Experimental Investigation of Solidification in the Cast Mold with a Consumable Cooler Introduced Inside
Abstract
:1. Introduction
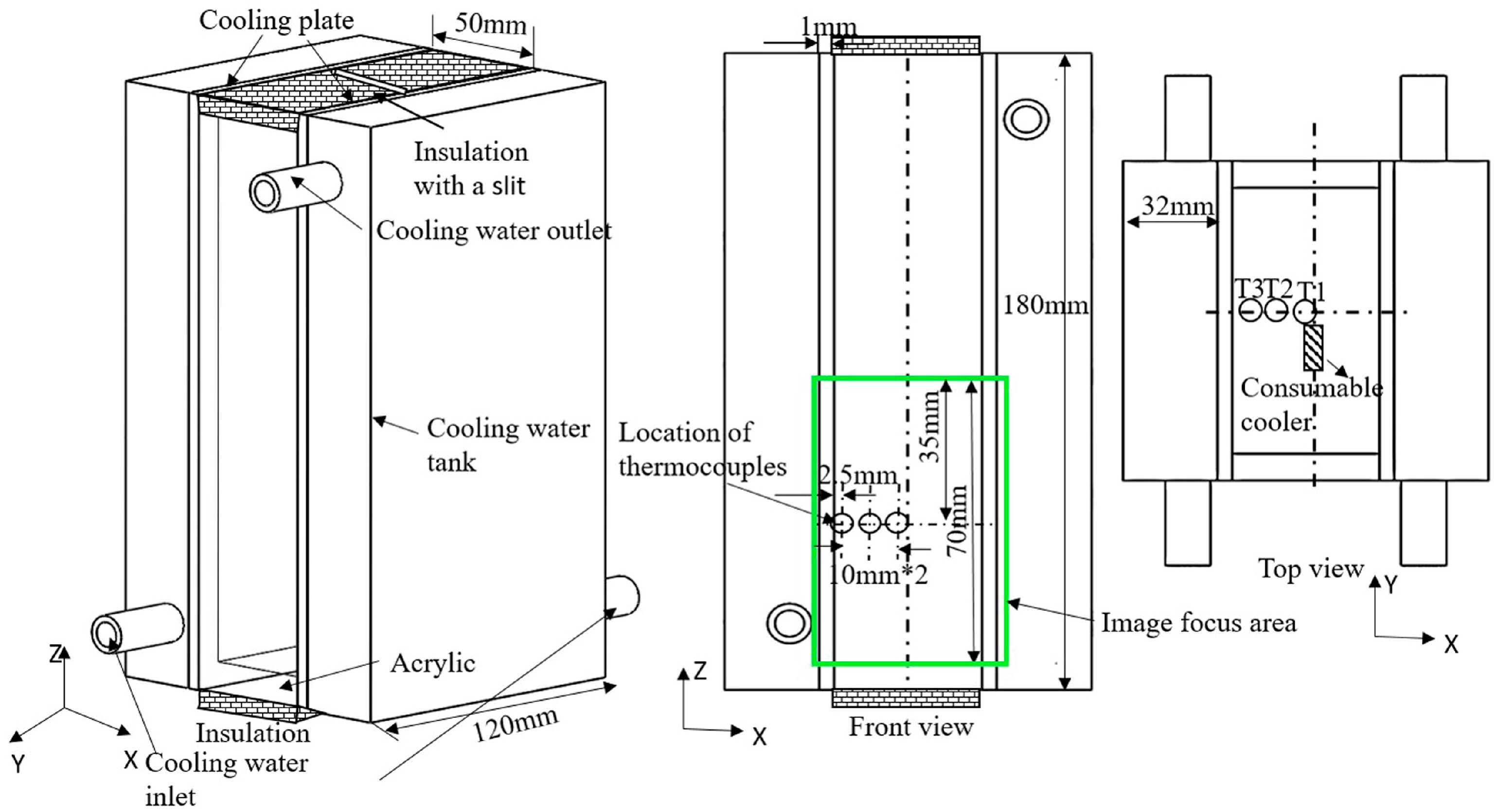
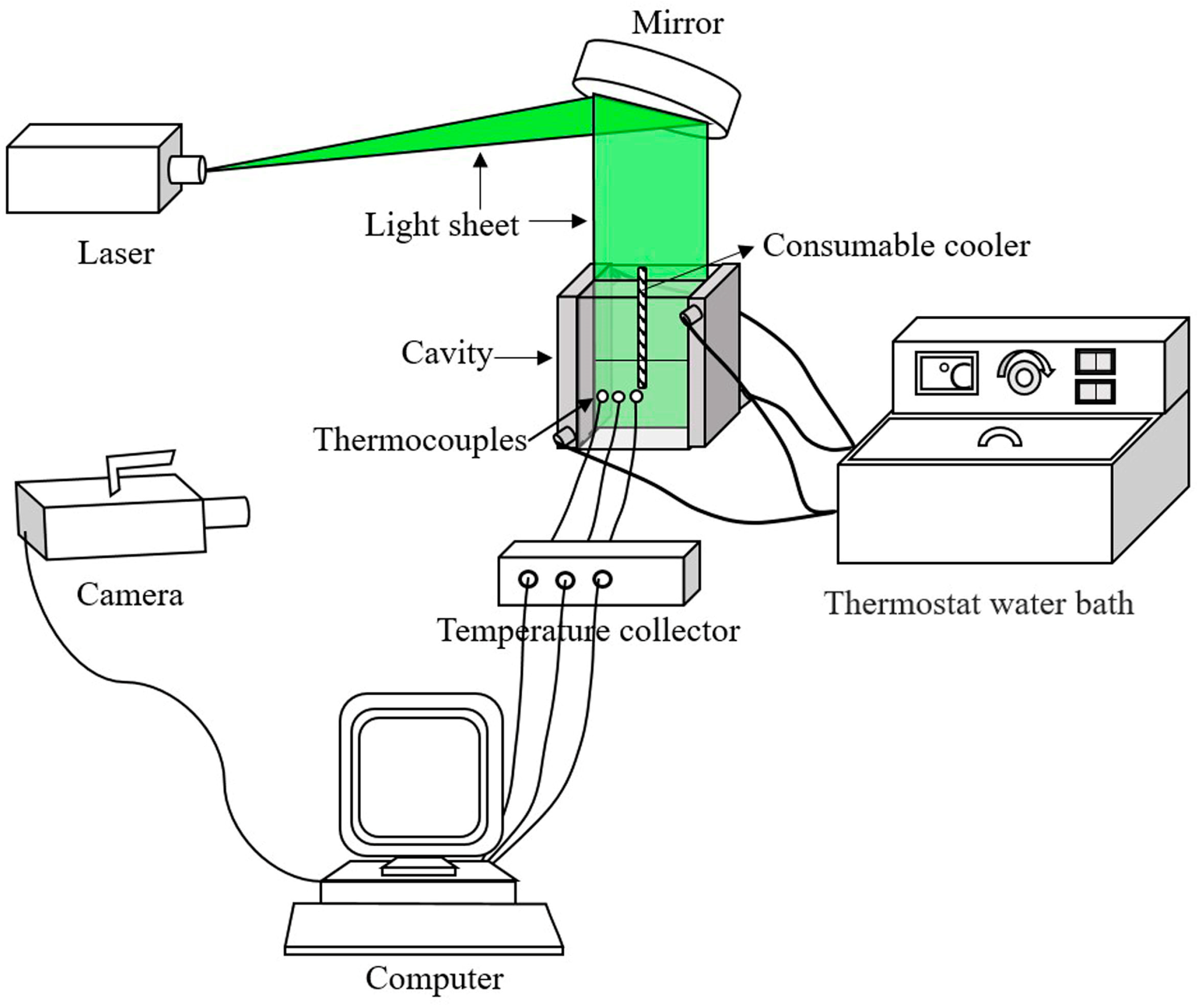
2. Experimental Procedure
3. Results and Discussion
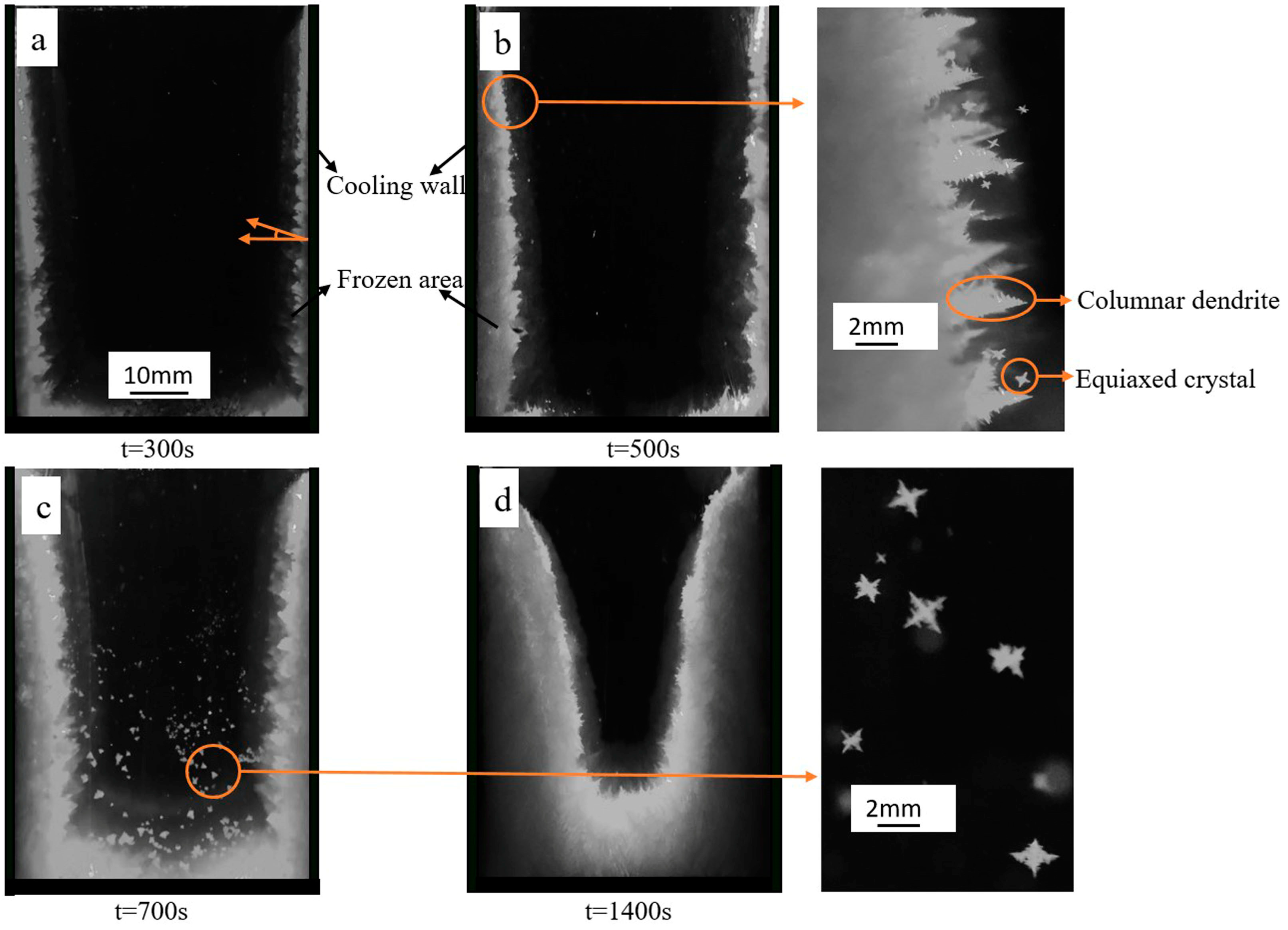
