Microstructures and Mechanical Properties of Mg-9Al/Ti Metallurgical Bonding Prepared by Liquid-Solid Diffusion Couples
Abstract
:1. Introduction
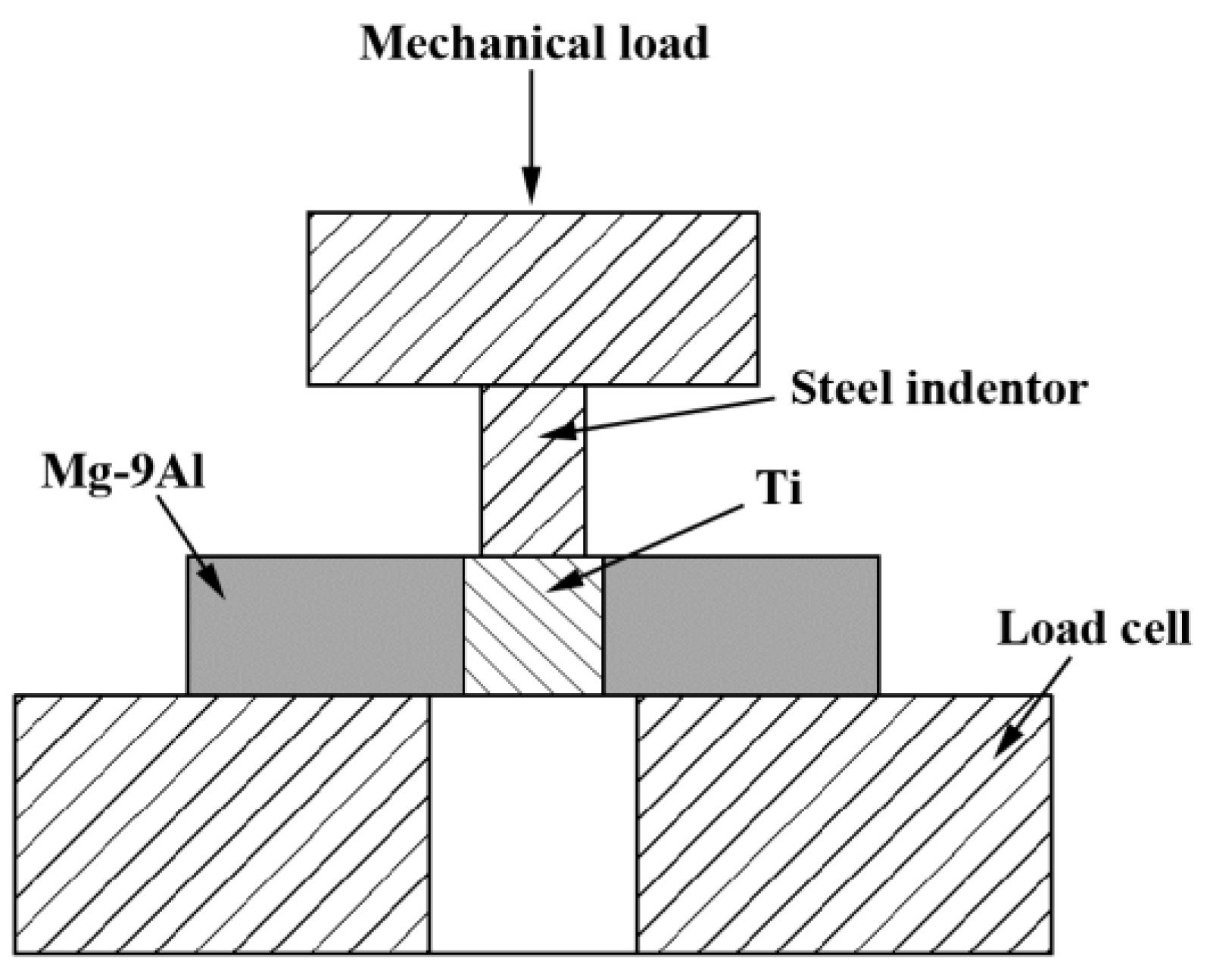
2. Experiments
3. Results and Discussion
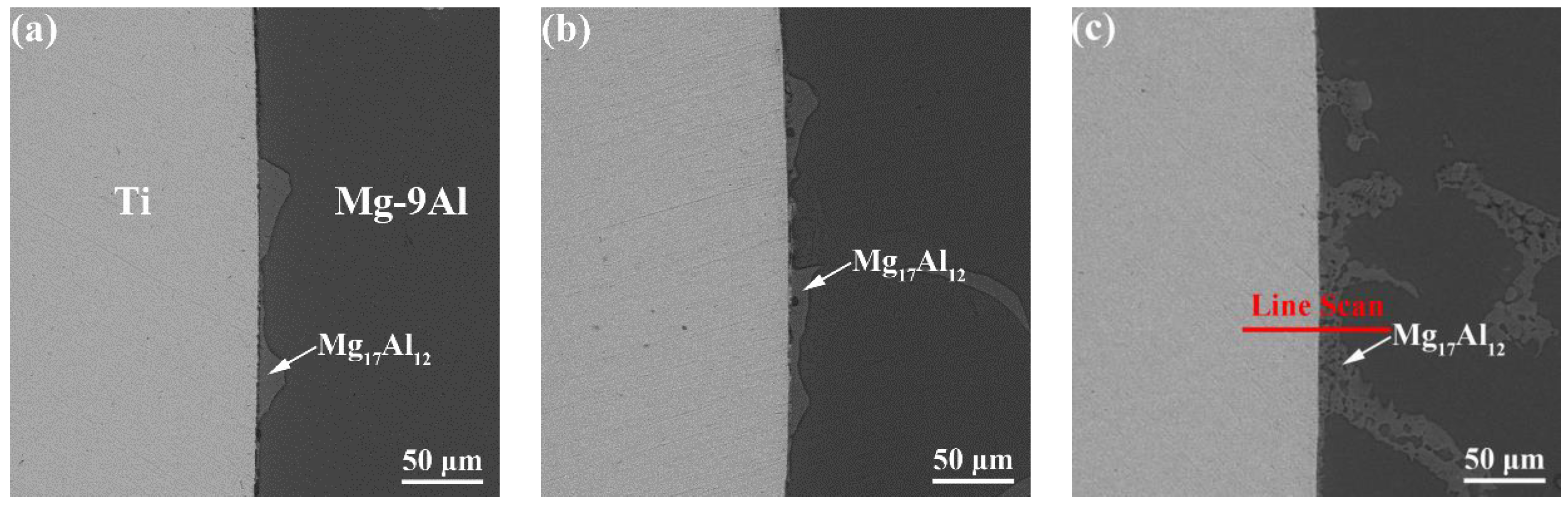
