Study of Nd Electrodeposition from the Aprotic Organic Solvent Dimethyl Sulfoxide
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Study of the Electrolyte
3.1.1. Dissolution Tests of NdCl3 in DMSO at Different Temperatures
3.1.2. Viscosity and Conductivity Study of NdCl3 and DMSO Solutions
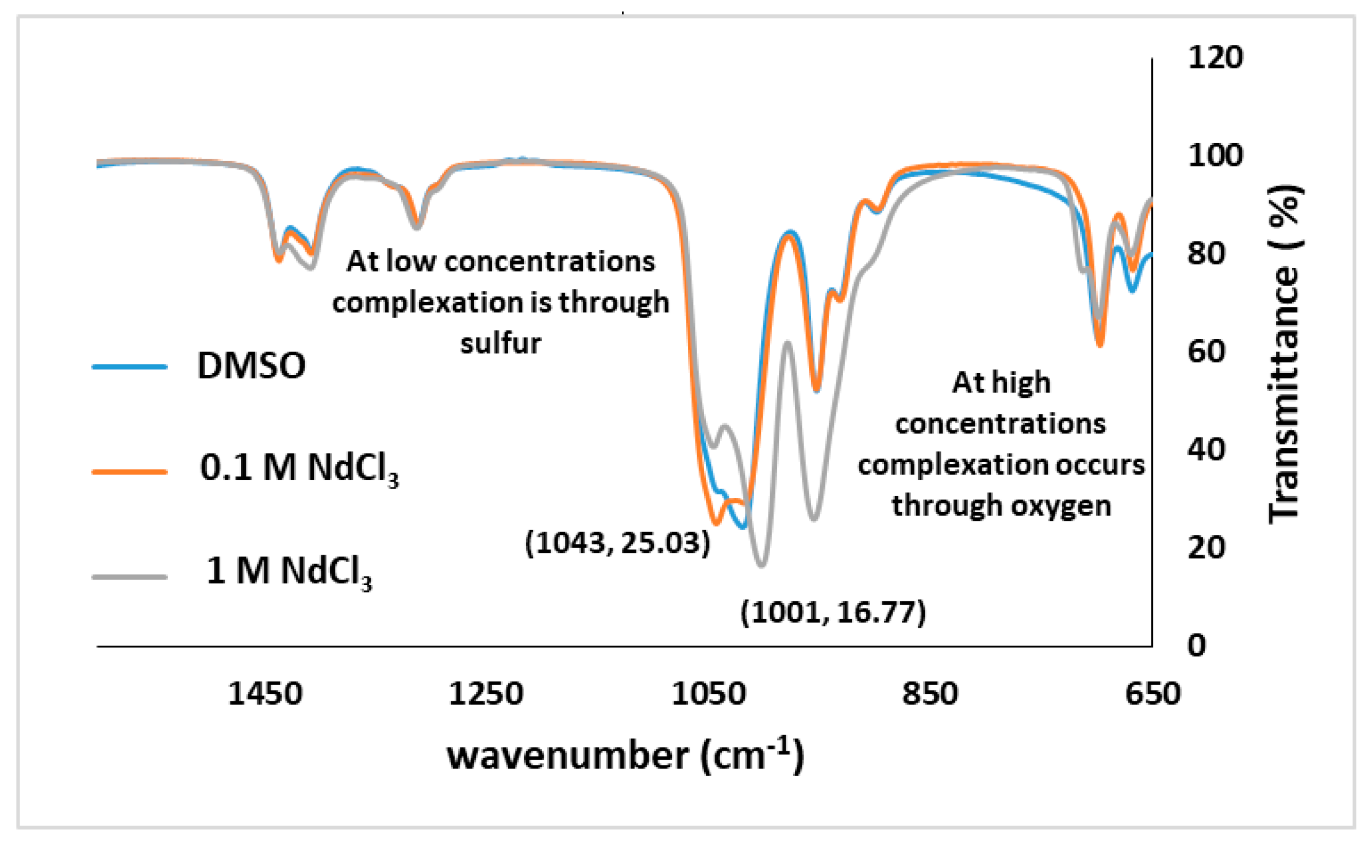
3.1.3. FTIR Study of NdCl3 and DMSO Solutions
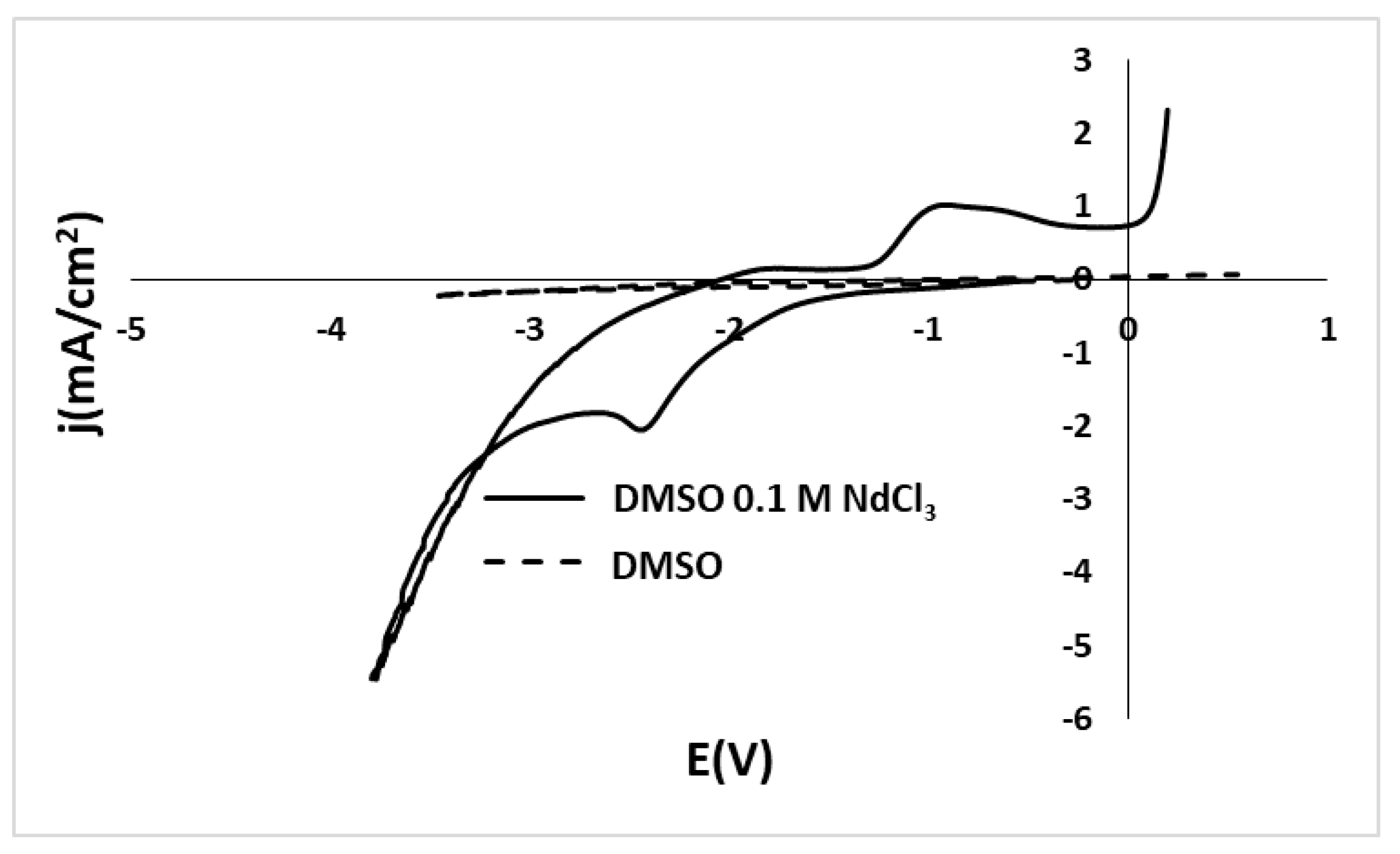
3.1.4. Cyclic Voltammetry of NdCl3 and DMSO Solutions
3.2. Study of Electrolysis Parameters
3.2.1. Electrolysis Mode
3.2.2. Supporting Electrolyte
3.2.3. Concentration
3.2.4. Temperature
3.2.5. Stirring
3.3. Optimum Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Union. European Commission, Study on the Review of the List of Critical Raw Materials. Critical Raw Materials Factsheets; Publications Office of the European Union: Luxembourg, 2017; pp. 403–411. ISBN 978-92-79-72119-9. [Google Scholar]