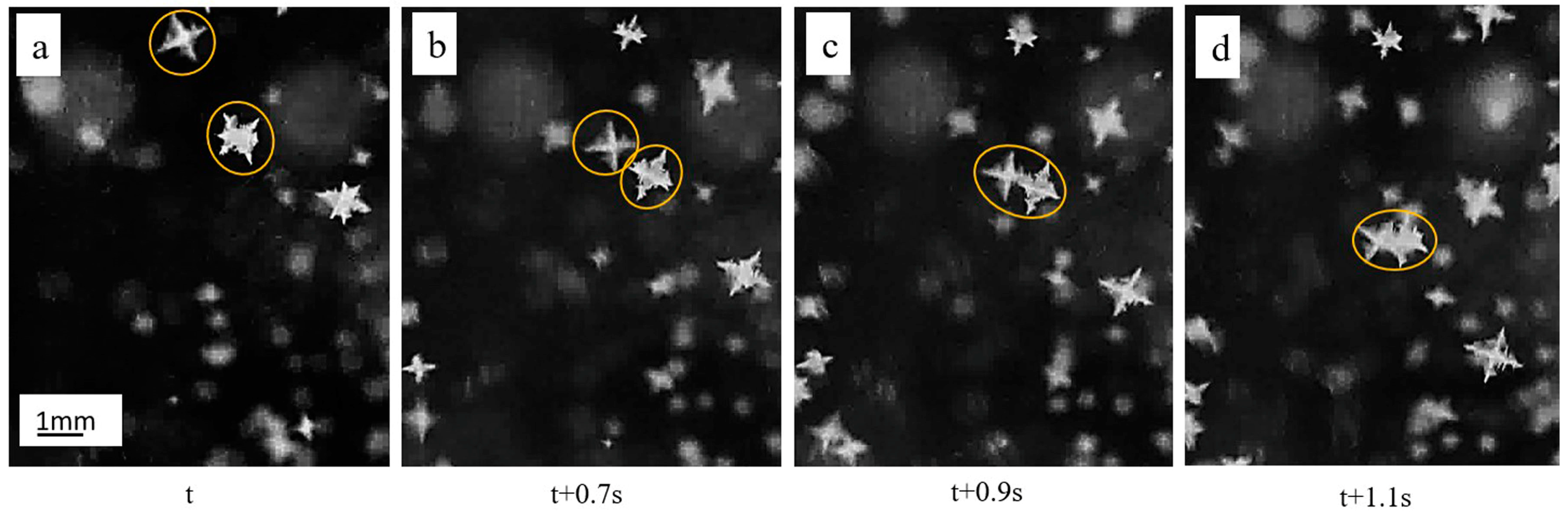
3.1. Dendrite Growth during Solidification
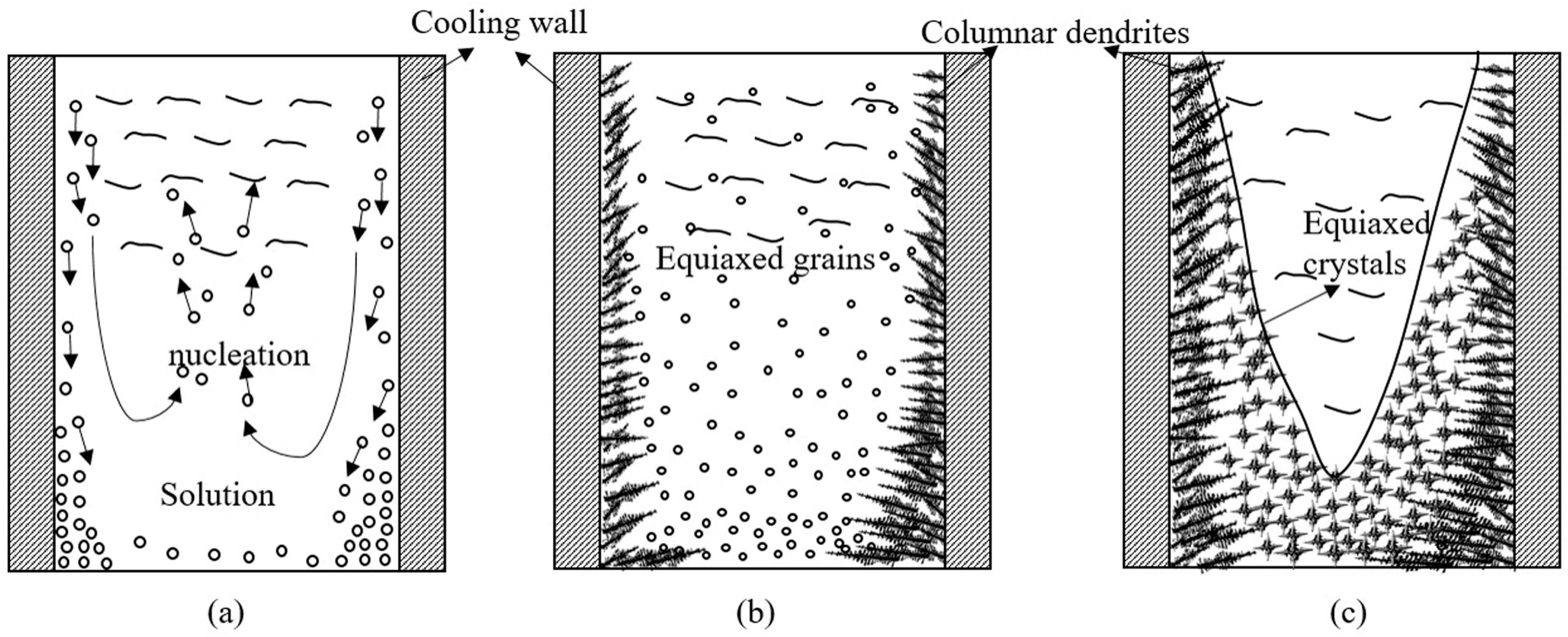