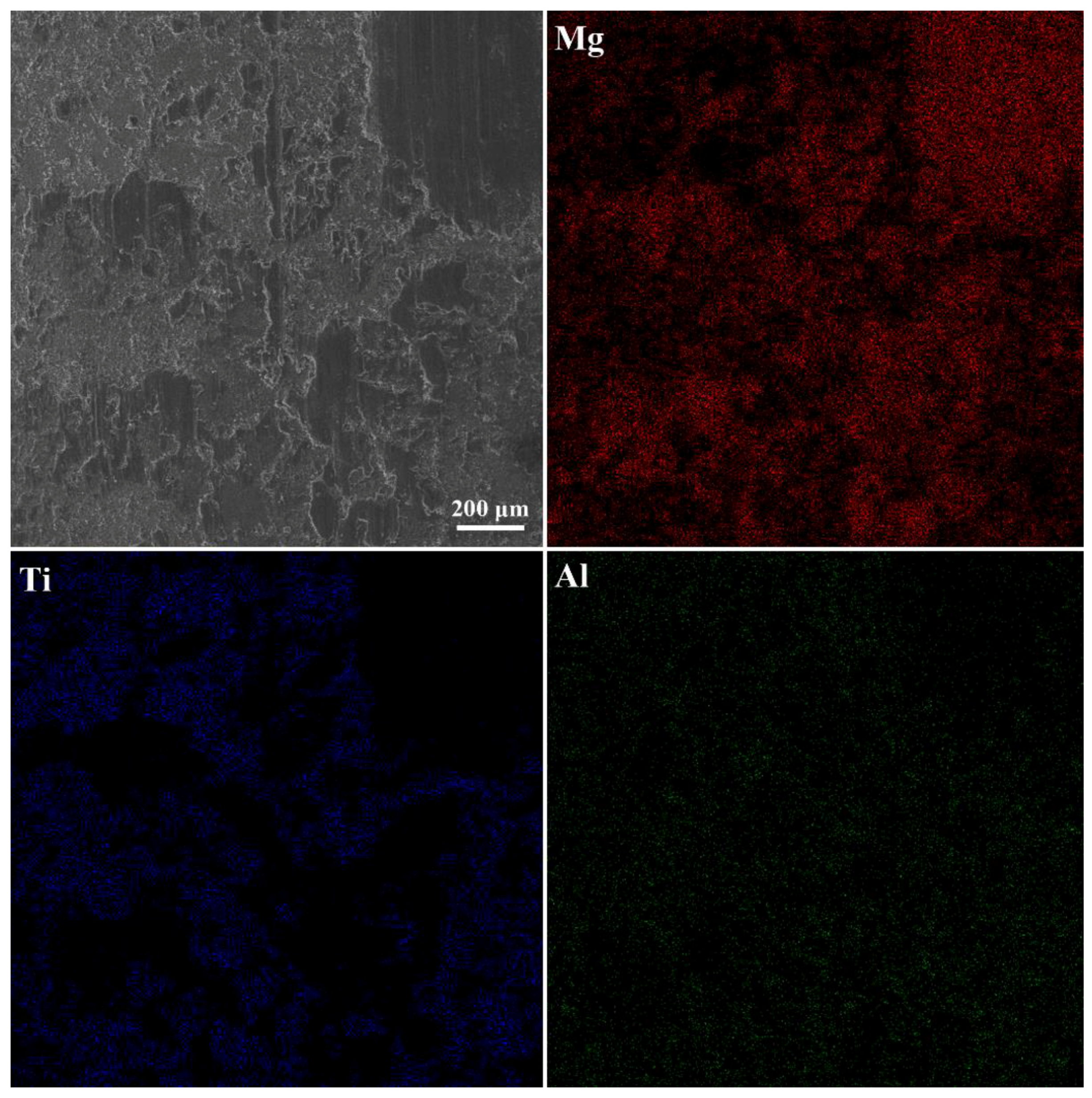
3.1. The Microstructure of the Interface
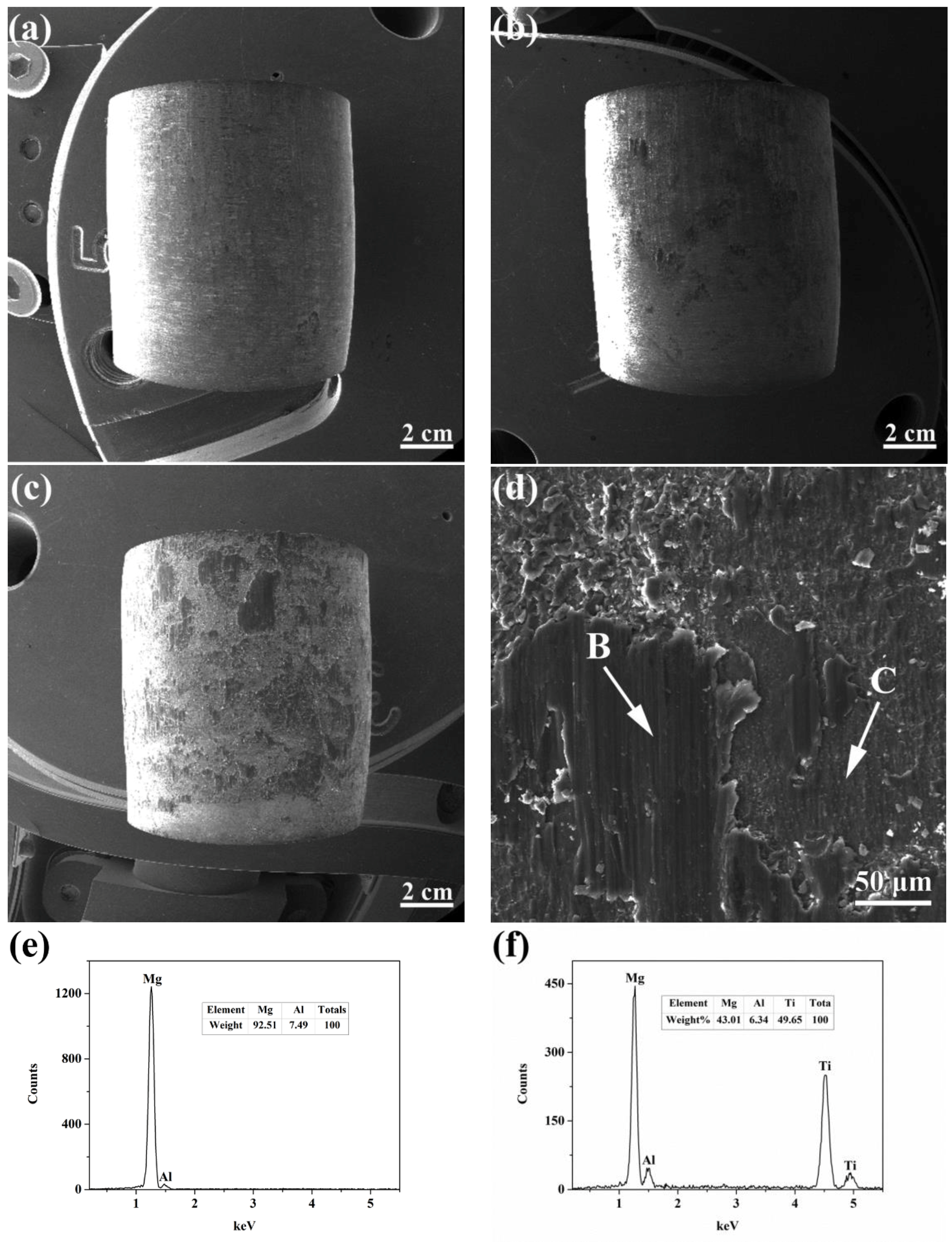
3.2. Mechanical Behaviors
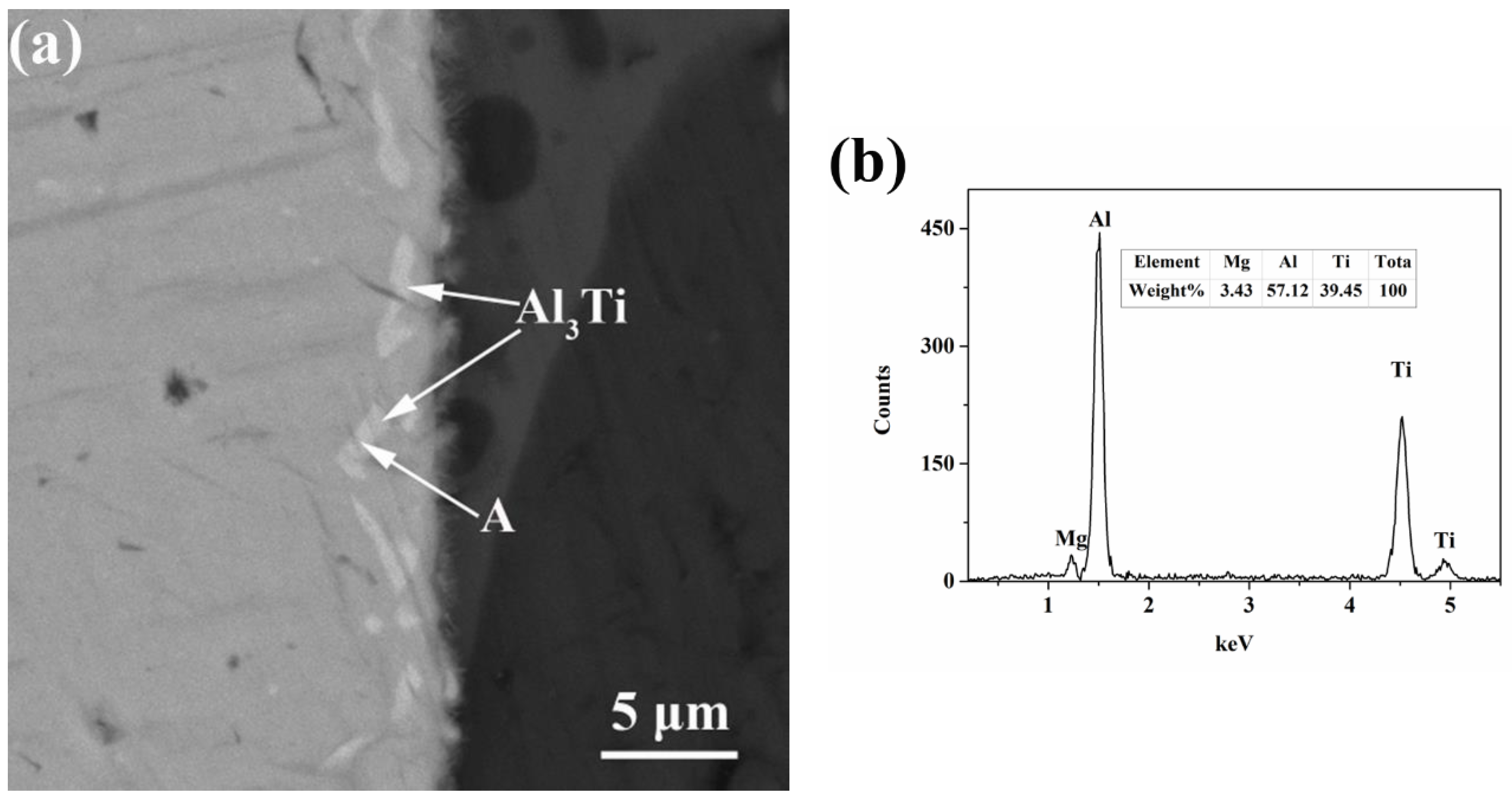
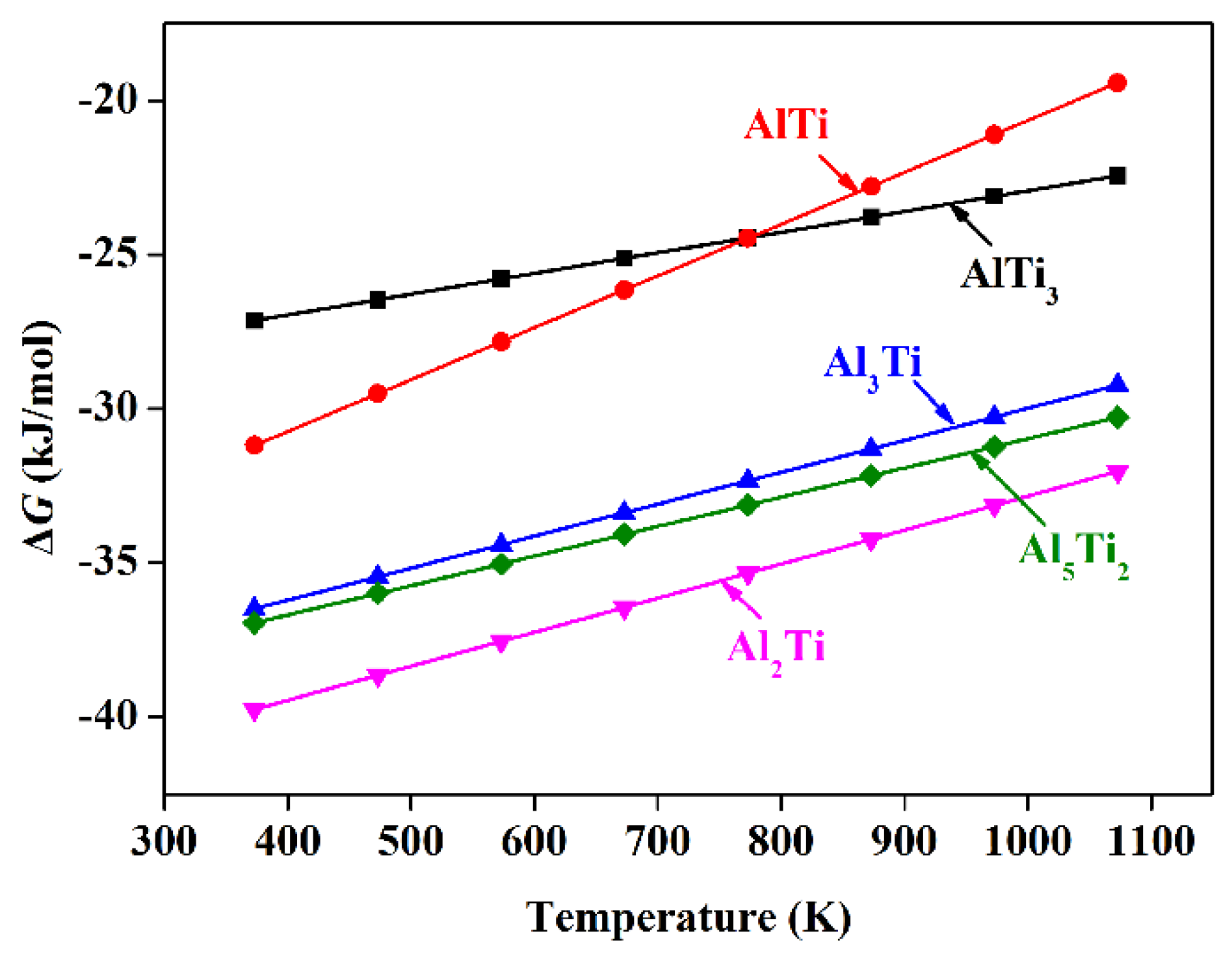
3.3. Thermodynamic Analysis of Intermetallic Compounds Formation at the Interface