- Rahman, M.A.; Slemon, G.R. Promising Applications of Neodymium Boron Iron Magnets in Electrical Machines. IEEE Trans. Magn. 1985, 21, 1712–1716. [Google Scholar] [CrossRef]
- Le Mercier, T. Applications of Rare Earth Luminescent Materials. In Rare Earths, Science, Technology, Production and Use, 1st ed.; Lucas, J., Lucas, P., Le Mercier, T., Rollat, A., Davenport, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 281–317. ISBN 978-0-444-62735-3. [Google Scholar]
- Davensport, W. Metallothermic Rare Earth Metal Reduction. In Rare Earths, Science, Technology, Production and Use, 1st ed.; Lucas, J., Lucas, P., Le Mercier, T., Rollat, A., Davenport, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 109–122. ISBN 978-0-444-62735-3. [Google Scholar]
- Firdaus, M.; Rhamdhani, M.A.; Durandet, Y.; Rankin, W.J.; McGregor, K. Review of high-temperature recovery of Rare Earth (Nd/Dy) from magnet waste. J. Sustain. Metall. 2016, 2, 276–295. [Google Scholar] [CrossRef]
- Miao, Y.W.; Horng, J.S.; Hoh, Y.C. The Preparation of Nd Metal from Taiwan Black Monazite. In Proceedings of the International Symposium on Production and Electrolysis of Light Metals, Halifax, NS, Canada, 20–24 August 1989. [Google Scholar]
- Vogel, H.; Friedrich, B. An estimation of PFC emission by rare earth electrolysis. In Light Metals 2018; Martin, O., Ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 1507–1516. ISBN 978-3-319-72283-2. [Google Scholar]
- Krishnamurthy, N.; Gupta, C.K. Rare earth metals and alloys by electrolytic methods. Min. Proc. Ext. Met. Rev. 2001, 22, 477–507. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 333–442. ISBN 978-1-4665-7638-4. [Google Scholar]
