Effects of Fe2O3 on Reduction Process of Cr-Containing Solid Waste Self-Reduction Briquette and Relevant Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Procedure
3. Results and Discussion
3.1. Reduction Yield
3.2. Morphology of Products
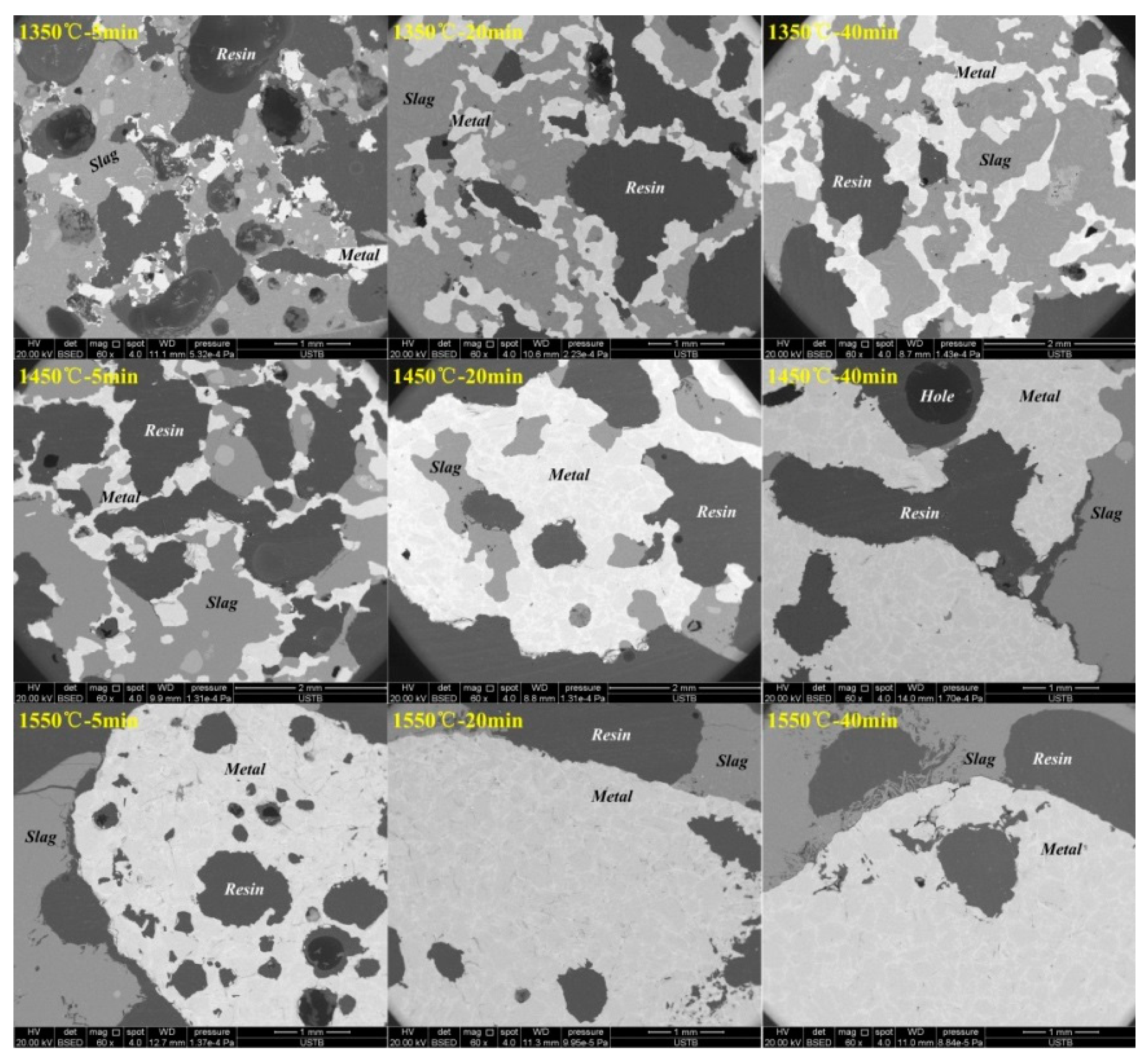
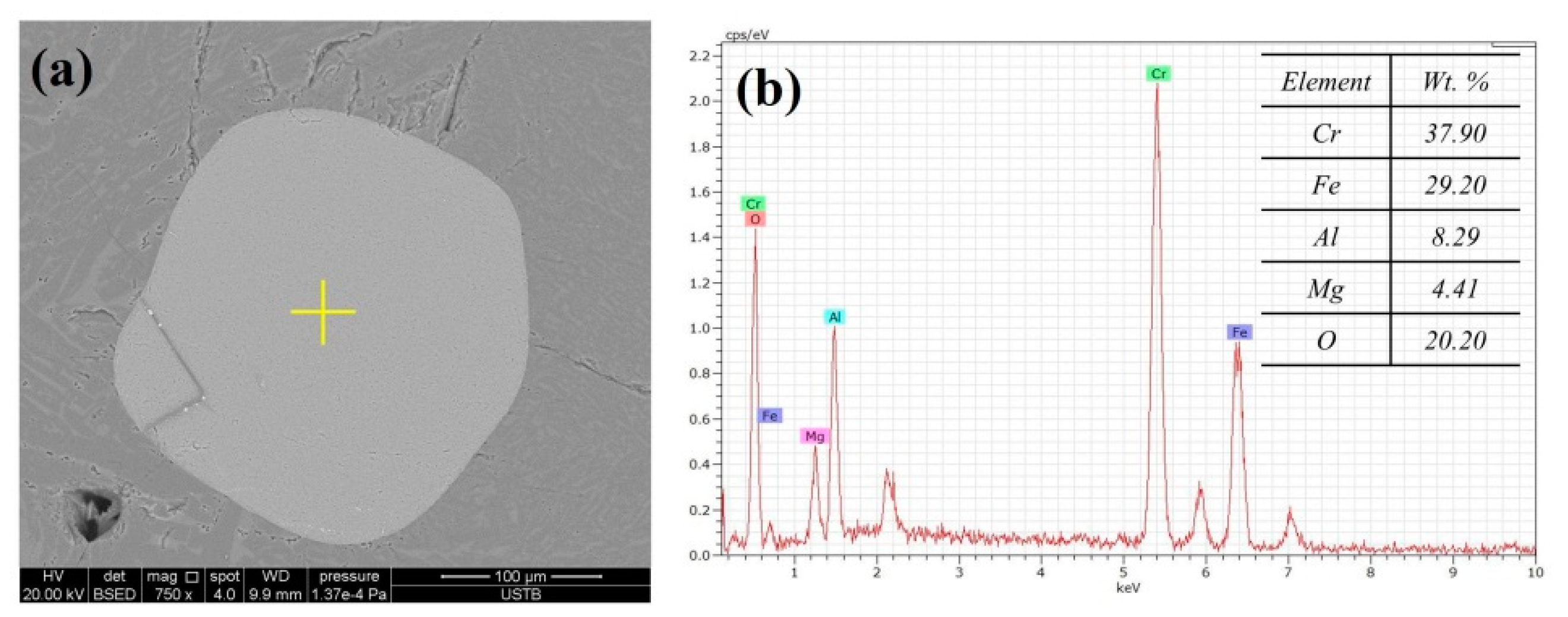
3.3. Variation of Product Morphology Observed by SEM
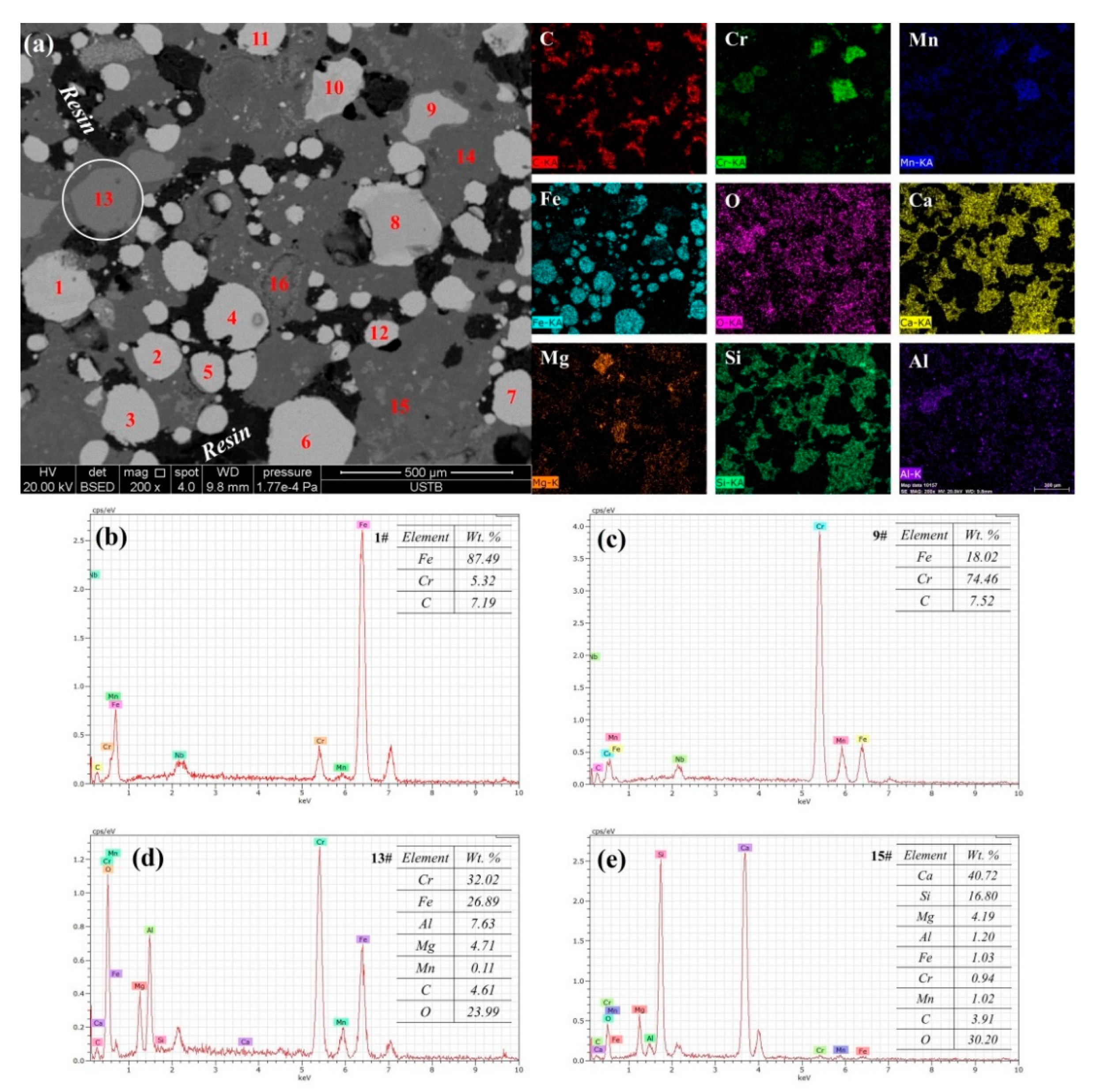
3.4. Variation of Metal Composition
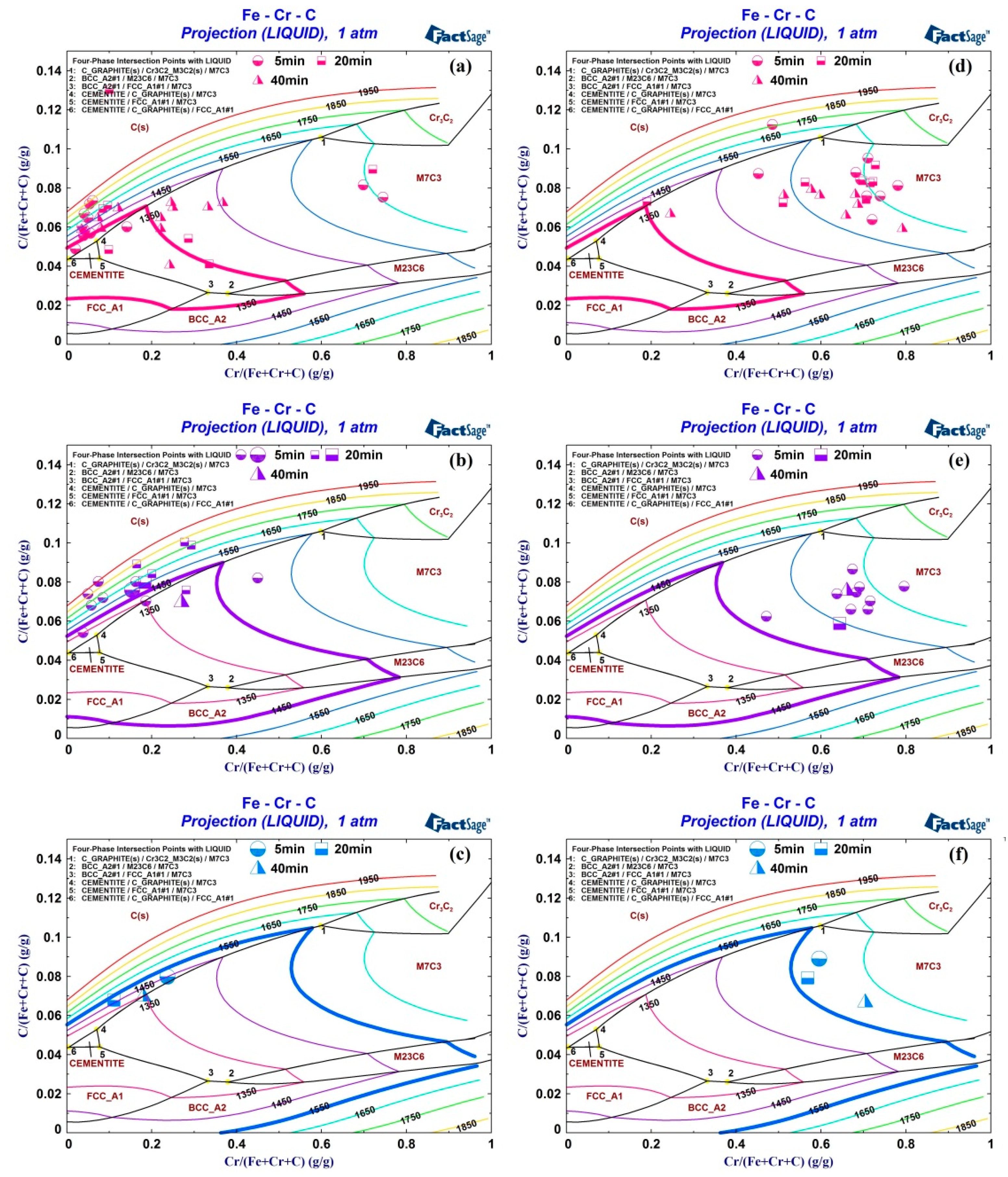
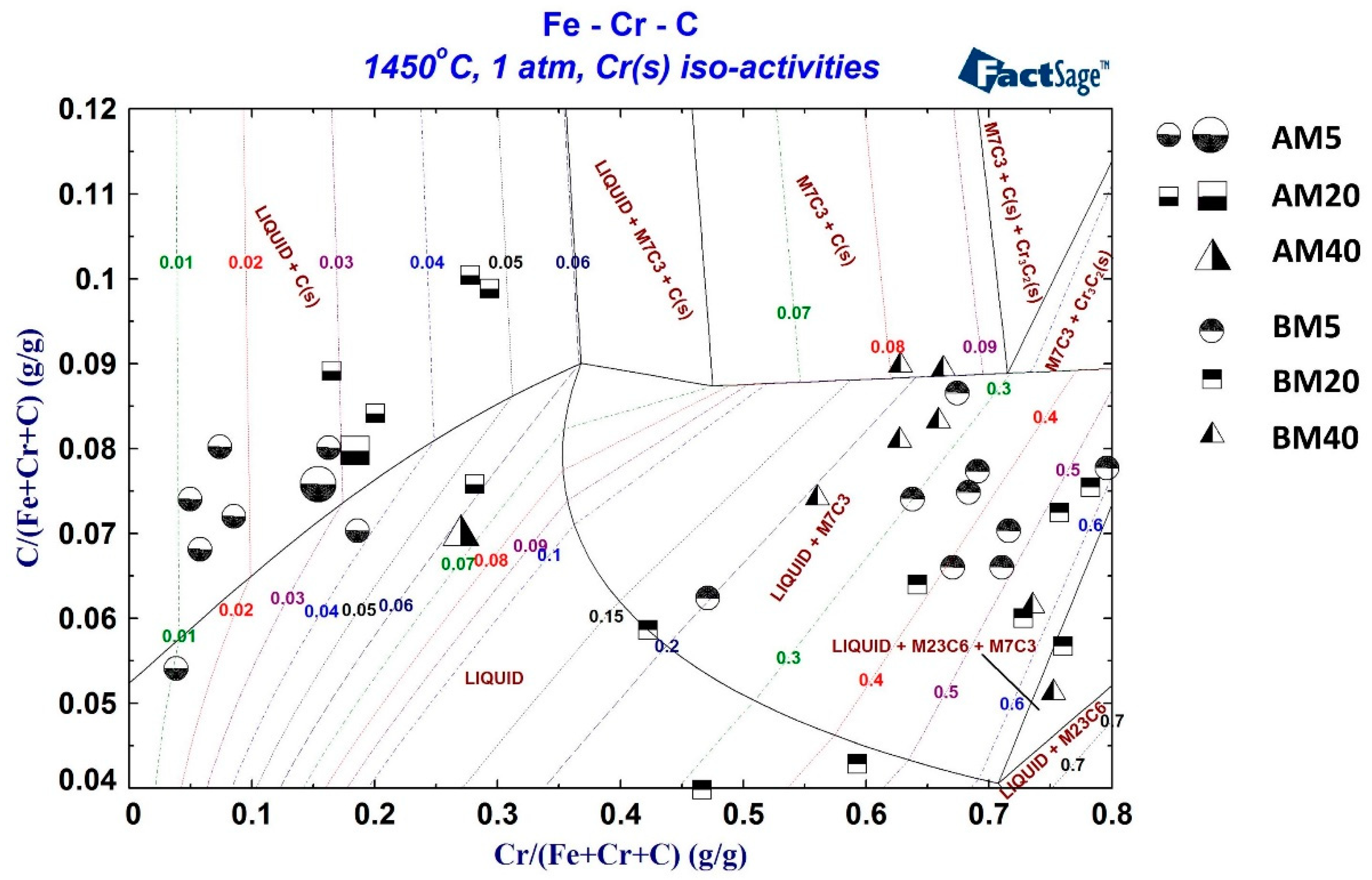
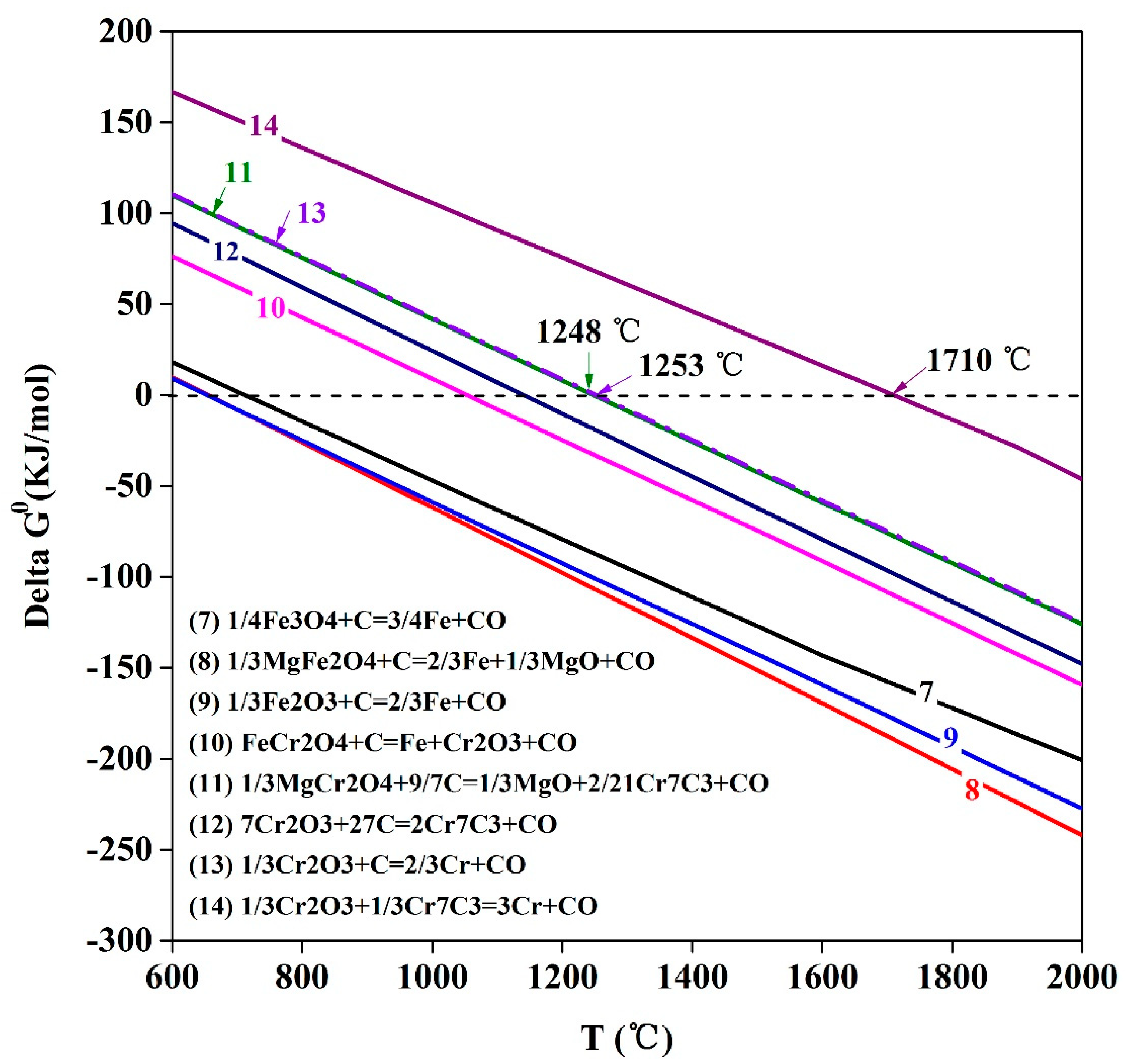
3.5. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, M.; Durinck, D.; Jones, P.T.; Heylen, G.; Hendrickx, R.; Beaten, R.; Blanpain, B.; Wollants, P. EAF stainless steel refining-Part I: Observational study on chromium recovery in an eccentric bottom tapping furnace and a spout tapping furnace. Steel Res. Int. 2007, 78, 117–124. [Google Scholar] [CrossRef]