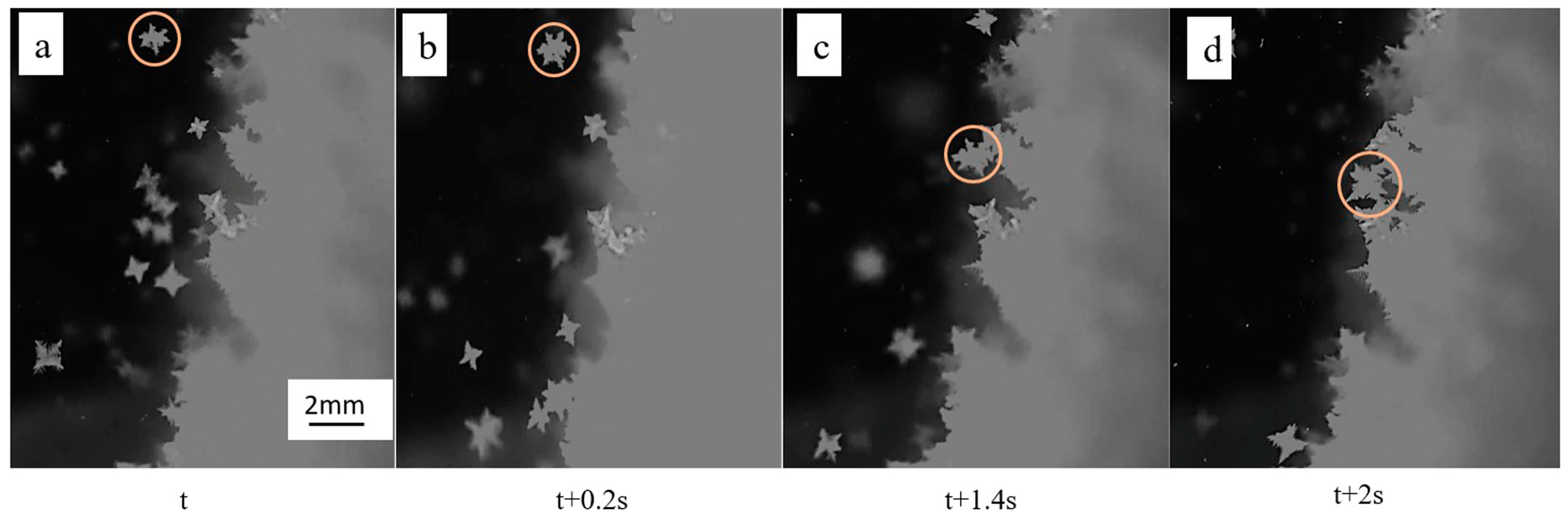
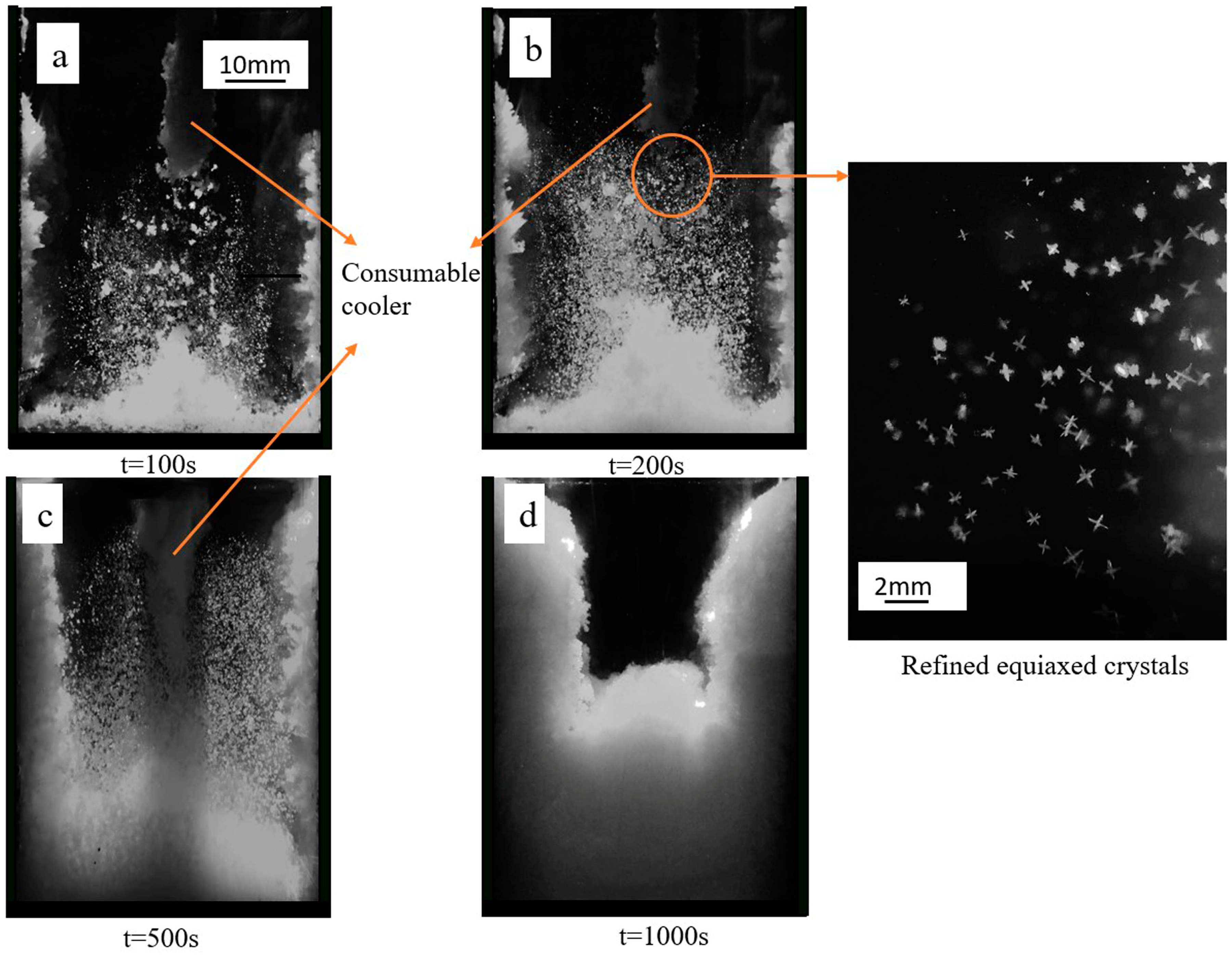
3.2. Effect of Cooler on the Solidification Morphology