- Keller, R. Electrolytic production of neodymium with and without emission of greenhouse gases. Electrochem. Soc. Proc. 1998, 97, 143–145. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Kovács, A. Thermodynamic Properties of the Lanthanide(III) Halides. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Gschneidner, K.A., Jr., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; Volume 33, pp. 147–248. ISBN 0-444-51323-33. [Google Scholar]
- Reynolds, W.L.; Silesky, H.S. Solubilities of potassium chloride and sodium iodide in dimethylsulfoxide—Water mixtures. J. Chem. Eng. Data 1960, 5, 250. [Google Scholar] [CrossRef]
- Reynolds, W.R. Dimethyl sulfoxide in inorganic chemistry. In Progress in Inorganic Chemistry, 1st ed.; Lippard, S.J., Ed.; Interscience: New York, NY, USA, 1970; Volume 12, pp. 3–21. ISBN 978-0-471-540823. [Google Scholar]
- Mercer, A.; Trotter, J. Crystal and molecular structure of dichlorotetrakis(dimethyl sulphoxide)ruthenium(II). J. Chem. Soc. Dalton Trans. 1975, 23, 2480–2483. [Google Scholar] [CrossRef]
- Meek, D.W.; Straub, D.K.; Drago, R.S. Transition metal ion complexes of dimethyl sulfoxide. J. Am. Chem. Soc. 1960, 82, 6013–6016. [Google Scholar] [CrossRef]
- Maxfield, M.; Eckhardt, H.; Iqbal, Z.; Reidinger, F.; Baughman, R.M. Bi-Sr-Ca-Cu-O and Pb-Bi-Sr-Ca-Cu-O superconductor films via an electrodeposition process. Appl. Phys. Lett. 1989, 54, 1932–1933. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Reddy, T.B. Polarography and Voltammetry in Dimethylsulfoxide. J. Electrochem. Soc. 1961, 108, 980–985. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Handley, T.H.; Cooper, J.H. Quantitative electrodeposition of actinides from dimethylsulfoxide. Anal. Chem. 1969, 41, 381–382. [Google Scholar] [CrossRef]