- Durinck, D.; Jones, P.T.; Guo, M.; Verhaeghe, F.; Heylen, G.; Hendrickx, R.; Beaten, R.; Blanpain, B.; Wollants, P. EAF stainless steel refining-Part II: Microstructural slag evolution and its implications for slag foaming and chromium recovery. Steel Res. Int. 2007, 78, 125–135. [Google Scholar] [CrossRef]
- Ma, G.; Garbers-Craig, A.M. Cr (VI) containing electric furnace dusts and filter cake from a stainless steel waste treatment plant: Part 1-Characteristics and microstructure. Ironmak. Steelmak. 2006, 33, 229–237. [Google Scholar] [CrossRef]
- Ma, G.; Garbers-Craig, A.M. Cr (VI) containing electric furnace dusts and filter cake from a stainless steel waste treatment plant: Part 2-Formation mechanisms and leachability. Ironmak. Steelmak. 2006, 33, 238–244. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J. Metal recovery from stainless steel mill scale by microwave heating. Met. Mater. Int. 2008, 14, 193–196. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hong, X. An overview for the utilization of wastes from stainless steel industries. Resour. Conserv. Recycl. 2011, 55, 745–754. [Google Scholar]
- Takano, C.; Cavallante, F.L.; Santos, D.M.D.; Mourão, M.B. Recovery of Cr, Ni and Fe from dust generated in stainless steelmaking. Miner. Process Extr. Metall. 2013, 114, 201–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Jia, X. Reduction of chromium oxides in stainless steel dust. Int. J. Min. Met. Mater. 2015, 22, 573–581. [Google Scholar] [CrossRef]
- Qi, Q.S.; Xu, A.J.; He, D.F.; Pan, J.T. Carbothermic reduction for recycling of iron and chromium from stainless steel slag. Iron Steel. 2017, 52, 82–87. [Google Scholar]
