Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Synthesis of Zinc Oxide Particles
2.2. Characterization of Zinc Oxide Particles
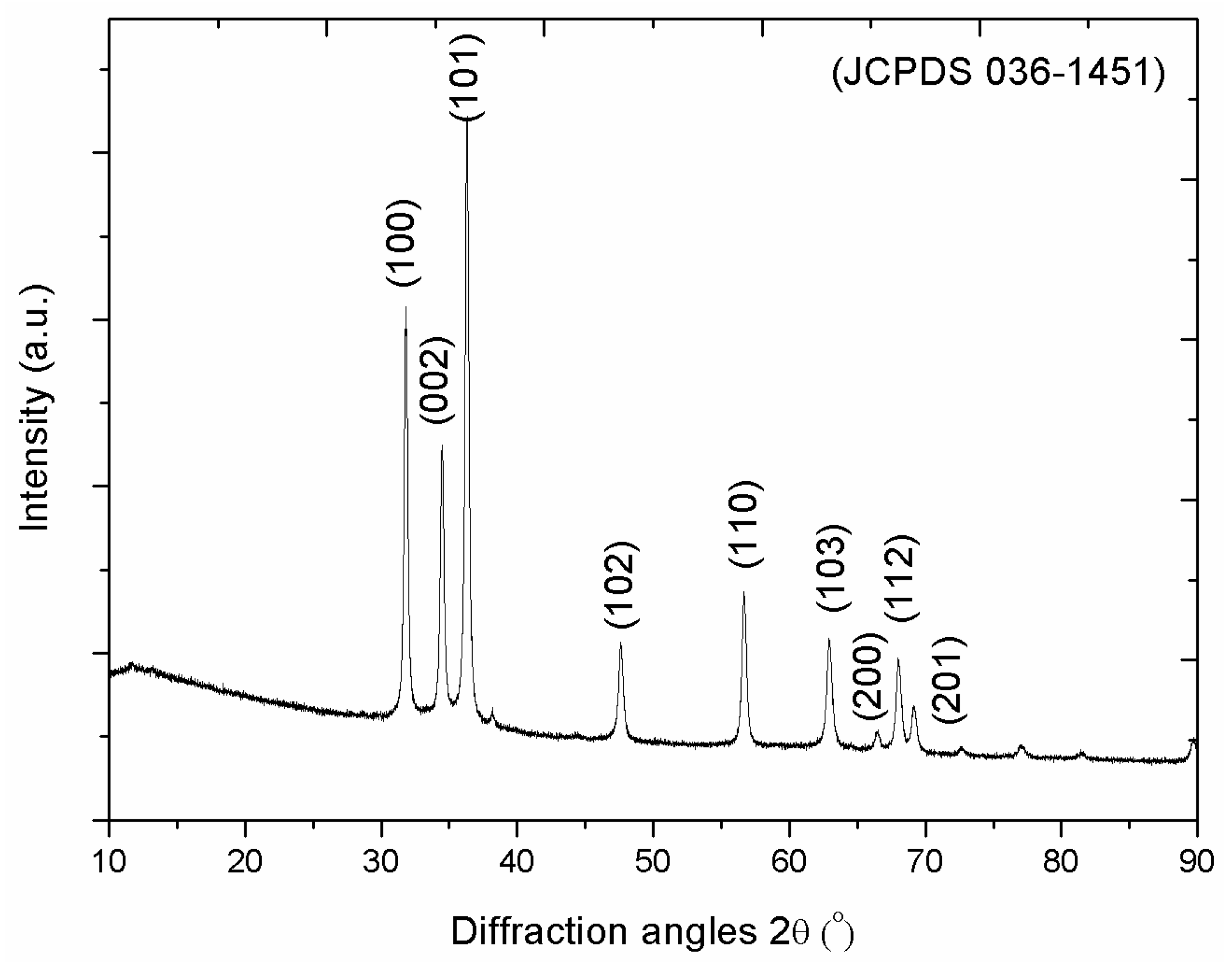
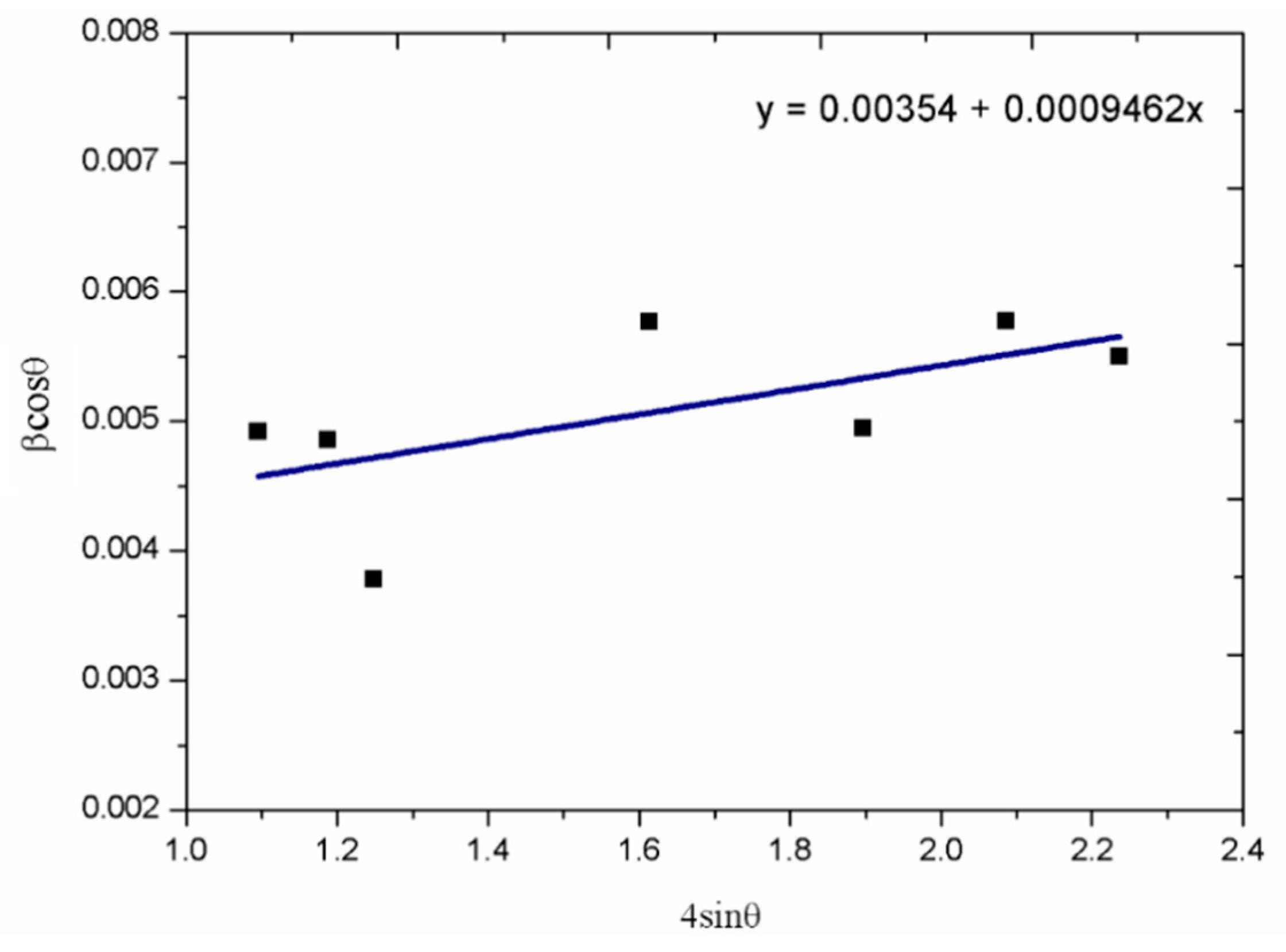
3. Results and Discussion
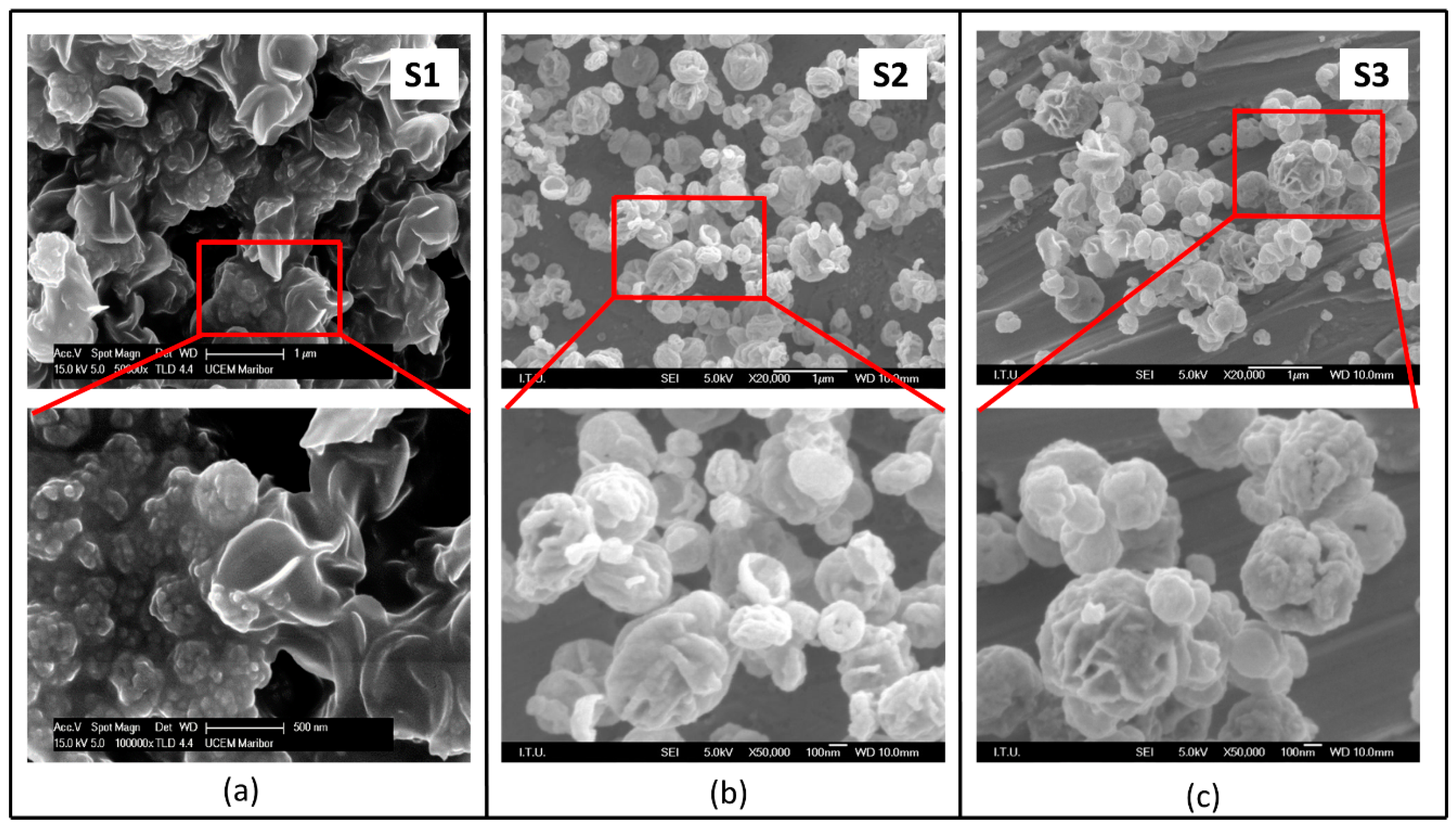
3.1. Effect of Precursor Concentration
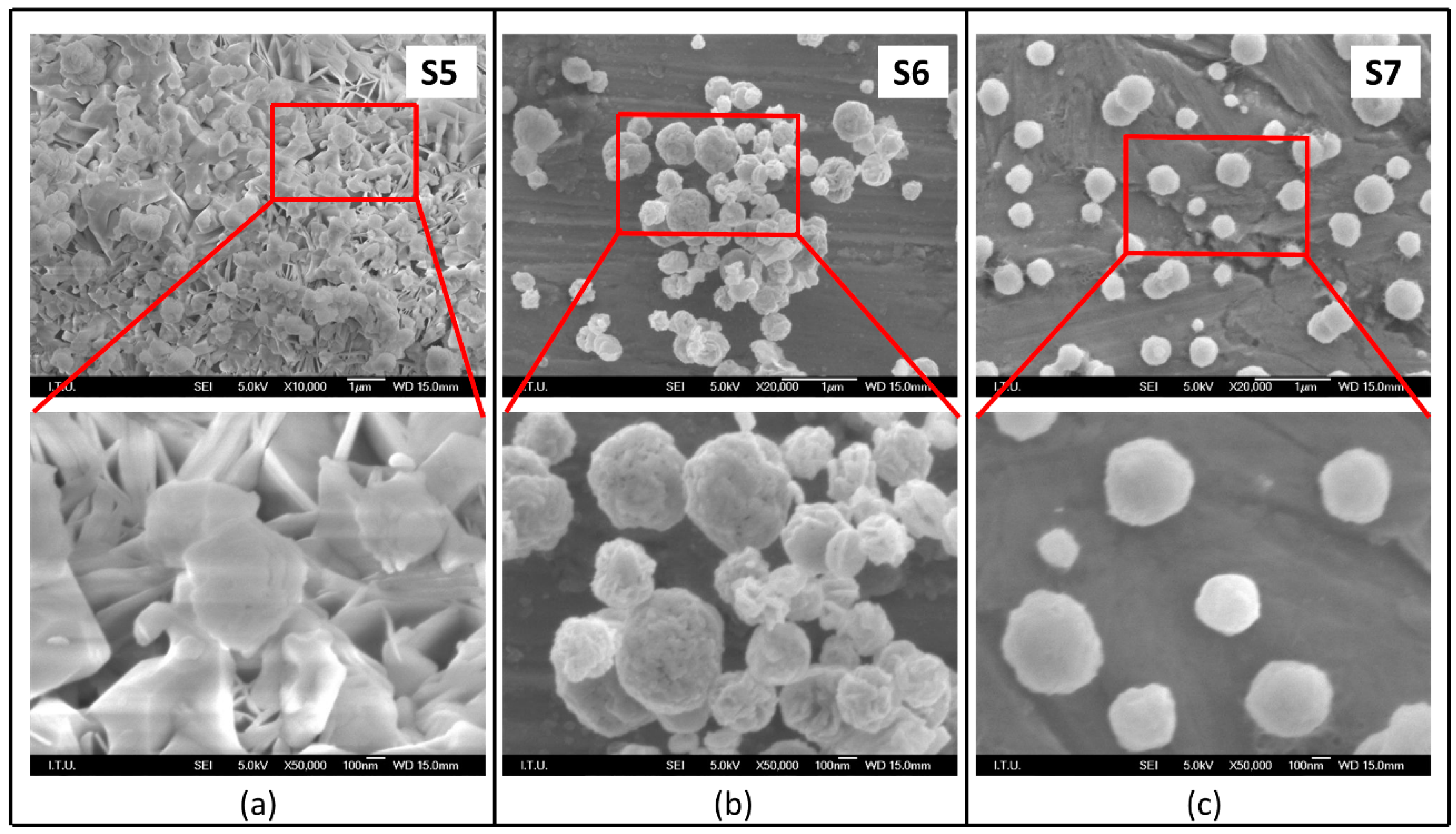
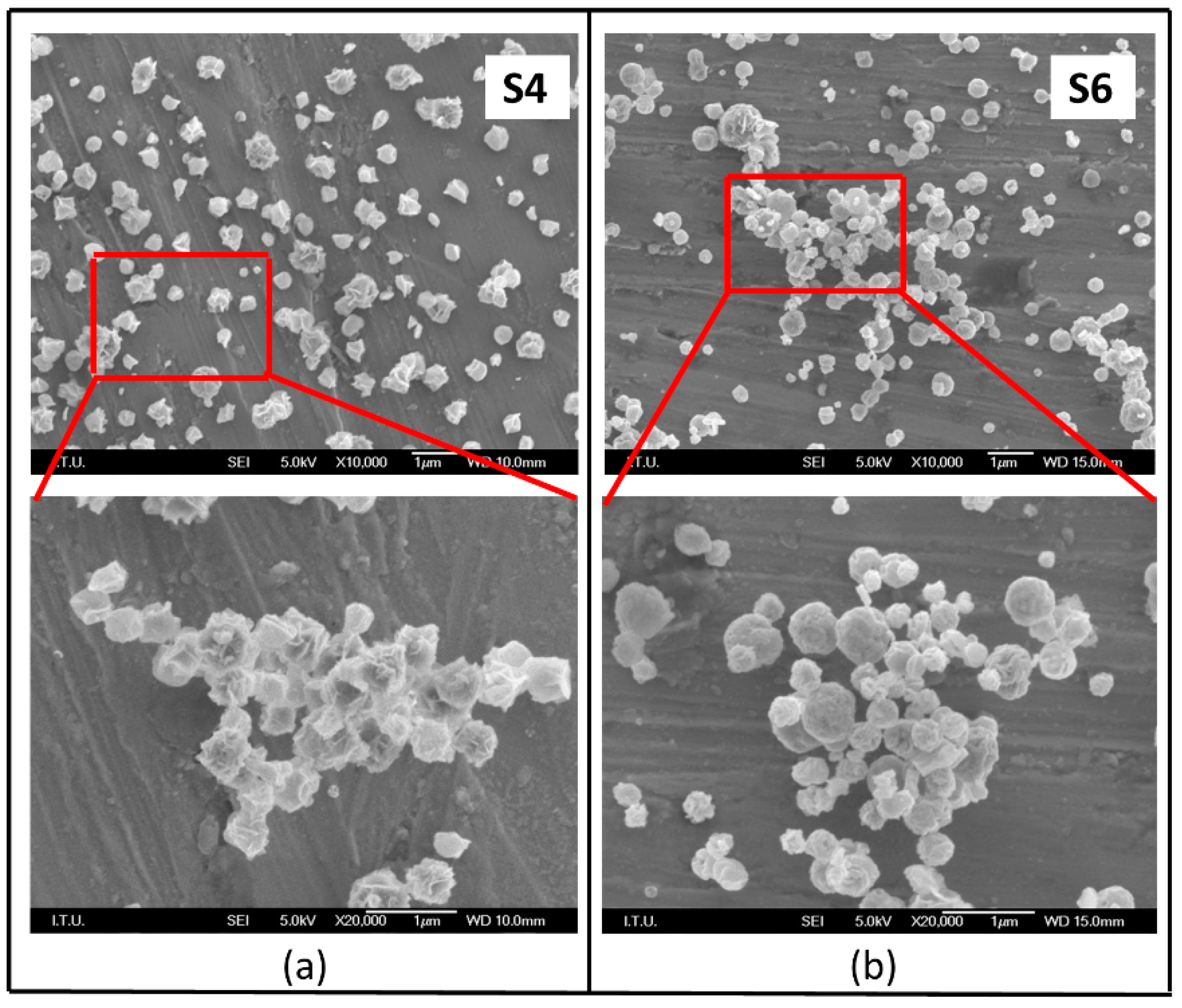
3.2. Effect of Reaction Temperature and N2 Gas Flow Rate
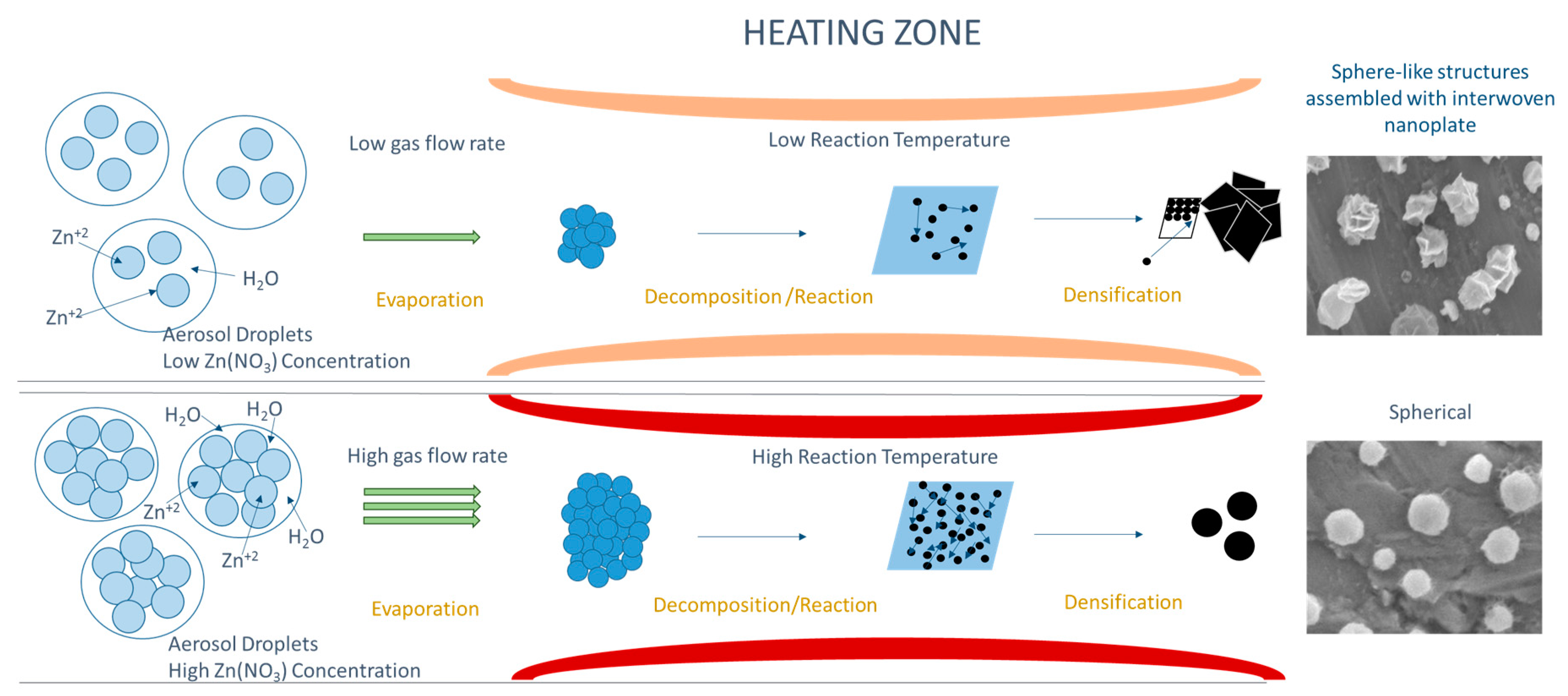
3.3. Formation Mechanism
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Ag | Silver |
| BF | Bright-Field |
| DS | Debye-Scherrer method |
| FE | SEM-Field Emission Scanning Electron Microscopy |
| HRTEM | High Resolution Transmission Electron Microscopy |
| N2 | nitrogen |
| OH− | Hydroxide ion |
| SAED | Selected Area Electron Diffraction |
| SEM | Scanning Electron Microscopy |
| UDM | Uniform Deformation Model |
| UV | Ultraviolet |
| USP | Ultrasonic Spray Pyrolysis |
| ZnO | Nanostructured zinc oxide particles |