- Li, G.; Tong, Y.; Liu, G. Preparation of Lu–Bi–Ni thin films in dimethylsulfoxide by cylic electrodeposition method. Mater. Lett. 2004, 58, 3839–3843. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, G.; Tong, Y. Electrochemical behavior of Tm3+ ion and cyclic electrodeposition of a Tm–Co alloy film in dimethylsulfoxide. J. Electroanal. Chem. 2002, 536, 123–127. [Google Scholar] [CrossRef]
- Li, G.; Tong, Y.; Wang, Y.; Liu, G. Electrodeposition of Lu–Ni alloy thin films. Electrochim. Acta 2003, 48, 4061–4067. [Google Scholar] [CrossRef]
- Giordano, M.C.; Bazan, J.C.; Arvia, A.J. The electrolysis of dimethylsulphoxide solutions of sodium chloride and sodium iodide. Electrochim. Acta 1966, 11, 741–747. [Google Scholar] [CrossRef]
- Giridhar, P.; Venkatesan, K.A.; Subramaniam, S.; Srinivasan, T.G.; Vasudeva Rao, P.R. Electrochemical behavior of uranium (VI) in 1-butyl-3-methylimidazolium chloride and in 0.05 M aliquat-336/chloroform. Radiochim. Acta 2006, 94, 415–420. [Google Scholar] [CrossRef]