- Adamczyk, B.; Brenneis, R.; Adam, C.; Mudersbach, D. Recovery of chromium from AOD-Converter slags. Steel Res. Int. 2010, 81, 1078–1083. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Li, H.; Wang, J. Experimental analysis on direct recycling Cr-Ni stainless steel making dust in EAF. Iron Steel. 2009, 44, 76–80. [Google Scholar]
- McClelland, J.M.; Metius, G.E. Recycling ferrous and nonferrous waste streams with FASTMET. JOM 2003, 55, 30–34. [Google Scholar] [CrossRef]
- Ye, G.; Burström, E.; Kuhn, M.; Piret, J. Reduction of steel-making slags for recovery of valuable metals and oxide materials. Scand. J. Metall. 2010, 32, 7–14. [Google Scholar] [CrossRef]
- Zhao, H.; Qi, Y.; Shi, Y.; Feng, H.; Na, X. A test research on treatment of stainless steel dust by Oxycup. J. Mat. Metal. 2017, 16, 58–62. [Google Scholar]
- Görnerup, M.; Lahiri, A.K. Reduction of electric arc furnace slags in stainless steelmaking Part 1 Observations. Ironmak. Steelmak. 1998, 25, 317–322. [Google Scholar]
- Görnerup, M.; Lahiri, A.K. Reduction of electric arc furnace slags in stainless steelmaking: Part 2 Mechanism of CrOx reduction. Ironmak. Steelmak. 1998, 25, 382–386. [Google Scholar]
- Chakraborty, D.; Ranganathan, S.; Sinha, S.N. Investigations on the carbothermic reduction of chromite ores. Metall. Mater. Trans. B 2005, 36, 437–444. [Google Scholar] [CrossRef]
- Hu, X.; Teng, L.; Wang, H.; Sundqvist Ökvist, L.; Yang, Q.; Björkma, B.; Seetharaman, S. Carbothermic reduction of synthetic chromite with/without the addition of iron powder. ISIJ Int. 2016, 56, 2147–2155. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Q.; Sundqvist Ökvist, L.; Björkma, B. Thermal analysis study on the carbothermic reduction of chromite ore with the addition of mill scale. Steel Res. Int. 2016, 87, 562–570. [Google Scholar] [CrossRef]
