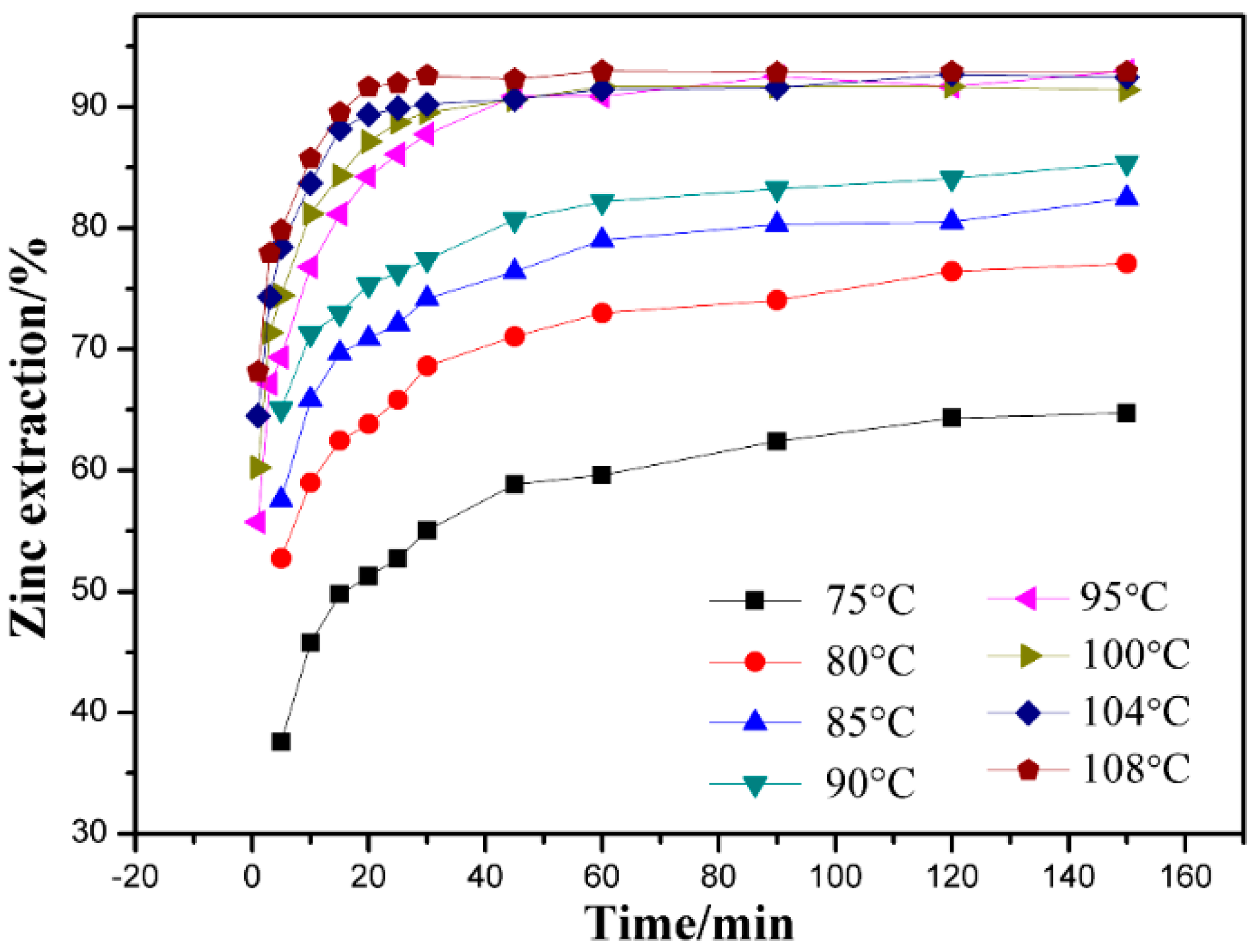
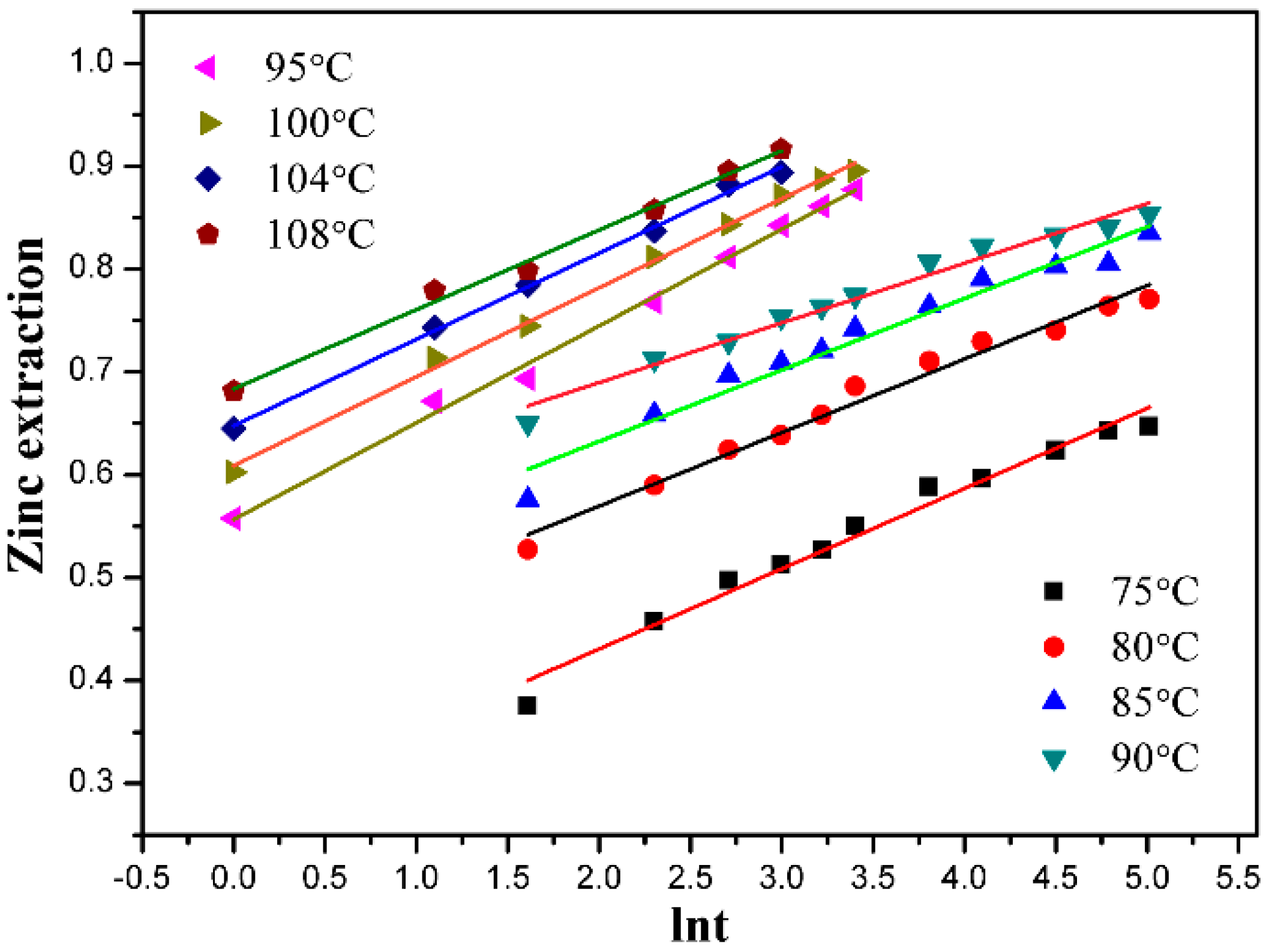
3.1. Effect of Leaching Temperature
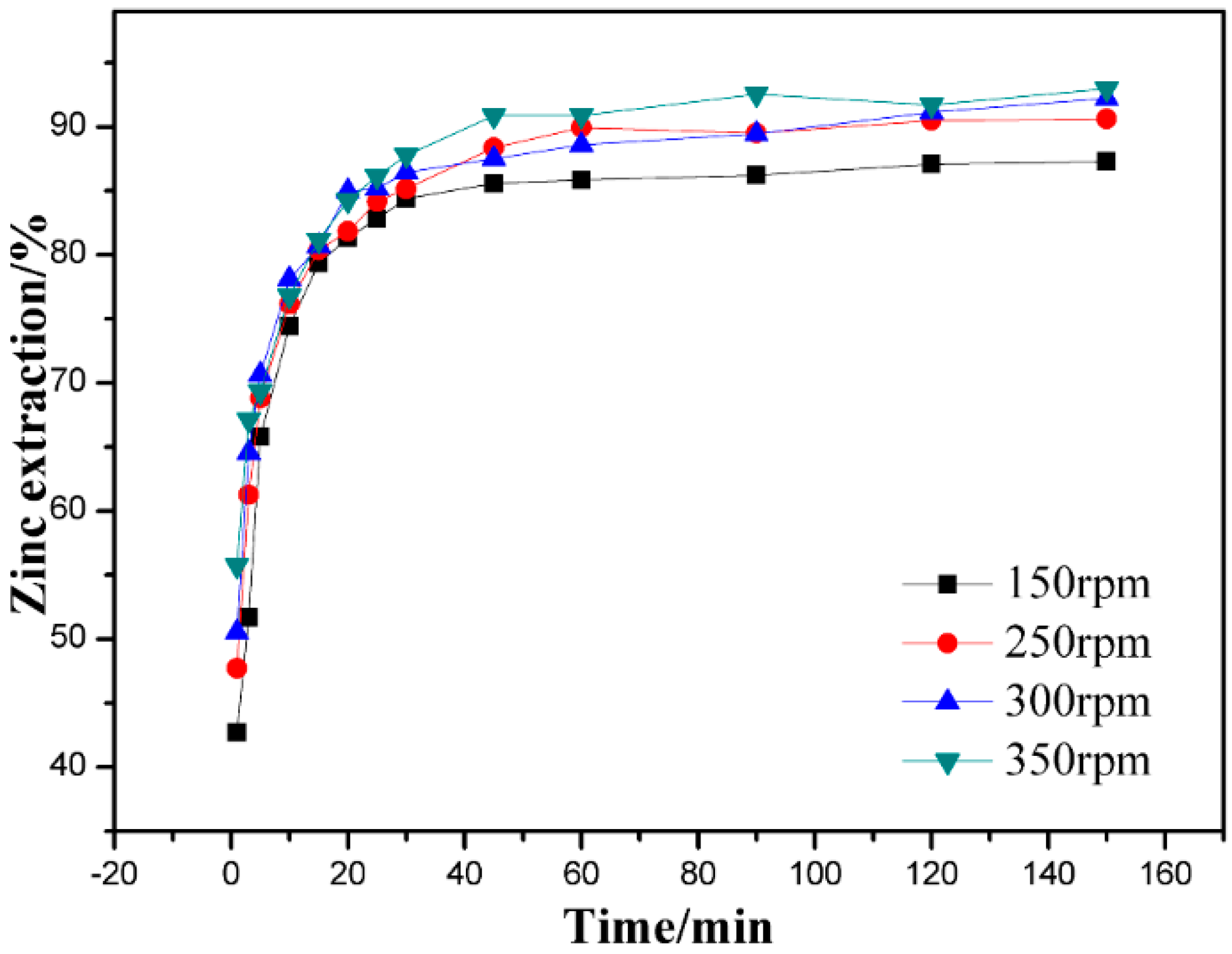
3.2. Effect of Stirring Speed
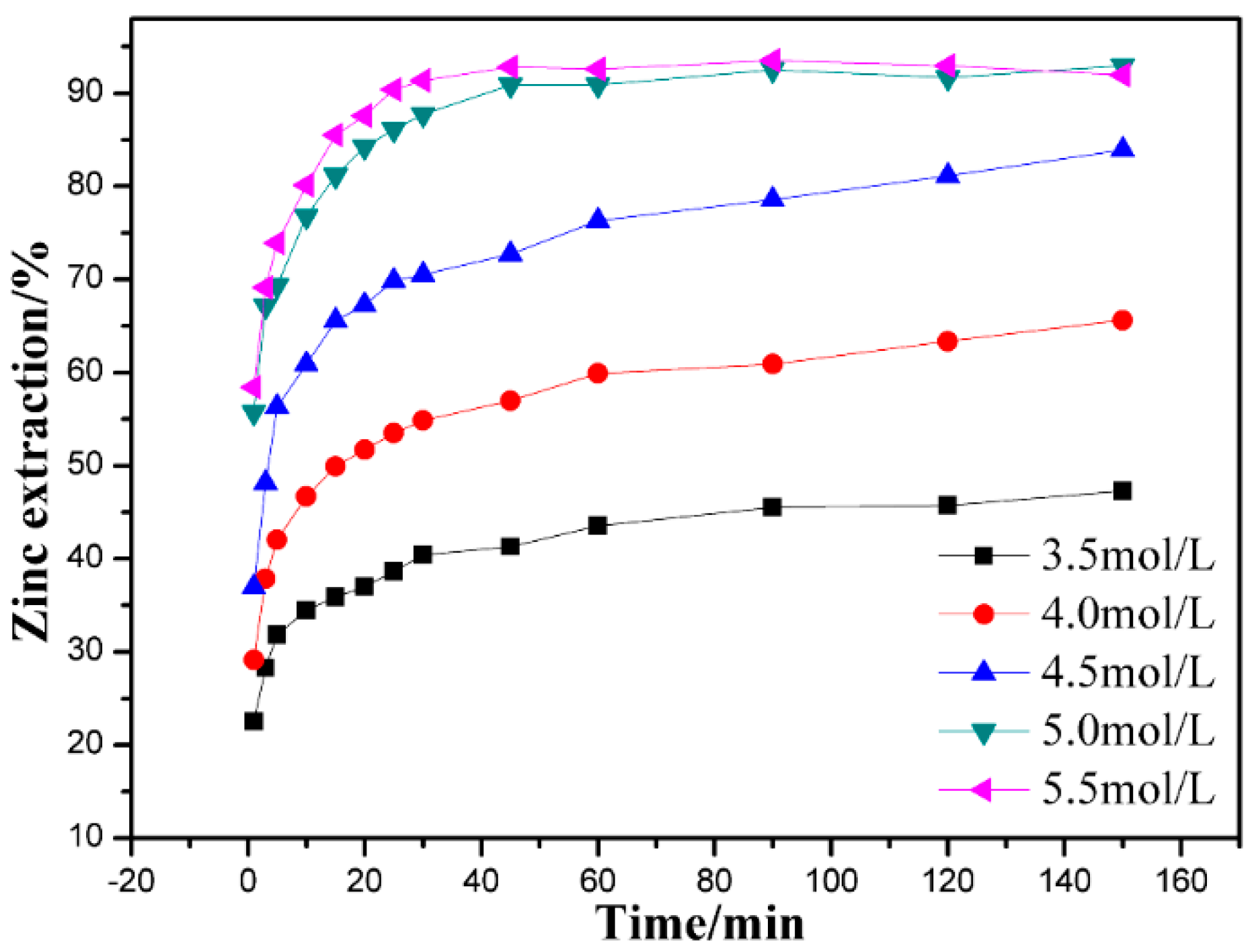
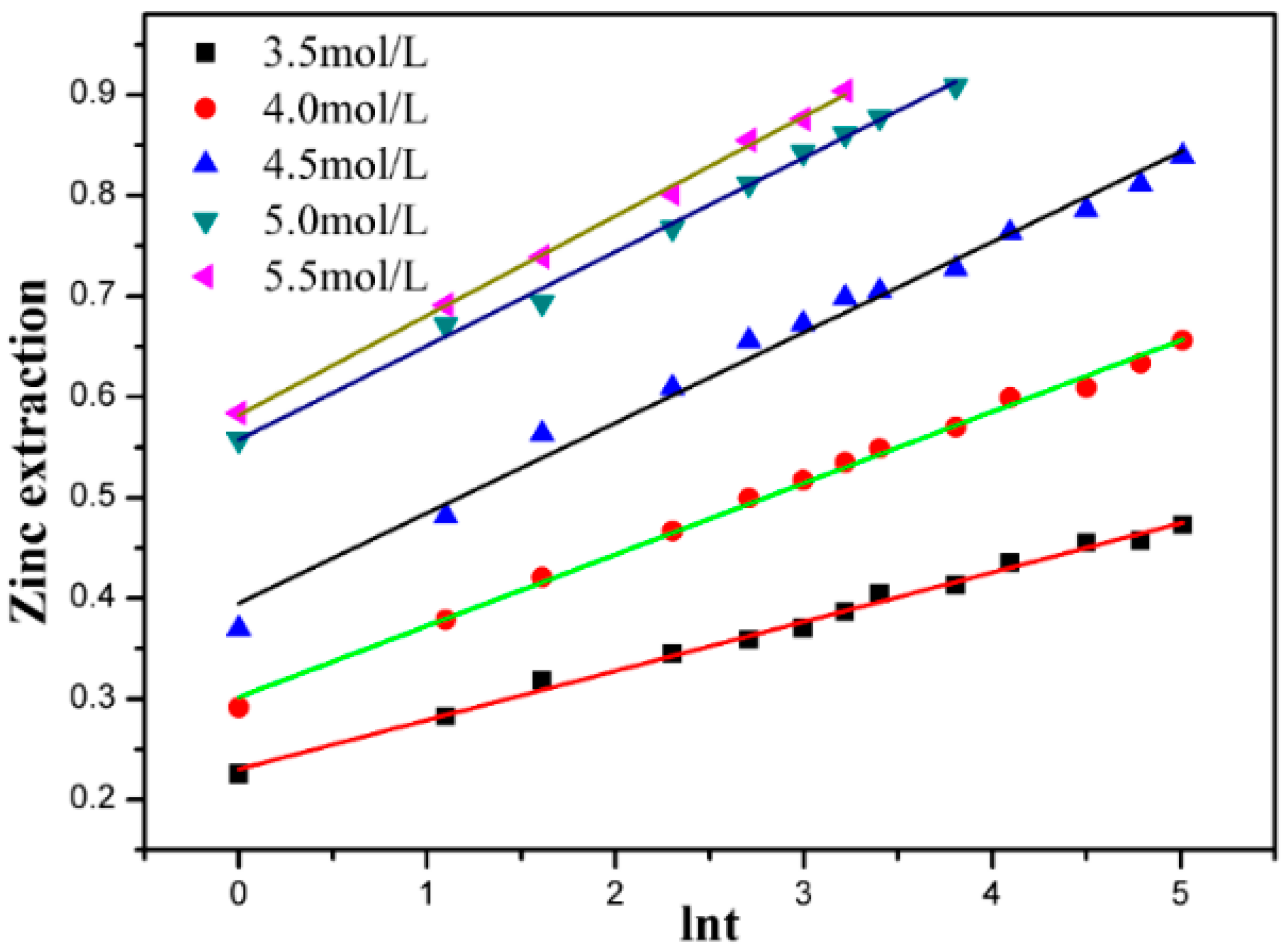
3.3. Effect of Ammonium Chloride Concentration
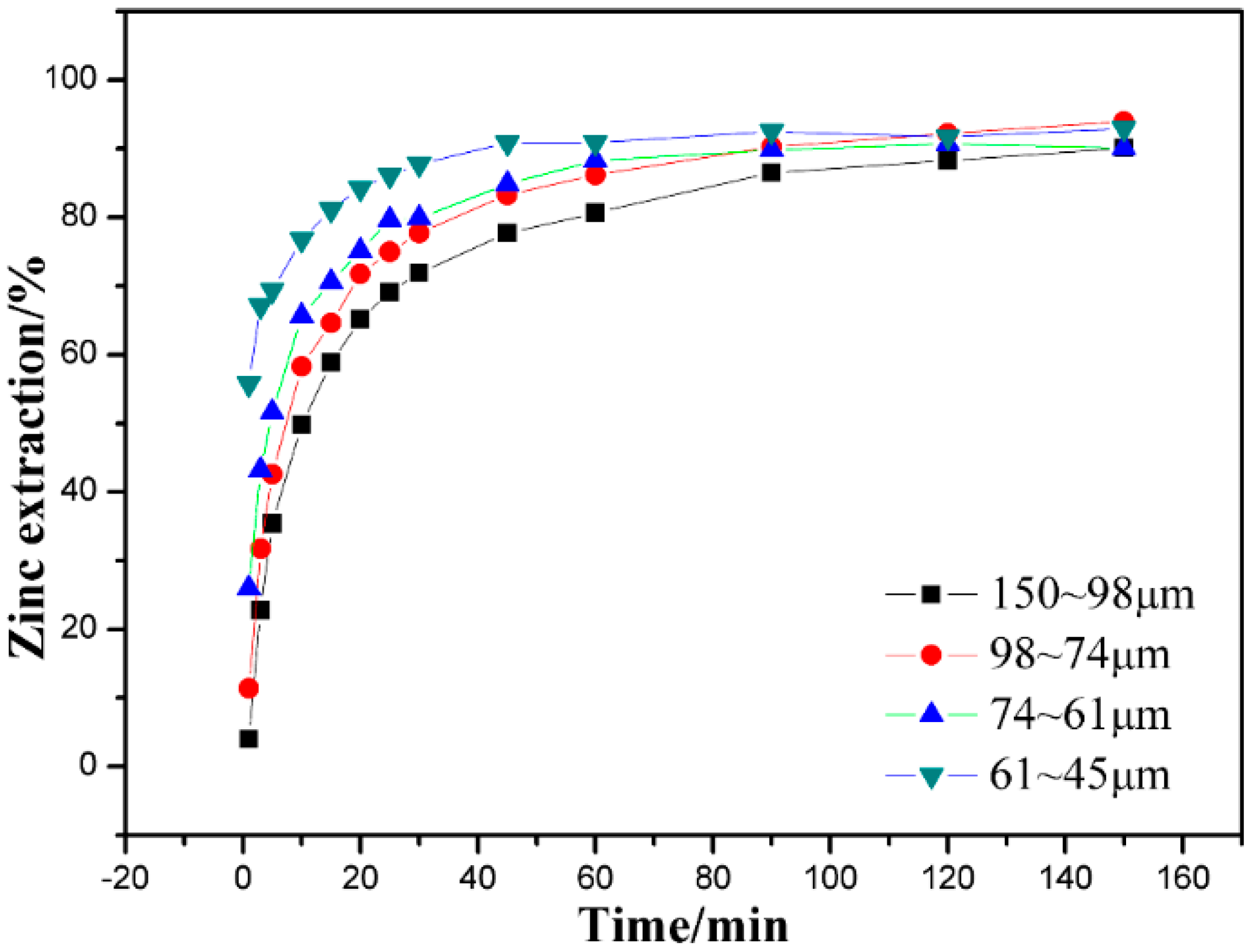
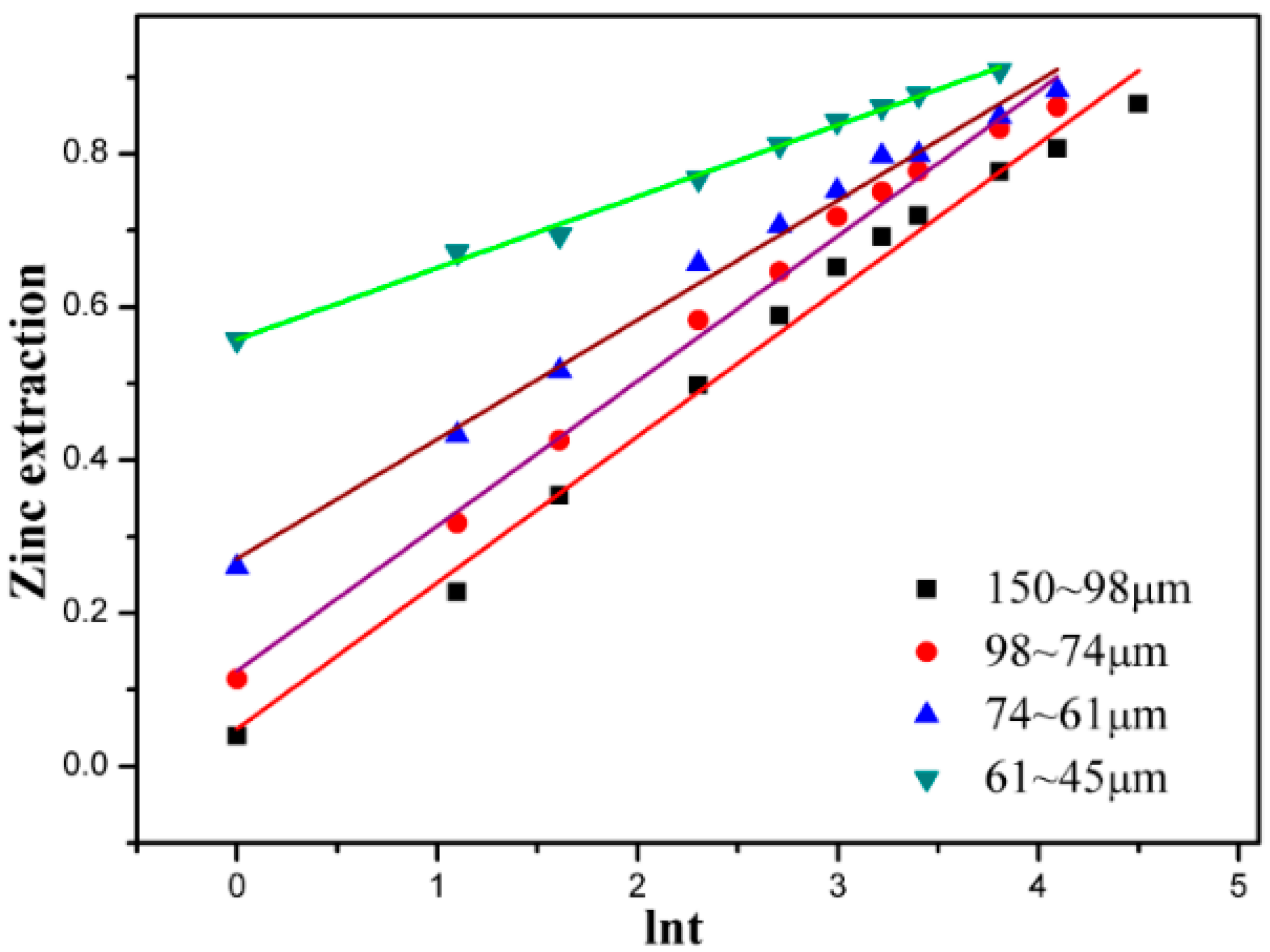
3.4. Effect of Particle Size
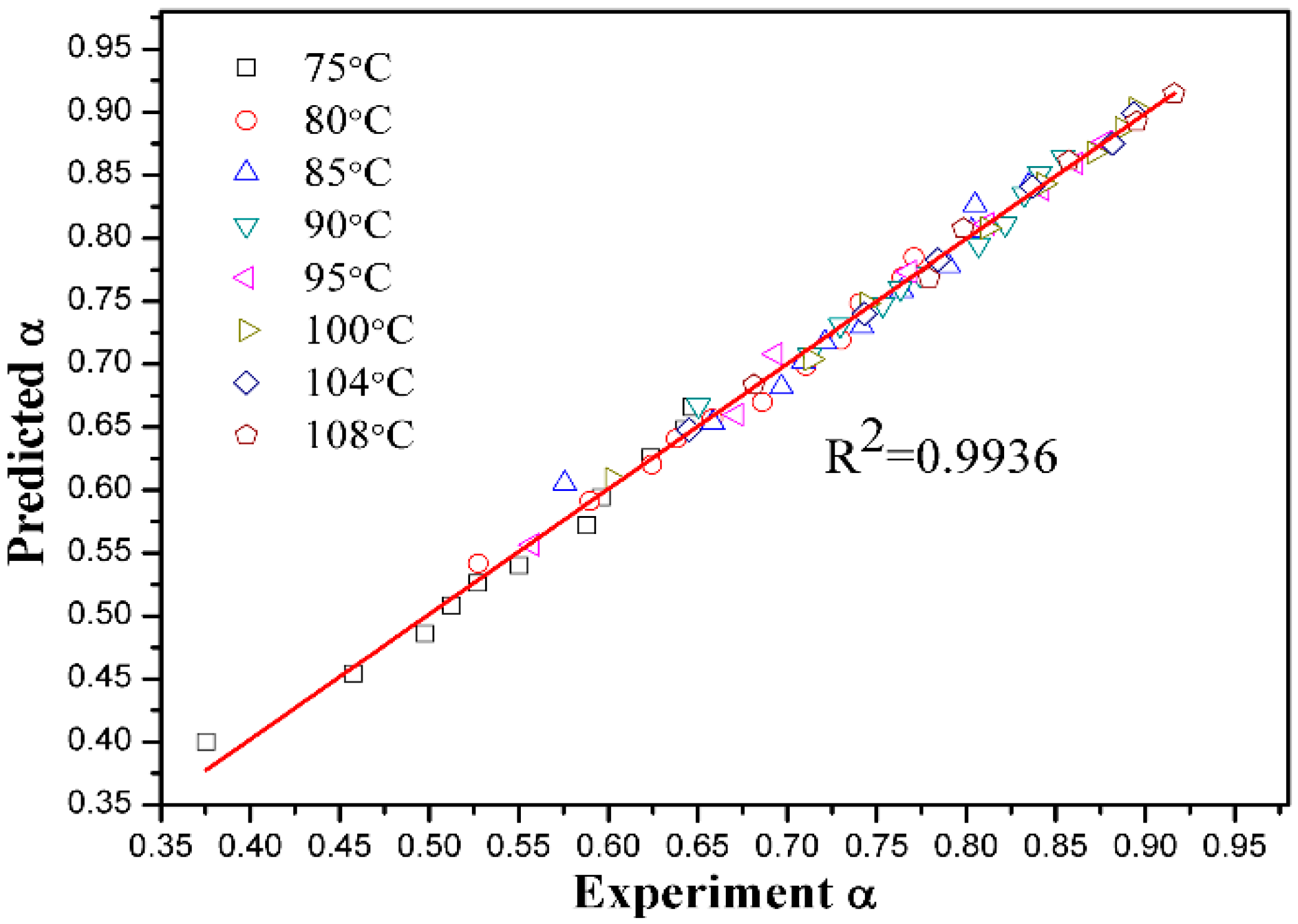
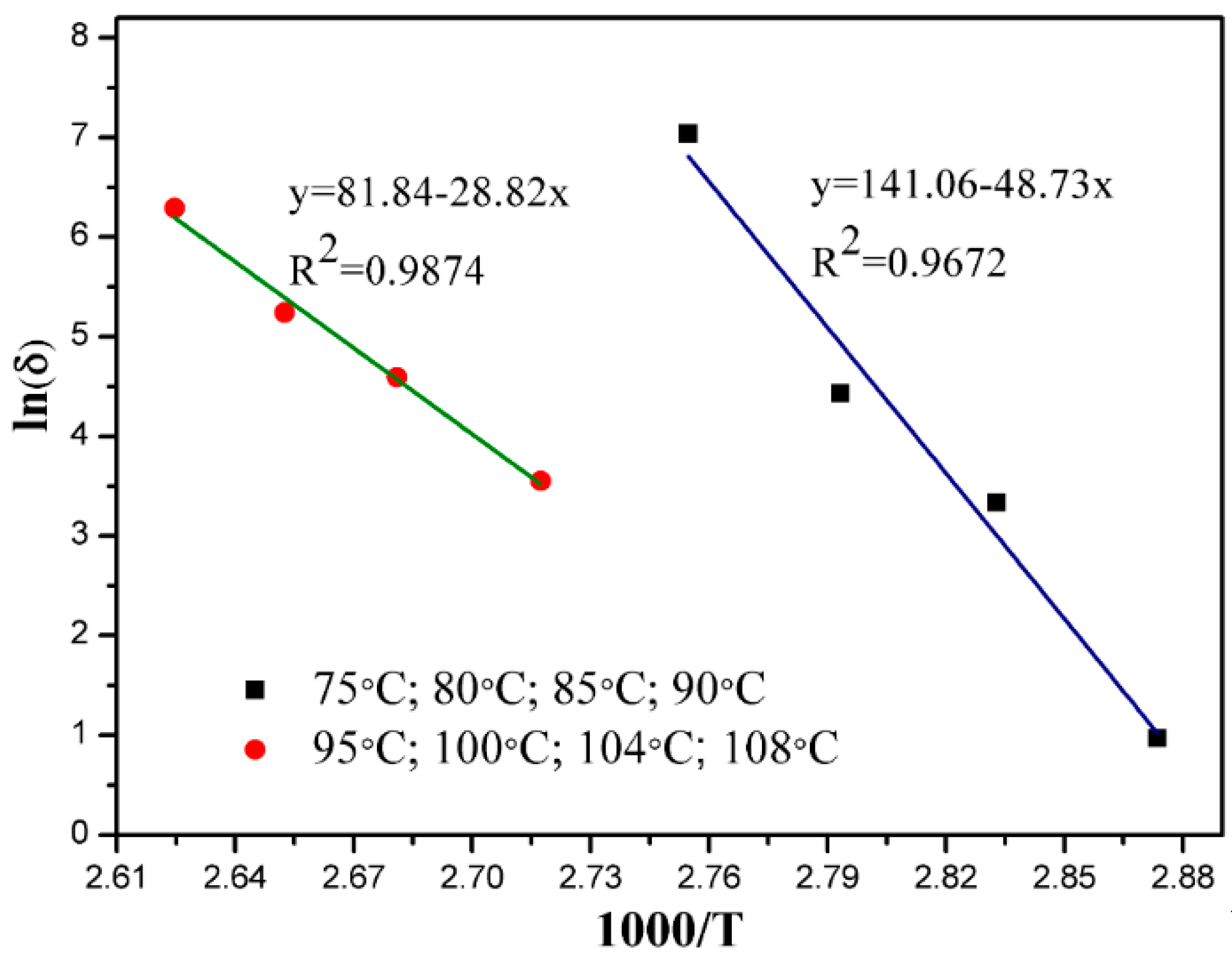
3.5. Kinetic Discussion
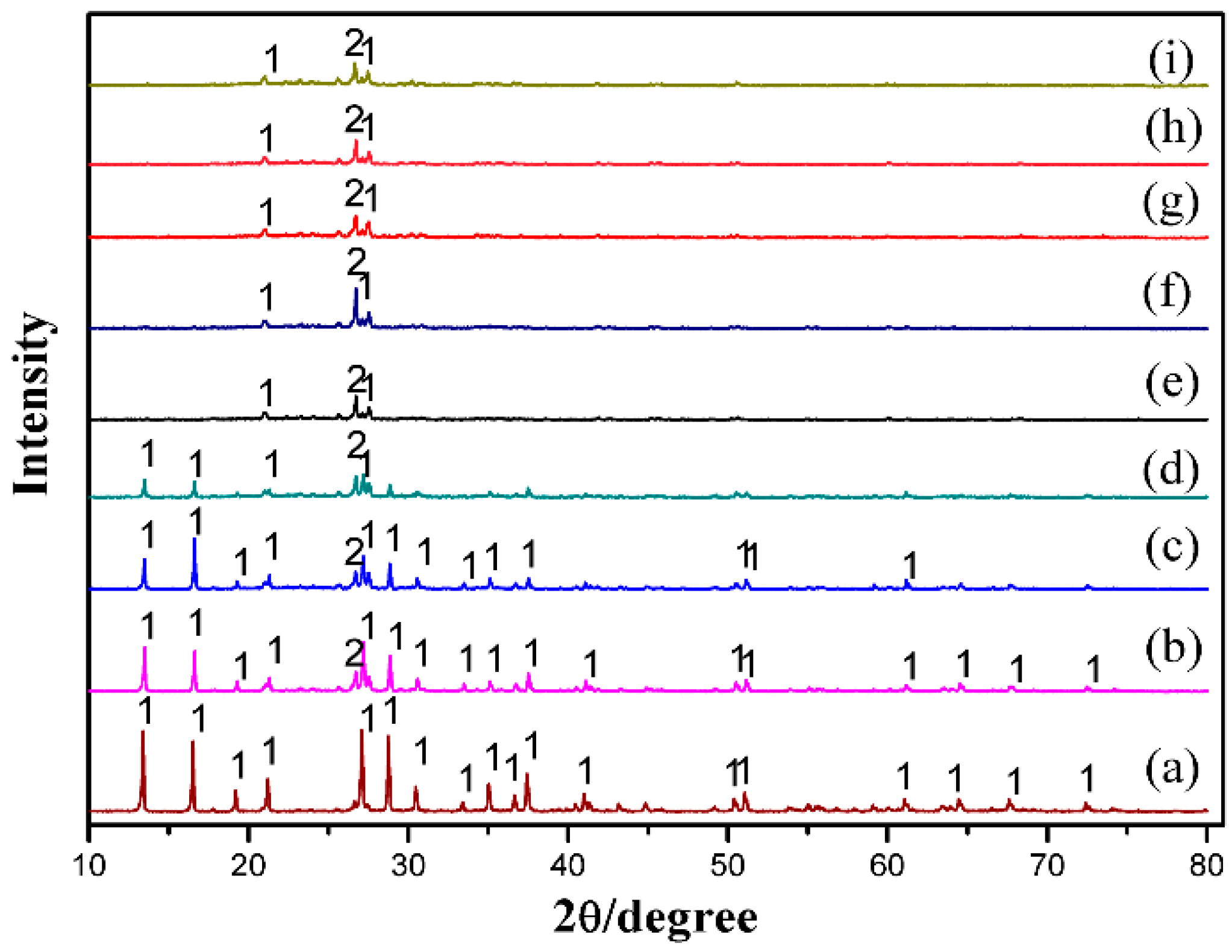
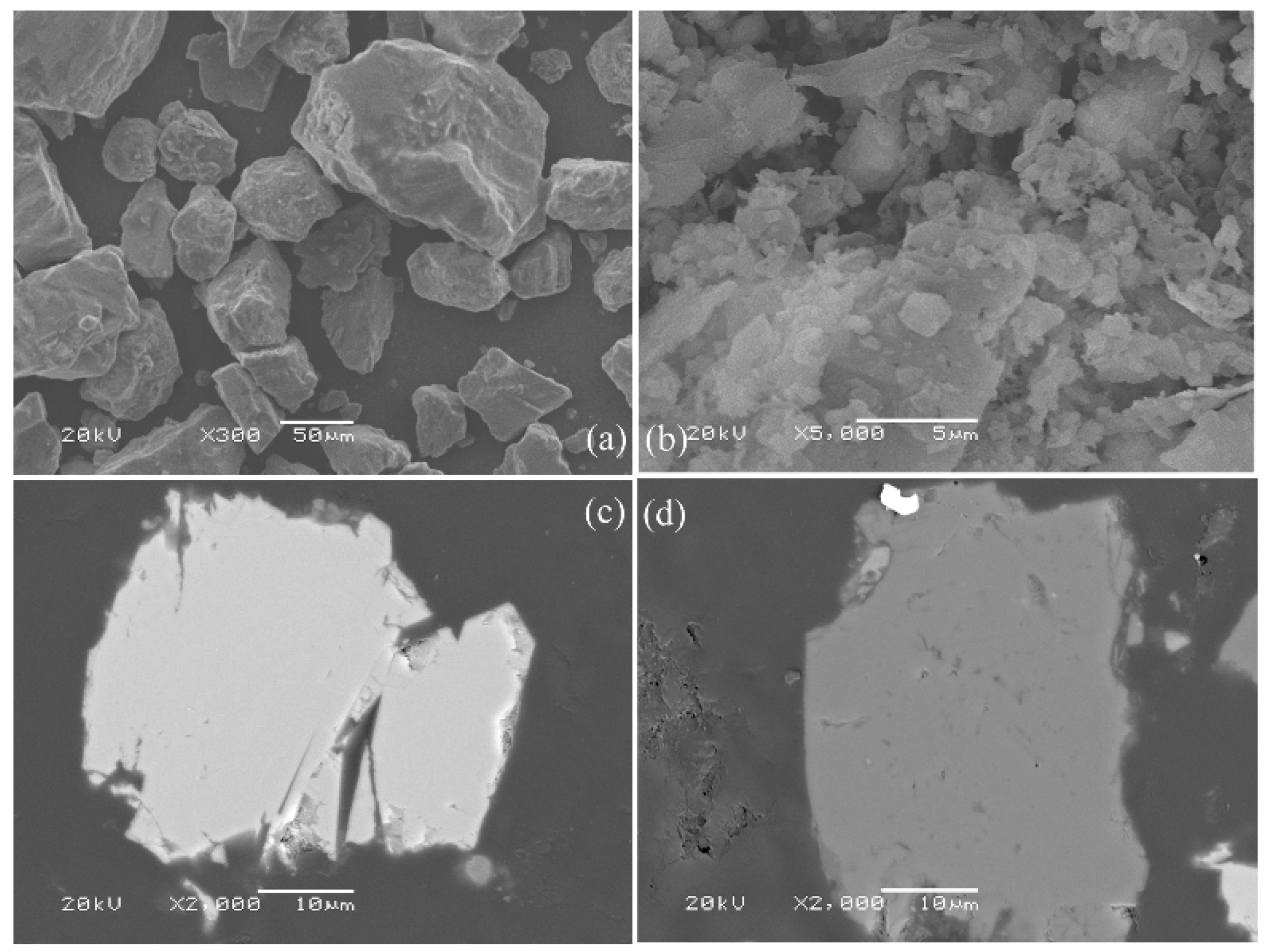
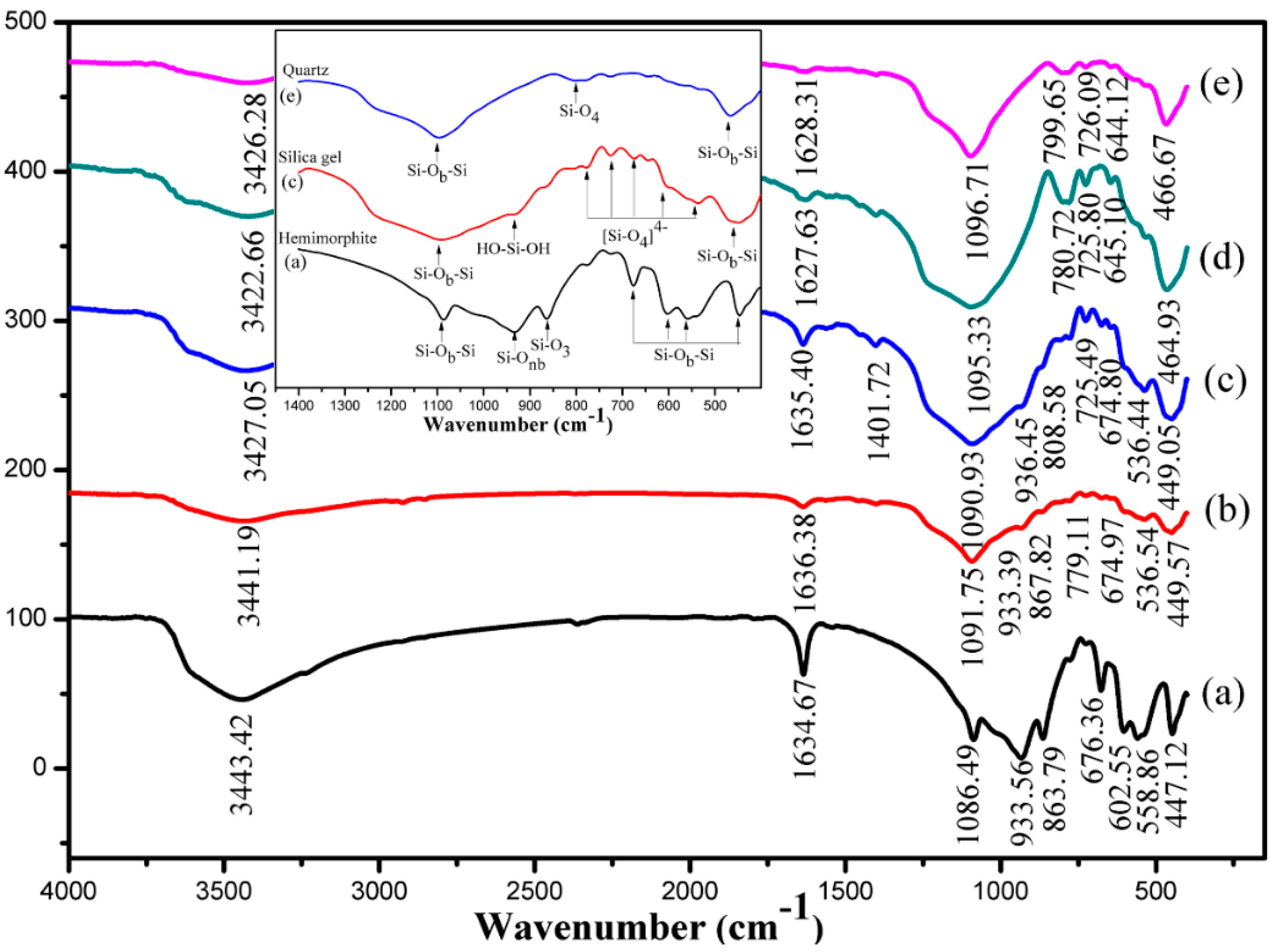
3.6. Characterization of the Leaching Residues