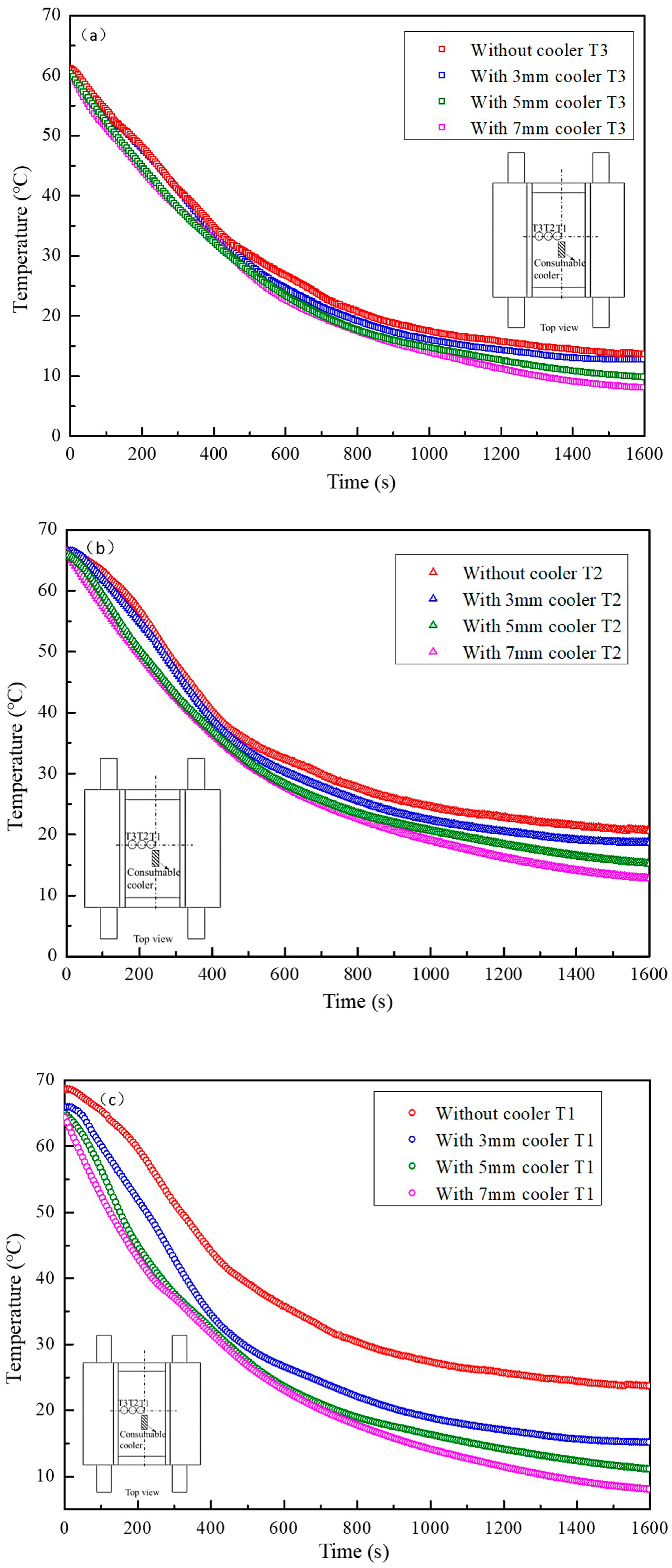
3.3. Effect of Adding Cooler on the Temperature Profile
4. Conclusions
- (1)
- The solidification process can be divided into three periods: pure columnar dendrites growth, equiaxed crystal rain occurrence, equiaxed crystal block the columnar growth to a steady state.