- Hu, X.; Sundqvist Ökvist, L.; Eriksson, J.; Yang, Q.; Björkma, B. Direct alloying steel with chromium by briquettes made from chromite ore, mill Scale, and petroleum coke. Steel Res. Int. 2017, 88, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wei, W. Products of carbothermic reduction of Fe-Cr-O and Fe-Cr-Ni-O systems. Trans. Nonferr. Met. Soc. China 2014, 24, 1210–1219. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wei, W. Carbothermal reduction process of the Fe-Cr-O system. Int. J. Min. Met. Mater. 2013, 20, 931–940. [Google Scholar] [CrossRef]
- Eric, R.H. Chapter 1.10-Production of Ferroalloys. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 477–532. [Google Scholar]
- Mori, Y.; Ototake, N. Viscosity of solid-liquid mixture. Chem. Eng. 1956, 20, 488. [Google Scholar] [CrossRef]
- Ohno, K.; Miki, T.; Hino, M. Kinetic analysis of iron carburizaiton during smelting reduciton. ISIJ Int. 2004, 44, 2033–2039. [Google Scholar] [CrossRef]
- Ohno, K.; Miki, T.; Sasaki, Y.; Hino, M. Carburization degree of iron nugget produced by rapid heating of powdery iron, iron oxide in slag and carbon mixture. ISIJ Int. 2008, 48, 1368–1372. [Google Scholar] [CrossRef]
- Mori, T.; Yang, J.; Kuwabara, M. Mechanism of carbothermic reduction of chromium oxide. ISIJ Int. 2007, 47, 1387–1393. [Google Scholar] [CrossRef]
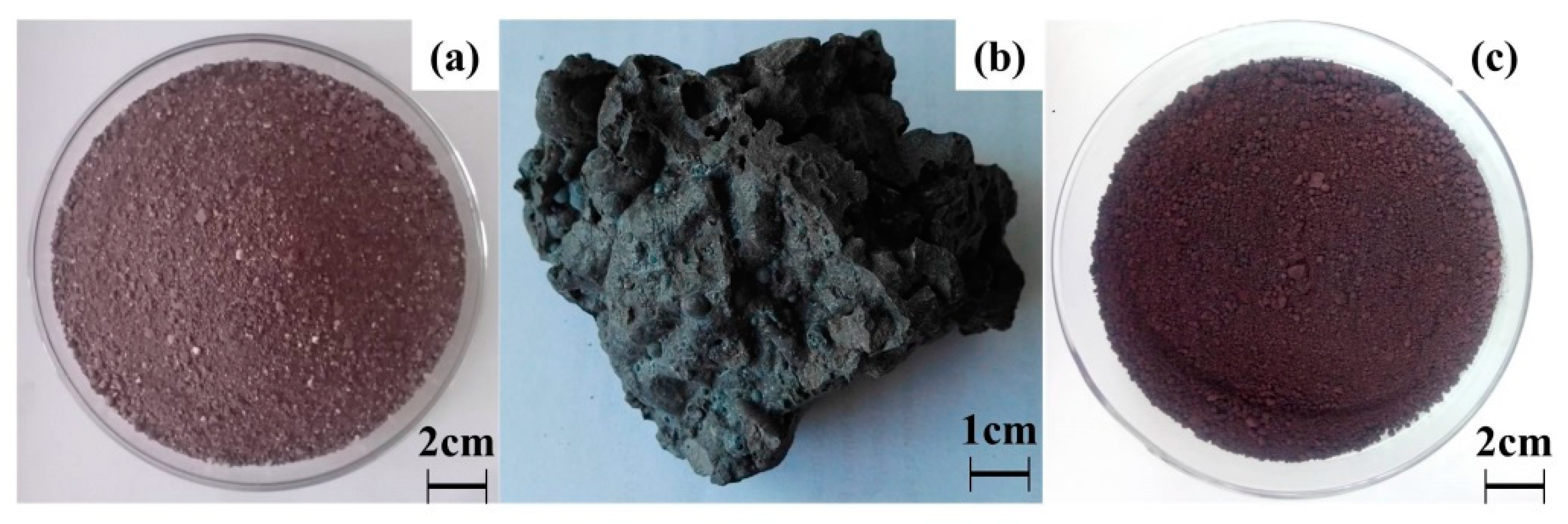
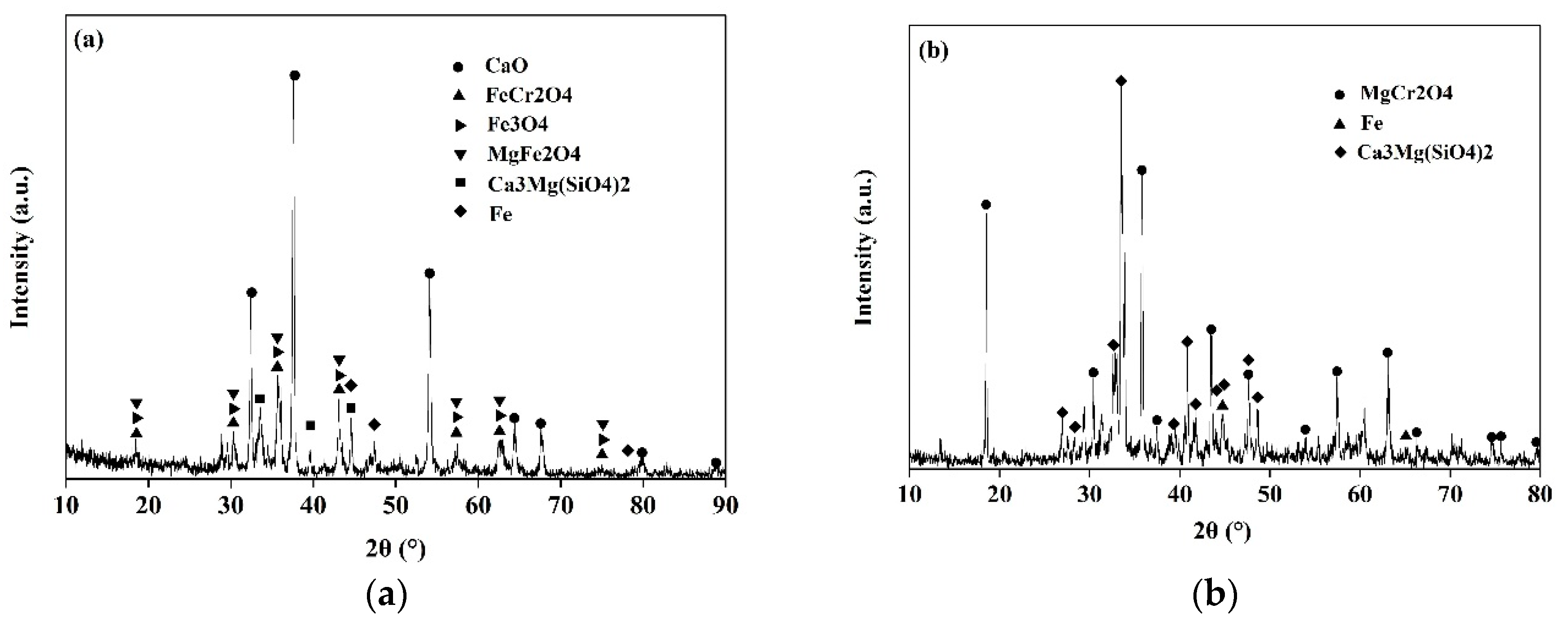






| Mater. | TFe | Cr | CaO | SiO2 | MgO | Al2O3 | NiO | MnO | MoO3 | V2O5 | F | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSD | 20.18 | 35.80 | 20.67 | 3.10 | 3.49 | 1.70 | 0.11 | 0.54 | 0.10 | Bal. | ||
| SSS | 3.20 | 9.12 | 41.01 | 28.22 | 9.43 | 2.14 | 1.50 | 0.28 | 0.64 | Bal. | ||
| Fe2O3 | ≥99% | Bal. |
| Proportion Scheme | Temperature | Holding Time/Min | Cr/(Cr + Fe) | ||
|---|---|---|---|---|---|
| A (30%Dust+20%SSS+50%Fe2O3): 100 g Graphite: nC:nO = 1.2 SiO2: R = 1.2 | L: 1350 | 5 | 20 | 40 | 0.23 |