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shirin, E.; Fereshteh, R.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar]
- Navidi Kashani, A.H.; Rashchi, F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents. Miner. Eng. 2008, 21, 967–972. [Google Scholar] [CrossRef]
- Xu, H.S.; Wei, C.; Li, C.X.; Deng, Z.G.; Li, X.B. Sulfuric acid leaching of zinc silicate ore under pressure. Hydrometallurgy 2010, 105, 186–190. [Google Scholar] [CrossRef]
- Souza, A.D.; Pina, P.S.; Lima, E.V.O.; Silva, C.A.; Leao, V.A. Kinetics of sulphuric acid leaching of a zinc silicate calcine. Hydrometallurgy 2007, 89, 337–345. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nayl, A.A.; Daoud, J.A. Leaching and recovery of zinc and copper from brass slag by sulfuric acid. J. Saudi Chem. Soc. 2016, 20, 280–285. [Google Scholar] [CrossRef]
- Chen, A.L.; Zhao, Z.W.; Jia, X.J.; Long, S.; Huo, G.S.; Chen, X.Y. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore. Hydrometallurgy 2009, 97, 228–232. [Google Scholar] [CrossRef]
- Zhao, Z.; Long, S.; Chen, A.; Huo, G.; Li, H.; Jia, X.; Chen, X. Mechanochemical leaching of refractory zinc silicate (hemimorphite) in alkaline solution. Hydrometallurgy 2009, 99, 255–258. [Google Scholar] [CrossRef]
- Harvey, T.G. The hydrometallurgical extraction of zinc by ammonium carbonate: A review of the Schnabel process. Miner. Process. Extr. Metall. Rev. 2006, 27, 231–279. [Google Scholar] [CrossRef]