- (2)
- Introduction of the consumable cooler can significantly reduce the temperature of the central zone of mold. Melting of the consumable cooler can supply large quantity of equiaxed grains and refine the crystal size. The introduced equiaxed crystals can block the growth of columnar dendrites, thereby promoting the CET.
- (3)
- In the reference experiment without a cooler, CET occurs when the temperature gradient decreased to 2.8 °C. In the experiment with a consumable cooler included, the CET occurs even if the temperature gradient is as high as 6.2 °C.
- (4)
- The consumable cooler with a larger thickness can introduce more equiaxed crystals with smaller sizes. Thus, the solidification rate and the compactness of the solidified structure increased.
Author Contributions
Funding
Conflicts of Interest
References
- Xu, Z.; Wang, X.; Jiang, M. Investigation on improvement of center porosity with heavy reduction in continuously cast thick slabs. Steel Res. Int. 2017, 88, 1600061. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Chunhui, H.U.; Mao, W. Research on fractal characteristics of primary phase morphology in semi-solid a356 alloy. Acta Metall. Sin. 2009, 535–537, 936–940. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, E.G.; Li, Z.; Deng, A.Y. Effects of vertical electromagnetic stirring on grain refinement and macrosegregation control of bearing steel billet in continuous casting. J. Iron Steel Res. Int. 2017, 24, 483–489. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, J.; Chen, W.; Yang, S. Effect of ultrasonic vibration on the grain refinement and sic particle distribution in Zn-based composite filler metal. Mater. Lett. 2008, 62, 2615–2618. [Google Scholar] [CrossRef]
- Ludwig, A.; Stefan-Kharicha, M.; Kharicha, A.; Wu, M. Massive formation of equiaxed crystals by avalanches of mushy zone segments. Metall. Mater. Trans. A 2017, 48, 2927–2931. [Google Scholar] [CrossRef]
- Rauter, D.I.W.; Reiter, J.; Srienc, K.; Brandl, W.; Erker, M.; Huemer, K. Soft reduction at a round bloom caster: Implementation and results. BHM Berg-und Hüttenmännische Monatshefte 2014, 159, 454–460. [Google Scholar] [CrossRef]
- Golenkov, M.A. Improvement of internal quality of continuously cast slabs by introducing consumable vibrating macrocoolers in a mold. Russ. Metall. 2014, 12, 961–965. [Google Scholar] [CrossRef]
- Nosochenko, O.V.; Isaev, O.B.; Lepikhov, L.S.; Degtyarev, P.A.; Kharchevnikov, V.P.; Mel’Nik, N.P. Reducing axial segregation in a continuous-cast semifinished product by micro-alloying. Metallurgist 2003, 47, 244–247. [Google Scholar] [CrossRef]
- Isaev, O.B. Effectiveness of using large cooling elements to alleviate axial segregation in continuous-cast ingots. Metallurgist 2005, 49, 324–331. [Google Scholar] [CrossRef]
- Habu, Y.; Itoyama, S.; Emi, T.; Sorimachi, K.; Kojima, H. Improvement cast structure and centerline segregation of continuously cast slabs by adding steel strip into mold. Tetsu-to-Hagane 1981, 67, 92–101. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Transparent compounds that freeze like metals. Acta Metall. 1965, 13, 1212–1215. [Google Scholar] [CrossRef]
- Christenson, M.S.; Incropera, F.P. Solidification of an aqueous ammonium chloride solution in a rectangular cavity. Int. J. Heat Mass Transfer 1989, 32, 47–68. [Google Scholar] [CrossRef]
- Tatsuo, N.; Masaki, F.; Namiko, H.; Hisashi, M. Temperature visualizations by use of liquid crystals of unsteady natural convection during supercooling and freezing of water in an enclosure with lateral cooling. Int. J. Heat Mass Transfer 1991, 34, 2663–2668. [Google Scholar] [CrossRef]