| XRD | X-ray diffraction analysis |
| W-H | Williamson-Hall analysis |
References
- Yang, F.; Liu, W.H.; Wang, X.W.; Zheng, J.; Shi, R.Y.; Zhao, H.; Yang, H.Q. Controllable low temperature vapor-solid growth and hexagonal disk enhanced field emission property of ZnO nanorod arrays and hexagonal nanodisk networks. ACS Appl. Mater. Interfaces, 2012, 4, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.I.; El-Molla, S.A.; Ibrahim, M.M.; Mahmoud, H.R.; Naghmash, M.A. Effect of preparation methods and optical band gap of ZnO nanomaterials on photodegradation studies. Opt. Mater. 2016, 58, 484–490. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Dedova, T.; Acik, I.O.; Krunks, M.; Mikli, V.; Volobujeva, O.; Mere, A. Effect of substrate morphology on the nucleation and growth of ZnO nanorods prepared by spray pyrolysis. Thin Solid Films 2012, 520, 4650–4653. [Google Scholar] [CrossRef]
- Klubnuan, S.; Suwanboon, S.; Amornpitoksuk, P. Effects of optical band gap energy, band tail energy and particle shape on photocatalytic activities of different ZnO nanostructures prepared by a hydrothermal method. Opt. Mater. 2016, 53, 134–141. [Google Scholar] [CrossRef]