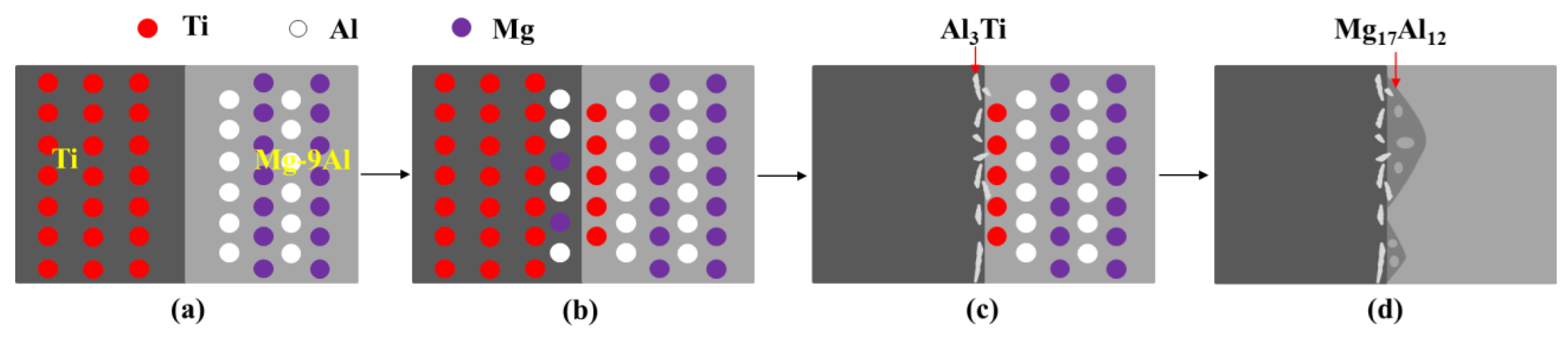
3.4. The Metallurgical Reaction Mechanism of the Solid/Liquid Interface
4. Conclusions
- (1)
- The Mg17Al12 in the Mg-9Al matrices was attached to the surface of Ti rods. Grain boundaries Ti and Mg-9Al wetting transition from incompletely wetted to completely wetted by Mg17Al12 phase. A metallurgical bond was established at the interface between Mg-9Al alloy and Ti matrices. The impurity diffusion coefficients of Mg in Ti and Al in Ti, and Ti in Mg-9Al are 1.70(±0.02) × 10−15 m2/s, 3.77(±0.12) × 10−15 m2/s, 1.60(±0.23) × 10−14 m2/s, respectively. The diffusion of Al and Ti elements play an important role during the interface of Mg-9Al/Ti metallurgical bonding.
- (2)
- Shear strength of Mg-9Al/Ti metallurgical bonding was presented an increasing tendency in perfect accordance with heat treatment time. Shear strength could reach the maximum value of 56 MPa. The fracture was took place along Mg-9Al matrix at the interface. Shear stress greatly improves when Al and Ti interdiffused and chemical interaction occurs between Mg-9Al and Ti.