- Rao, S.; Yang, T.Z.; Zhang, D.C.; Liu, W.F.; Chen, L. Leaching of low grade zinc oxide ores in NH4Cl-NH3 solution with nitrilotriacetic acid as complexing agents. Hydrometallurgy 2015, 158, 101–106. [Google Scholar] [CrossRef]
- He, S.M.; Wang, J.K.; Yang, J.F. Pressure leaching of synthetic zinc silicate in sulfuric acid medium. Hydrometallurgy 2011, 108, 171–176. [Google Scholar] [CrossRef]
- Santos, F.M.F.; Pina, P.S.; Porcaro, R.; Oliveira, V.A.; Silva, C.A.; Leão, V.A. The kinetics of zinc silicate leaching in sodium hydroxide. Hydrometallurgy 2010, 102, 43–49. [Google Scholar] [CrossRef]
- Yang, S.H.; Li, H.; Sun, Y.W.; Chen, Y.M.; Tang, C.B.; He, J. Leaching kinetics of zinc silicate in ammonium chloride solution. Trans. Nonferrous Met. Soc. China 2016, 26, 1688–1695. [Google Scholar] [CrossRef]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical extraction of zinc from Cao treated EAF dust in ammonium chloride solution. J. Hazard. Mater. 2016, 302, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.A.; Margarido, F. Selective process of zinc extraction from spent Zn-MnO2 batteries by ammonium chloride leaching. Hydrometallurgy 2015, 157, 13–21. [Google Scholar] [CrossRef]
- Tang, F.L.; Li, X.B.; Wei, C. Synergistic extraction of zinc from ammoniacal/ammonia sulfate solution by a mixture of β-diketone and 2-hydroxy-5-nonylacetophenone oxime. Hydrometallurgy 2016, 162, 42–48. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, Z.H.; Li, Q.H.; Yang, T.Z.; Zhang, X. Leaching of hemimorphite in NH3-(NH4)2SO4-H2O system and its mechanism. Hydrometallurgy 2012, 8, 137–143. [Google Scholar] [CrossRef]