| M: 1450 | 5 | 20 | 40 | ||
| H: 1550 | 5 | 20 | 40 | ||
| B (60%Dust+40%SSS): 100 g Graphite: nC:nO = 1.2 SiO2: R = 1.2 | L: 1350 | 5 | 20 | 40 | 0.65 |
| M: 1450 | 5 | 20 | 40 | ||
| H: 1550 | 5 | 20 | 40 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Zhang, Y.; Zhao, Z.; Yuan, F. Effects of Fe2O3 on Reduction Process of Cr-Containing Solid Waste Self-Reduction Briquette and Relevant Mechanism. Metals 2019, 9, 51. https://doi.org/10.3390/met9010051
Wu T, Zhang Y, Zhao Z, Yuan F. Effects of Fe2O3 on Reduction Process of Cr-Containing Solid Waste Self-Reduction Briquette and Relevant Mechanism. Metals. 2019; 9(1):51. https://doi.org/10.3390/met9010051
Chicago/Turabian StyleWu, Tuo, Yanling Zhang, Zheng Zhao, and Fang Yuan. 2019. "Effects of Fe2O3 on Reduction Process of Cr-Containing Solid Waste Self-Reduction Briquette and Relevant Mechanism" Metals 9, no. 1: 51. https://doi.org/10.3390/met9010051