- Beckermann, C.; Wang, C.Y. Equiaxed dendritic solidification of a NH4Cl-H2O solution with convection 3 comparisons with NH4Cl-H2O experiments. Metall. Mater. Trans. A 1998, 27, 2784–2795. [Google Scholar] [CrossRef]
- Shigeo, A.; Takahiko, S.; Iwwo, M. Theoretical analysis and model study of the formation of A-type segregation in ingot. ISIJ Int. 1977, 63, 1512–1519. [Google Scholar]
- Kharicha, A.; Stefan-Kharicha, M.; Ludwig, A.; Wu, M. Simultaneous observation of melt flow and motion of equiaxed crystals during solidification using a dual phase particle image velocimetry technique. part ii: Relative velocities. Metall. Mater. Trans. A 2013, 44, 661–668. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Y.; Chen, X.; Wu, C.; Wei, Z.; Zhai, Q. In situ observation of crystal rain and its effect on columnar to equiaxed transition. Metals 2016, 6, 271. [Google Scholar] [CrossRef]
- Hellawell, A.; Liu, S.; Lu, S.Z. Dendrite fragmentation and the effects of fluid flow in castings. JOM 1997, 49, 18–20. [Google Scholar] [CrossRef]
- Hellawell, A.; Sarazin, J.R.; Steube, R.S. Channel convection in partly solidified systems. Philos. Trans. Phys. Sci. Eng. 1993, 345, 507–544. [Google Scholar]
- Jie, W.; Zhou, X. Experimental study on the columnar to equiaxed transformation process. J. Northwest. Polytech. Univ. 1988, 6, 35–46. [Google Scholar]
- Glicksmann, M.E. The solidification of metals; The Iron and Steel Institute: London, UK, 1967; p. 43. [Google Scholar]
- Huang, W.D.; Wang, W.L.; Lin, X.; Wang, M. In situ observation of SSM microstructure formation during cooling slope casting of an NH4Cl-H2O alloy with vibrating the slope. Solid State Phenom. 2006, 116–117, 193–196. [Google Scholar] [CrossRef]
- Trivedi, R.; Kurz, W. Dendritic growth. Int. Met. Rev. 1994, 39, 49–74. [Google Scholar] [CrossRef]
- Lu, C.W.; Chen, J.C.; Hu, L.J. A numerical investigation of the thermal distribution effects in a heat-exchanger-method crystal growth system. Model. Simul. Mater. Sci. Eng. 2002, 10, 147. [Google Scholar] [CrossRef]
- Shi, Y.; Qingyan, X.U.; Gong, M. Simulation of NH4Cl-H2O dendritic growth in directional solidification. Acta Metall. Sin. 2011, 47, 620–627. [Google Scholar]


| Property | Value |
|---|---|
| Density of the liquid phase, ρf (kg/m3) | 1078 |
| Density of the solid ammonium chloride, ρs (kg/m3) | 1527.4 |
| Dynamic viscosity of the liquid phase, µf (N s/m2) | 0.0013 |
| Thermal conductivity of the liquid phase, kf (W/m K) | 0.468 |
| Thermal conductivity of the solid phase, kfs (W/m K) | 2.7 |
| Specific heat of the liquid phase, cf (J/kg K) | 3249 |
| Specific heat of the solid phase, cs (J/kg K) | 1827 |
| Thermal expansion coefficient, βT (1/K) | 3.832 × 10−4 |
| Latent heat of fusion at TE, Δh (J/kg) | 3.138 × 105 |
| Eutectic temperature, TE (K) | 259.2 |
| Liquidus temperature, TL (K) | 310.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, R.; Li, B.; Liu, Z.; Bu, L.; Li, X.; Yang, X.; Tsukihashi, F. Experimental Investigation of Solidification in the Cast Mold with a Consumable Cooler Introduced Inside. Metals 2019, 9, 55. https://doi.org/10.3390/met9010055
Niu R, Li B, Liu Z, Bu L, Li X, Yang X, Tsukihashi F. Experimental Investigation of Solidification in the Cast Mold with a Consumable Cooler Introduced Inside. Metals. 2019; 9(1):55. https://doi.org/10.3390/met9010055
Chicago/Turabian StyleNiu, Ran, Baokuan Li, Zhongqiu Liu, Lifu Bu, Xianglong Li, Xiao Yang, and Fumitaka Tsukihashi. 2019. "Experimental Investigation of Solidification in the Cast Mold with a Consumable Cooler Introduced Inside" Metals 9, no. 1: 55. https://doi.org/10.3390/met9010055