- (3)
- By EDS and XRD analysis, these results were found that Al3Ti is the only intermetallic compound at the interface of the Mg-9Al/Ti compound castings. Gibbs free energies of formation of AlTi3, AlTi, Al3Ti, Al2Ti and Al5Ti2 were calculated. The result was shown that Al3Ti is expected to be the first phase to form in the Al-Ti system.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.J.; Xu, D.K.; Wu, R.Z.; Chen, X.B.; Peng, Q.M.; Jin, L.; Xin, Y.C.; Zhang, Z.Q.; Liu, Y.; Chen, X.H. What is going on in magnesium alloys? J. Mater. Sci. Technol. 2017, 34, 245–247. [Google Scholar] [CrossRef]
- Pan, H.; Ren, Y.; Fu, H.; Zhao, H.; Wang, L.; Meng, X.; Qin, G. Recent developments in rare-earth free wrought magnesium alloys having high strength: A review. J. Alloys Compd. 2015, 663, 321–331. [Google Scholar] [CrossRef]
- Pan, F.; Chen, X.; Yan, T.; Liu, T.; Mao, J.; Luo, W.; Wang, Q.; Peng, J.; Tang, A.; Jiang, B. A novel approach to melt purification of magnesium alloys. J. Magnes. Alloys 2016, 4, 8–14. [Google Scholar] [CrossRef]
- Gorynin, I.V. Titanium alloys for marine application. Mater. Sci. Eng. A 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Ren, Y.M.; Lin, X.; Fu, X.; Tan, H.; Chen, J.; Huang, W.D. Microstructure and deformation behavior of Ti-6Al-4V alloy by high-power laser solid forming. Acta Mater. 2017, 132, 82–95. [Google Scholar] [CrossRef]
- Neugebauer, J. Theory-guided bottom-up design of beta-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar]
- Chen, M.C.; Hsieh, H.C.; Wu, W. The evolution of microstructures and mechanical properties during accumulative roll bonding of Al/Mg composite. J. Alloys Compd. 2006, 416, 169–172. [Google Scholar] [CrossRef]
- Feng, B.; Xin, Y.; Guo, F.; Yu, H.; Wu, Y.; Liu, Q. Compressive mechanical behavior of Al/Mg composite rods with different types of Al sleeve. Acta Mater. 2016, 120, 379–390. [Google Scholar] [CrossRef]
- Gao, M.; Mei, S.; Li, X.; Zeng, X. Characterization and formation mechanism of laser-welded Mg and Al alloys using Ti interlayer. Scripta Mater. 2012, 67, 193–196. [Google Scholar] [CrossRef]
- Jiang, W.; Li, G.; Fan, Z.; Wang, L.; Liu, F. Investigation on the Interface Characteristics of Al/Mg Bimetallic Castings Processed by Lost Foam Casting. Metall. Mater. Trans. A 2016, 47, 2462–2470. [Google Scholar] [CrossRef]
- Zhao, K.N.; Liu, J.C.; Nie, X.Y.; Li, Y.; Li, H.X.; Du, Q.; Zhuang, L.Z.; Zhang, J.S. Interface formation in magnesium–magnesium bimetal composites fabricated by insert molding method. Mater. Des. 2016, 91, 122–131. [Google Scholar] [CrossRef]
- Nie, X.Y.; Liu, J.C.; Li, H.X.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. An investigation on bonding mechanism and mechanical properties of Al/Ti compound materials prepared by insert moulding. Mater. Des. 2014, 63, 142–150. [Google Scholar] [CrossRef]
- Predel, B. Mg-Ti (Magnesium-Titanium); Springer: Berlin, Germany, 1997. [Google Scholar]
- Cao, R.; Wang, T.; Wang, C.; Feng, Z.; Lin, Q.; Chen, J.H. Cold metal transfer welding–brazing of pure titanium TA2 to magnesium alloy AZ31B. J. Alloys Compd. 2014, 605, 12–20. [Google Scholar] [CrossRef]
- Tan, C.; Chen, B.; Meng, S.; Zhang, K.; Song, X.; Zhou, L.; Feng, J. Microstructure and mechanical properties of laser welded-brazed Mg/Ti joints with AZ91 Mg based filler. Mater. Des. 2016, 99, 127–134. [Google Scholar] [CrossRef]
- Tan, C.; Song, X.; Chen, B.; Li, L.; Feng, J. Enhanced interfacial reaction and mechanical properties of laser welded-brazed Mg/Ti joints with Al element from filler. Mater. Lett. 2016, 167, 38–42. [Google Scholar] [CrossRef]
- Straumal, B.B.; Gornakova, A.S.; Kogtenkova, O.A.; Protasova, S.G.; Sursaeva, V.G.; Baretzky, B. Continuous and discontinuous grain-boundary wetting in ZnxAl1−x. Phys. Rev. B 2008, 78, 054202. [Google Scholar] [CrossRef]