- Limpo, J.L.; Figueiredo, J.M.; Amer, S.; Luis, A. The CENIM-LNETI process: A new process for the hydrometallurgical treatment of complex sulphides in ammonium chloride solutions. Hydrometallurgy 1992, 28, 149–161. [Google Scholar] [CrossRef]
- Alafara, A.B.; Ayo, F.B.; Daud, T.O.; Rafiu, B.B.; Folahan, A.A.; Abdul, G.G.A. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 2017, 34, 1133–1140. [Google Scholar]
- Baba, A.A.; Ghosh, M.K.; Pradhan, S.R.; Rao, D.S.; Baral, A.; Adekola, F.A. Characterization and kinetic study on ammonia leaching of complex copper ore. Trans. Nonferrous Met. Soc. China 2014, 24, 1587–1595. [Google Scholar] [CrossRef]
- Ekmekyapar, A.; Demirk1ran, N.; Kunkul, A.; Aktas, E. Leahcing of malachite ore in ammonium sulfate solutions and production of copper oxide. Braz. J. Chem. Eng. 2015, 32, 155–165. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Yin, Z.L.; Hu, H.P.; Chen, Q.Y. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution. Hydrometallurgy 2010, 104, 201–206. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Ying, Y.P.; Wang, S.T.; Li, C.G.; Han, X.L.; Li, Z.; Li, Y.Q., Translators; Science Press: Beijing, China, 1982; p. 428. [Google Scholar]
- Wen, L.; Liang, W.; Zhang, Z.; Huang, J. The Infrared Spectroscopy of Minerals; Chongqing University Press: Chongqing, China, 1989; p. 190. [Google Scholar]
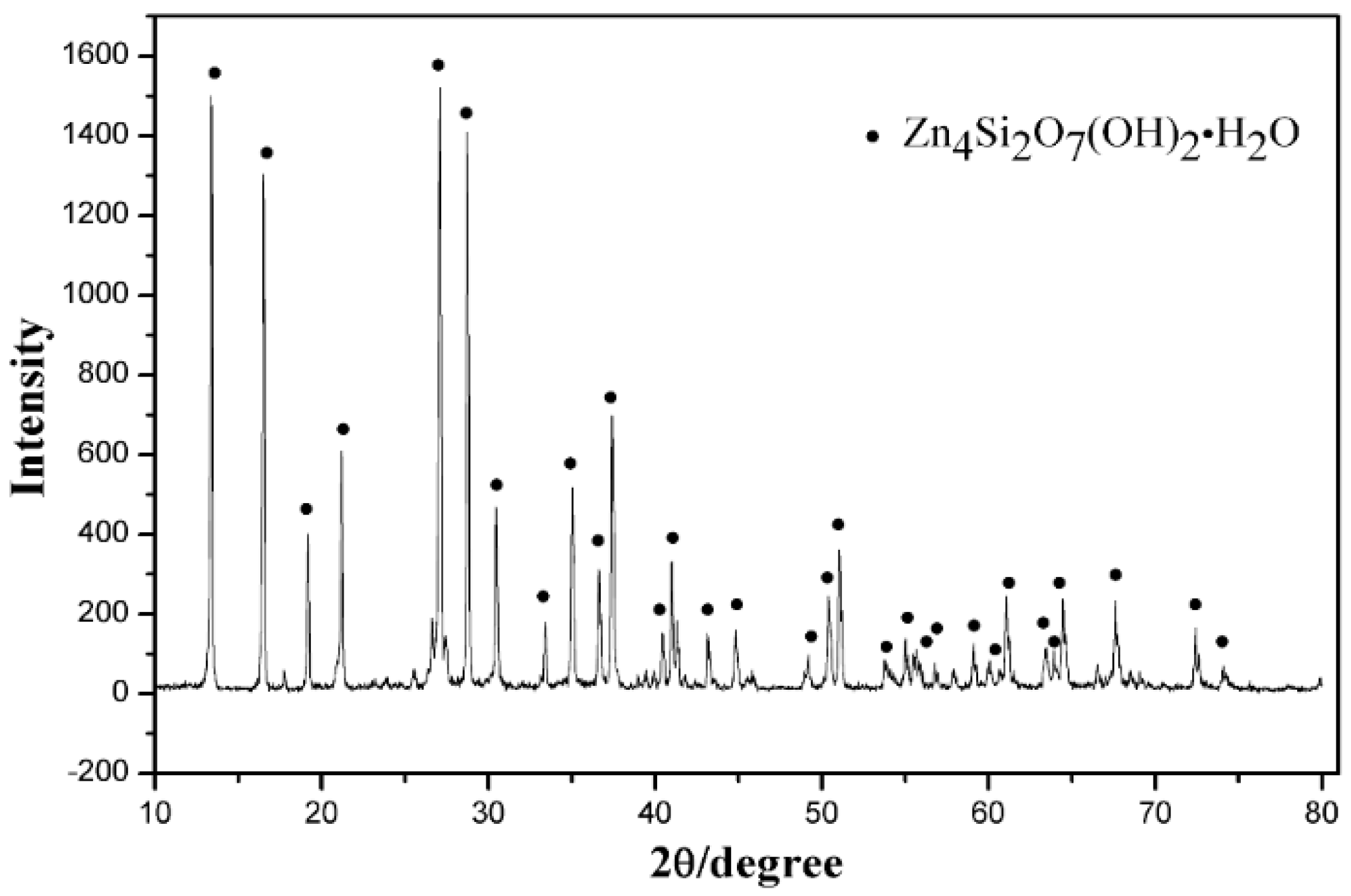
| Phase | ZnSO4 | Zn4Si2O7(OH)2·H2O | ZnS | ZnFe2O4 | Zn (total) |
|---|---|---|---|---|---|
| Content (%) 1 | 0.08 | 41.90 | 0.20 | 0.06 | 42.24 |
| Occupancy (%) 2 | 0.19 | 99.20 | 0.47 | 0.14 | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Yang, S.; Chen, Y.; Tang, C.; He, J.; Li, H. Leaching Kinetics of Hemimorphite in Ammonium Chloride Solution. Metals 2017, 7, 237. https://doi.org/10.3390/met7070237
Zhao D, Yang S, Chen Y, Tang C, He J, Li H. Leaching Kinetics of Hemimorphite in Ammonium Chloride Solution. Metals. 2017; 7(7):237. https://doi.org/10.3390/met7070237
Chicago/Turabian StyleZhao, Duoqiang, Shenghai Yang, Yongming Chen, Chaobo Tang, Jing He, and Hao Li. 2017. "Leaching Kinetics of Hemimorphite in Ammonium Chloride Solution" Metals 7, no. 7: 237. https://doi.org/10.3390/met7070237