- Litaiem, Y.; Dhahbi, M. Measurements and correlations of viscosity, conductivity and density of a hydrophobic ionic liquid (Aliquat 336) mixtures with a non-associated dipolar aprotic solvent (DMC). J. Mol. Liq. 2012, 169, 54–62. [Google Scholar] [CrossRef]
- Otaa, H.; Matsumiya, M.; Sasayaa, N.; Nishihatab, K. Investigation of electrodeposition behavior for Nd(III) in [P2225][TFSA] ionic liquid by EQCM methods with elevated temperatures. Electrochim. Acta 2016, 222, 20–26. [Google Scholar] [CrossRef]


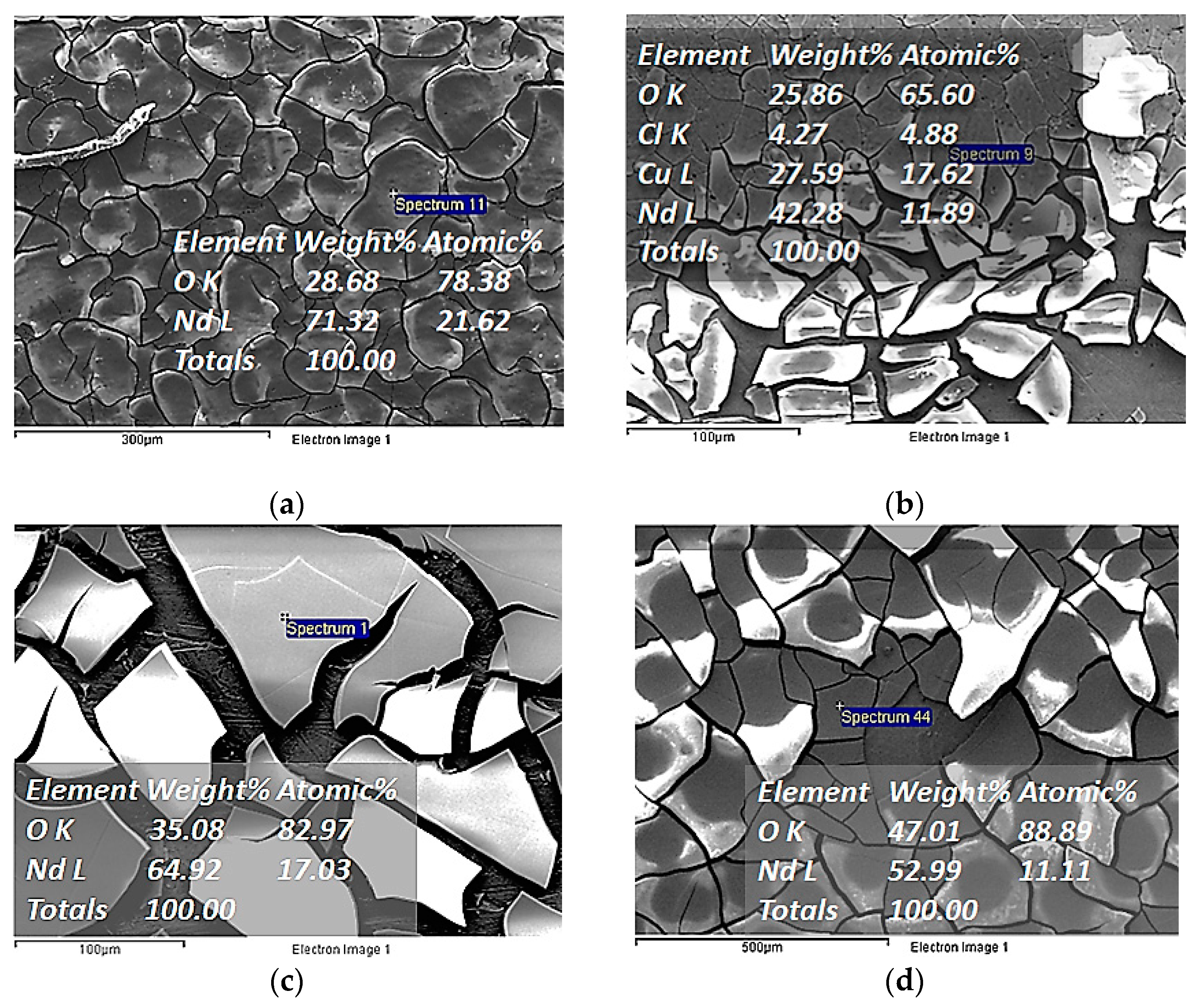

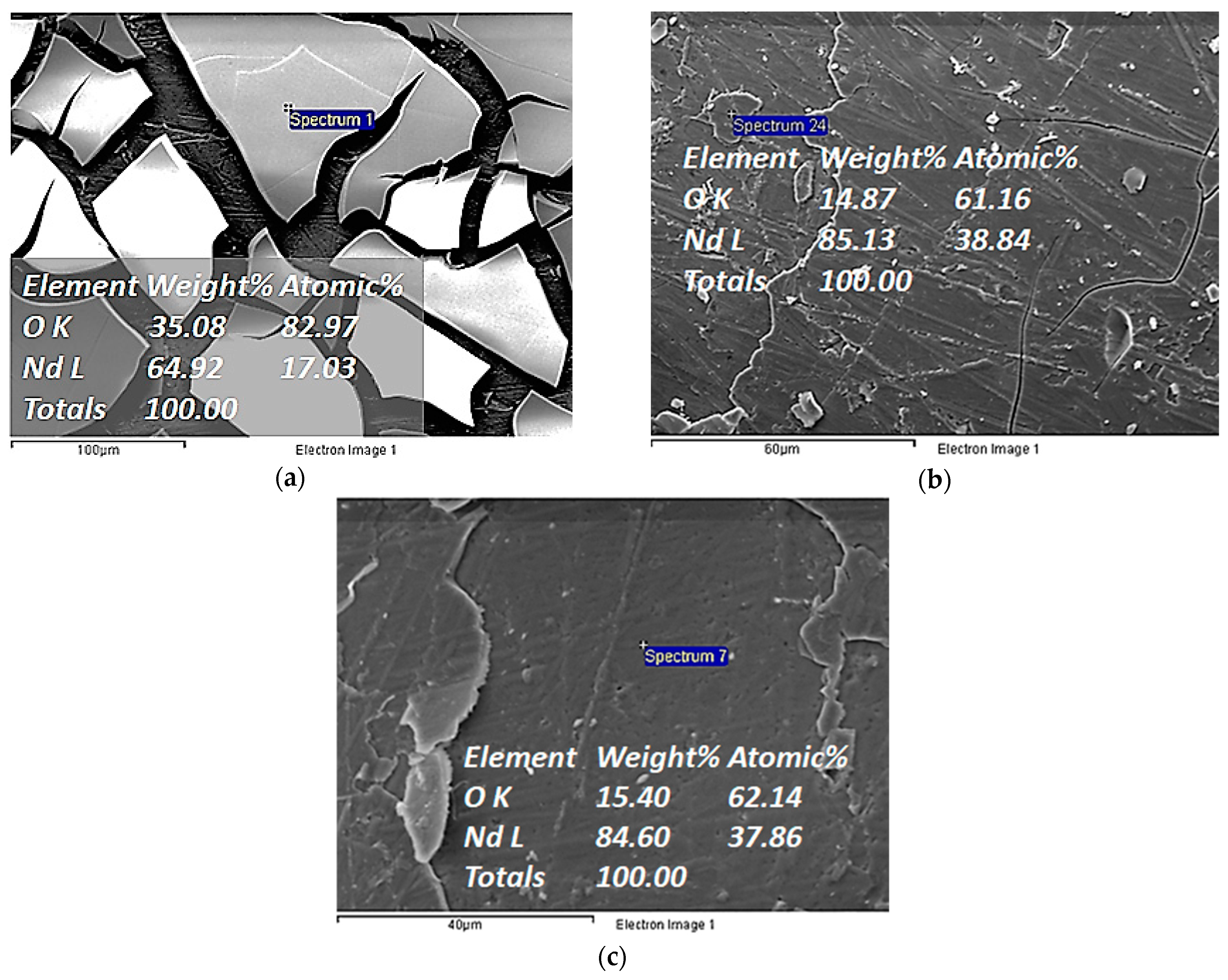
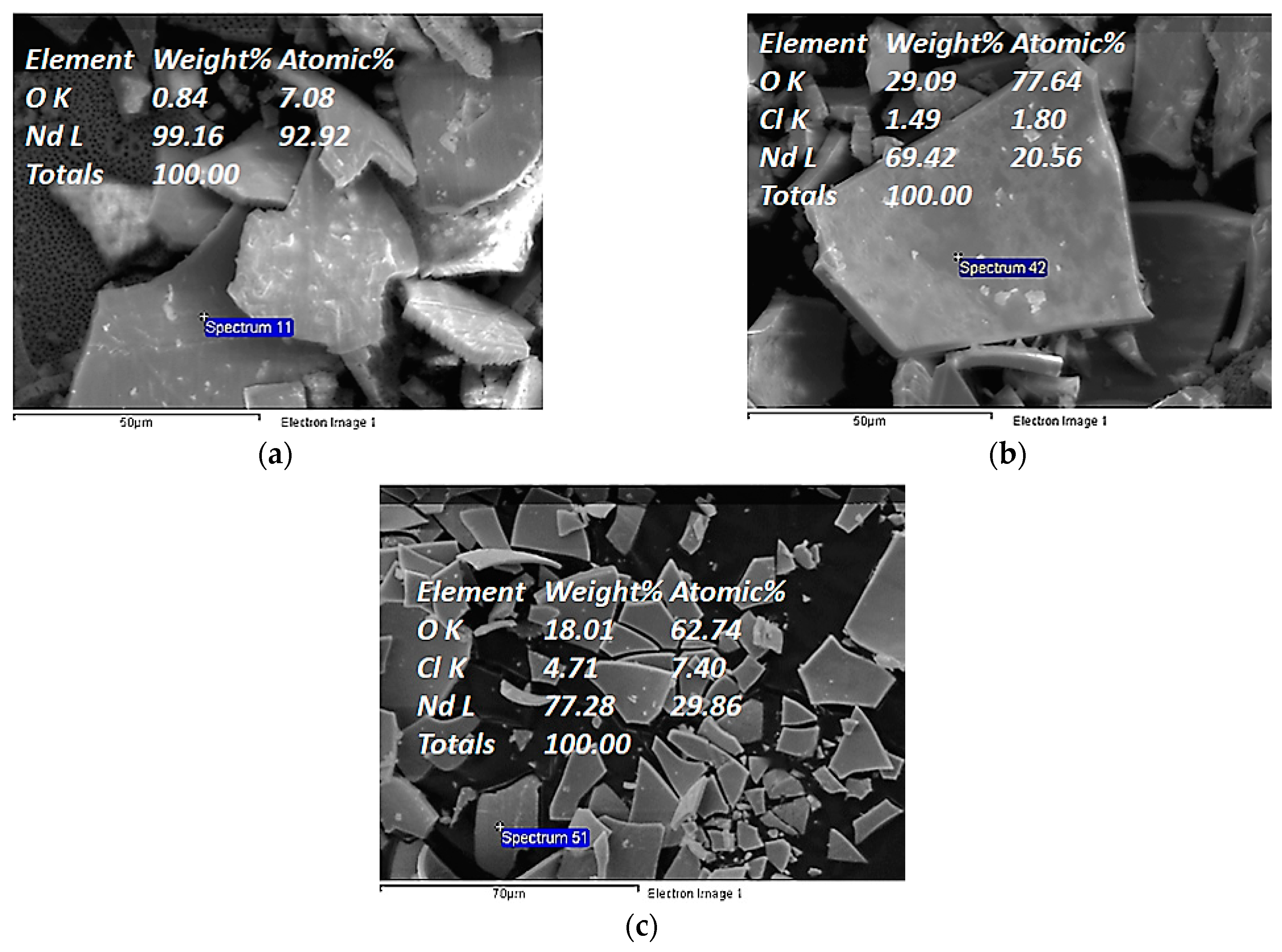

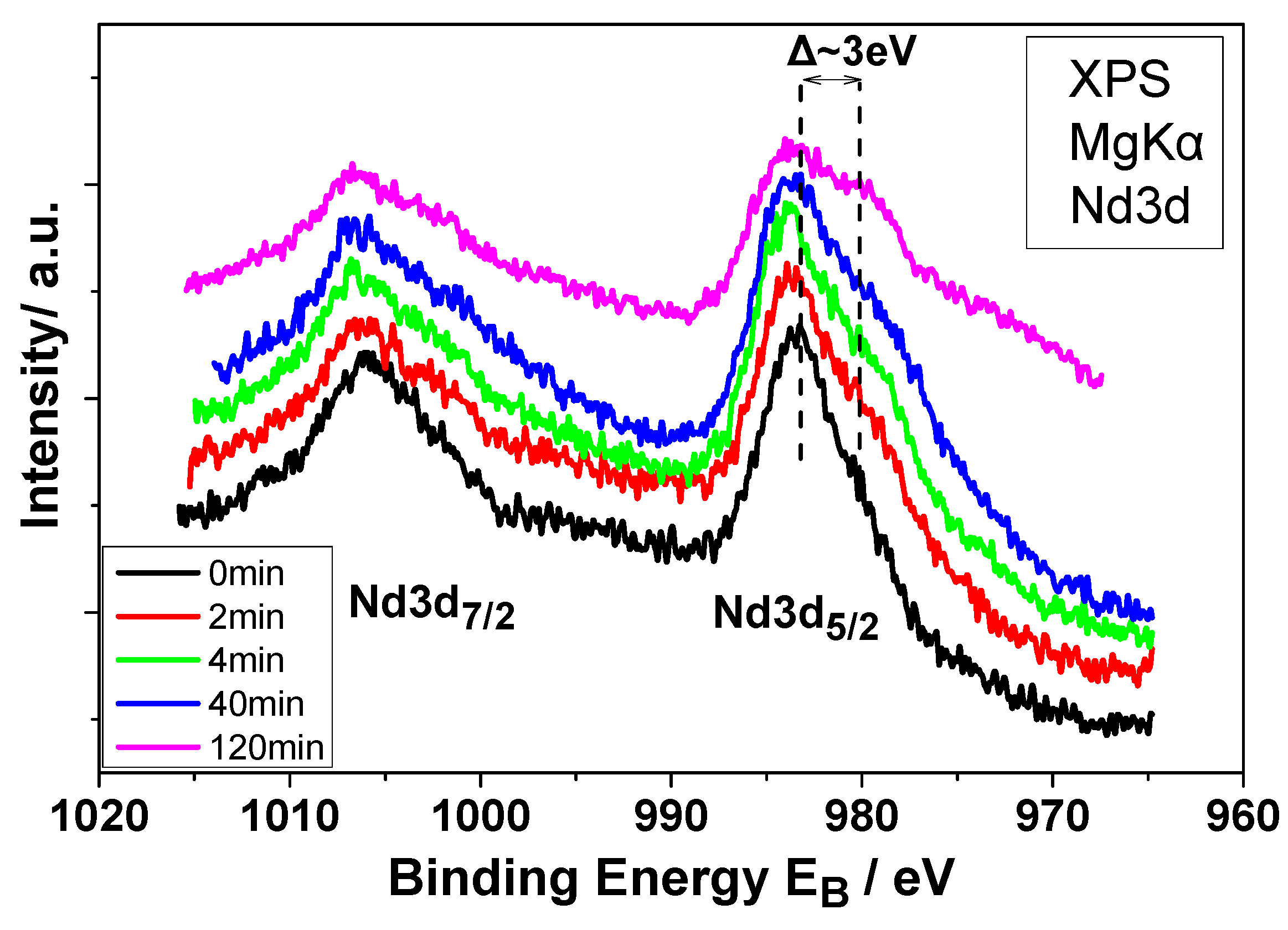
| Electrochemical Mode | Galvanostatic Polarization | Potentiostatic Polarization | Potential Pulsed Electrolysis | Current Pulsed Electrolysis |
|---|---|---|---|---|
| Mass | 3 | 3 | 8 | 12 |
| Concentration | 0.1 | 0.5 | 1 |
|---|---|---|---|
| Mass | 12 | 14 | 19 |
| Temperature | 30 | 50 | 70 |
|---|---|---|---|
| Mass | 4 | 4 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourbos, E.; Karantonis, A.; Sygellou, L.; Paspaliaris, I.; Panias, D. Study of Nd Electrodeposition from the Aprotic Organic Solvent Dimethyl Sulfoxide. Metals 2018, 8, 803. https://doi.org/10.3390/met8100803
Bourbos E, Karantonis A, Sygellou L, Paspaliaris I, Panias D. Study of Nd Electrodeposition from the Aprotic Organic Solvent Dimethyl Sulfoxide. Metals. 2018; 8(10):803. https://doi.org/10.3390/met8100803
Chicago/Turabian StyleBourbos, Evangelos, Antonis Karantonis, Labrini Sygellou, Ioannis Paspaliaris, and Dimitrios Panias. 2018. "Study of Nd Electrodeposition from the Aprotic Organic Solvent Dimethyl Sulfoxide" Metals 8, no. 10: 803. https://doi.org/10.3390/met8100803