- Gu, C.; Huang, J.; Wu, Y.; Zhai, M.; Sun, Y.; Liu, J. Preparation of porous flower-like ZnO nanostructures and their gas-sensing property. J. Alloys Compd. 2011, 509, 4499–4504. [Google Scholar] [CrossRef]
- Singh, N.K.; Shrivastava, S.; Rath, S.; Annapoorni, S. Optical and room temperature sensing properties of highly oxygen deficient flower-like ZnO nanostructures. Appl. Surf. Sci. 2010, 257, 1544–1549. [Google Scholar] [CrossRef]
- Kennedy, O.W.; Coke, M.L.; White, E.R.; Shaffer, M.S.P.; Warburton, P.A. MBE growth and morphology control of ZnO nanobelts with polar axis perpendicular to growth direction. Mater. Lett. 2018, 212, 51–53. [Google Scholar] [CrossRef]
- Ding, J.; Fang, X.; Yang, R.; Kan, B.; Li, X.; Yuan, N. Transformation of ZnO polycrystalline sheets into hexagon-like mesocrystalline ZnO rods (tubes) under ultrasonic vibration. Nanoscale Res. Lett. 2014, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Ashraf Talesh, S.S. The effect of ultrasonic irradiation on the structure, morphology and photocatalytic performance of ZnO nanoparticles by sol-gel method. Ultrason. Sonochem. 2017, 39, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, J.; Yan, K.; Duan, M. Growth Mechanism of Different Morphologies of ZnO Crystals Prepared by Hydrothermal Method. J. Mater. Sci. Technol. 2011, 27, 153–158. [Google Scholar] [CrossRef]
- Huang, J.; Xia, C.; Cao, L.; Zeng, X. Facile microwave hydrothermal synthesis of zinc oxide one-dimensional nanostructure with three-dimensional morphology. Mater. Sci. Eng. B Solid-State Mater. Adv. Tec. 2008, 150, 187–193. [Google Scholar] [CrossRef]
- Htay, M.T.; Hashimoto, Y.; Ito, K. Growth of ZnO submicron single-crystalline platelets, wires, and rods by ultrasonic spray pyrolysis. Jpn. J. Appl. Phys. Part 1 Regul. Pap. and Short Notes and Rev. Pap. 2007, 46, 440–448. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Ghorbani, H.; Paravar, A.; Samberg, J.P.; Kajbafvala, E.; Sadrnezhaad, S.K. Effects of morphology on photocatalytic performance of Zinc oxide nanostructures synthesized by rapid microwave irradiation methods. Superlattices Microstruct. 2012, 51, 512–522. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Self-assembly fabrication of ZnO hierarchical micro/nanospheres for enhanced photocatalytic degradation of endocrine-disrupting chemicals. Mater. Sci. Semicond. Process. 2013, 16, 1542–1550. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Shangguan, E.; Li, Y.; Li, L.; Wang, D.; Wang, M.; Chang, Z.; Li, Q. Enhancing the rate and cycling performance of spherical ZnO anode material for advanced zinc-nickel secondary batteries by combined in-situ doping and coating with carbon. Electrochim. Acta 2017, 236, 180–189. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Alkan, G.; Milošević, O.; Rabanal, M.E.; Friedrich, B. Synthesis and characterisation of spherical core-shell Ag/ZnO nanocomposites using single and two-steps ultrasonic spray pyrolysis (USP). Catal. Today 2017. [Google Scholar] [CrossRef]
- Hidayat, D.; Ogi, T.; Iskandar, F.; Okuyama, K. Single crystal ZnO:Al nanoparticles directly synthesized using low-pressure spray pyrolysis. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2008, 151, 231–237. [Google Scholar] [CrossRef]
- Gurmen, S.; Ebin, B. Production and characterization of the nanostructured hollow iron oxide spheres and nanoparticles by aerosol route. J. Alloys Compd.. 2010, 492, 585–589. [Google Scholar] [CrossRef]
- Gurmen, S.; Guven, A.; Ebin, B.; Stopic, S.; Friedrich, B. Synthesis of nano-crystalline spherical cobalt-iron (Co-Fe) alloy particles by ultrasonic spray pyrolysis and hydrogen reduction. J. Alloys Compd. 2009, 481, 600–604. [Google Scholar] [CrossRef]
- Lojpur, V.; Mancic, L.; Rabanal, M.E.; Dramicanin, M.D.; Tan, Z.; Hashishin, T.; Ohara, S.; Milosevic, O. Structural, morphological and luminescence properties of nanocrystalline up-converting Y1.89Yb0.1Er0.01O3 phosphor particles synthesized through aerosol route. J. Alloys Compd. 