- Protasova, S.G.; Kogtenkova, O.A.; Straumal, B.B.; Baretzky, B. Inversed solid-phase grain boundary wetting in the Al–Zn system. J. Mater. Sci. 2011, 46, 4349–4353. [Google Scholar] [CrossRef]
- Jie, W.Q.; Kandalova, E.G.; Zhang, R.J. Al3Ti/Al composites prepared by SHS. Rare Metal Mat. Eng. 2000, 29, 145–148. [Google Scholar]
- Palm, M.; Zhang, L.C.; Stein, F.; Sauthoff, G. Phases and phase equilibria in the Al-rich part of the Al–Ti system above 900 °C. Intermetallics 2002, 10, 523–540. [Google Scholar] [CrossRef]
- Paul, W.; Oliver, D.; Miyahara, Y.; Grutter, P. Transient adhesion and conductance phenomena in initial nanoscale mechanical contacts between dissimilar metals. Nanotechnology 2013, 24, 475704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutheil, P.; Thomann, A.L.; Lecas, T.; Brault, P.; Vayer, M. Sputtered Ag thin films with modified morphologies: Influence on wetting property. Appl. Surf. Sci. 2015, 347, 101–108. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F. Nanoscale structure of submicron-thick sputter-deposited Pd films: Effect of the adatoms diffusivity by the film-substrate interaction. Surf. Coat. Tech. 2017, 315, 123–129. [Google Scholar] [CrossRef]
- Sarafianos, N. An analytical method of calculating variable diffusion coefficients. J. Mater. Sci. 1986, 21, 2283–2288. [Google Scholar] [CrossRef]
- Rawers, J.C.; Wrzesinski, W.R. Reaction-sintered hot-pressed TiAl. J. Mater. Sci. 1992, 27, 2877–2886. [Google Scholar] [CrossRef]
- Abboud, J.H.; West, D.R.F. Microstructures of titanium-aluminides produced by laser surface alloying. J. Mater. Sci. 1992, 27, 4201–4207. [Google Scholar] [CrossRef]
- Sujata, M.; Bhargava, S.; Sangal, S. On the formation of TiAl3 during reaction between solid Ti and liquid Al. J. Mater. Sci. Lett. 1997, 16, 1175–1178. [Google Scholar] [CrossRef]
- Kattner, U.R.; Lin, J.C.; Chang, Y.A. Thermodynamic assessment and calculation of the Ti-Al System. Metall. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Chen, S.H.; Li, L.Q.; Chen, Y.B.; Liu, D.J. Si diffusion behavior during laser welding-brazing of Al alloy and Ti alloy with Al-12Si filler wire. T. Nonferr. Metal. Soc. 2010, 20, 64–70. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.X.; Pan, F.; Li, Z.; Luo, X. Effect of composition on the microstructure and mechanical properties of Mg–Zn–Al alloys. Mater. Sci. Eng. A 2007, 456, 43–51. [Google Scholar] [CrossRef]







| Sample | Maximum Stress (N) | Maximum Shear Strength (MPa) |
|---|---|---|
| 0 min-1# | 3478 | 16 |
| 0 min-2# | 3482 | 16 |
| 30 min-1# | 7086 | 32 |
| 30 min-2# | 7742 | 35 |
| 60 min-1# | 12,174 | 54 |
| 60 min-2# | 12,531 | 56 |
| Compound | Free Energy of Formation, ΔG |
|---|---|
| AlTi3 | −9,633.6 + 6.70801T |
| AlTi | −37,445.1 + 16.79376T |
| Al3Ti | −40,349.6 + 10.36525T |
| Al2Ti | −43,858.4 + 11.02077T |
| Al5Ti2 | −40,495.4 + 9.52964T |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Jiang, B.; Yan, Q.; Xie, H.; Jiang, Z.; Yang, Q.; Chen, Q.; Peng, C.; Pan, F. Microstructures and Mechanical Properties of Mg-9Al/Ti Metallurgical Bonding Prepared by Liquid-Solid Diffusion Couples. Metals 2018, 8, 778. https://doi.org/10.3390/met8100778
Dai J, Jiang B, Yan Q, Xie H, Jiang Z, Yang Q, Chen Q, Peng C, Pan F. Microstructures and Mechanical Properties of Mg-9Al/Ti Metallurgical Bonding Prepared by Liquid-Solid Diffusion Couples. Metals. 2018; 8(10):778. https://doi.org/10.3390/met8100778
Chicago/Turabian StyleDai, Jiahong, Bin Jiang, Qiong Yan, Hongmei Xie, Zhongtao Jiang, Qingshan Yang, Qiaowang Chen, Cheng Peng, and Fusheng Pan. 2018. "Microstructures and Mechanical Properties of Mg-9Al/Ti Metallurgical Bonding Prepared by Liquid-Solid Diffusion Couples" Metals 8, no. 10: 778. https://doi.org/10.3390/met8100778