2013, 580, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Emil, E.; Gürmen, S.; Hall, W. Estimation of yttrium oxide microstructural parameters using the Williamson–Hall analysis. Mater. Sci. Technol. 2018, 836. [Google Scholar] [CrossRef]
- Ebin, B.; Arig, E.; Özkal, B.; Gürmen, S. Production and characterization of ZnO nanoparticles and porous particles by ultrasonic spray pyrolysis using a zinc nitrate precursor. Inter. J. Miner. Metall. Mater. 2012, 19, 651–656. [Google Scholar] [CrossRef]
- Yassitepe, E.; Yatmaz, H.C.; Öztürk, C.; Öztürk, K.; Duran, C. Photocatalytic efficiency of ZnO plates in degradation of azo dye solutions. J. Photochem. Photobiol. A Chem. 2008, 198, 1–6. [Google Scholar] [CrossRef]
- Tartaj, P.; Tartaj, J. Preparation, characterization and sintering behavior of spherical iron oxide doped alumina particles. Acta Mater. 2002, 50, 5–12. [Google Scholar] [CrossRef]
- Neamjan, N.; Sricharussin, W.; Threepopnatkul, P. Effect of various shapes of ZnO nanoparticles on cotton fabric via electrospinning for UV-blocking. J. Nanosci. Nanotechnol. 2012, 12, 525–530. [Google Scholar] [CrossRef] [PubMed]

| Sample Name | Zn(NO3)2·6H2O Concentration (mol/L) | Reaction Temperature (°C) | N2 Gas Flow Rate (L/min) |
|---|---|---|---|
| S1 | 0.01875 | 800 | 0.5 |
| S2 | 0.02875 | 800 | 0.5 |
| S3 | 0.03750 | 800 | 0.5 |
| S4 | 0.02875 | 600 | 0.5 |
| S5 | 0.02875 | 400 | 0.75 |
| S6 | 0.02875 | 600 | 0.75 |
| S7 | 0.02875 | 800 | 0.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emil, E.; Alkan, G.; Gurmen, S.; Rudolf, R.; Jenko, D.; Friedrich, B. Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process. Metals 2018, 8, 569. https://doi.org/10.3390/met8080569
Emil E, Alkan G, Gurmen S, Rudolf R, Jenko D, Friedrich B. Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process. Metals. 2018; 8(8):569. https://doi.org/10.3390/met8080569
Chicago/Turabian StyleEmil, Elif, Gözde Alkan, Sebahattin Gurmen, Rebeka Rudolf, Darja Jenko, and Bernd Friedrich. 2018. "Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process" Metals 8, no. 8: 569. https://doi.org/10.3390/met8080569







