Effect of Small Additions of Cr, Ti, and Mn on the Microstructure and Hardness of Al–Si–Fe–X Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
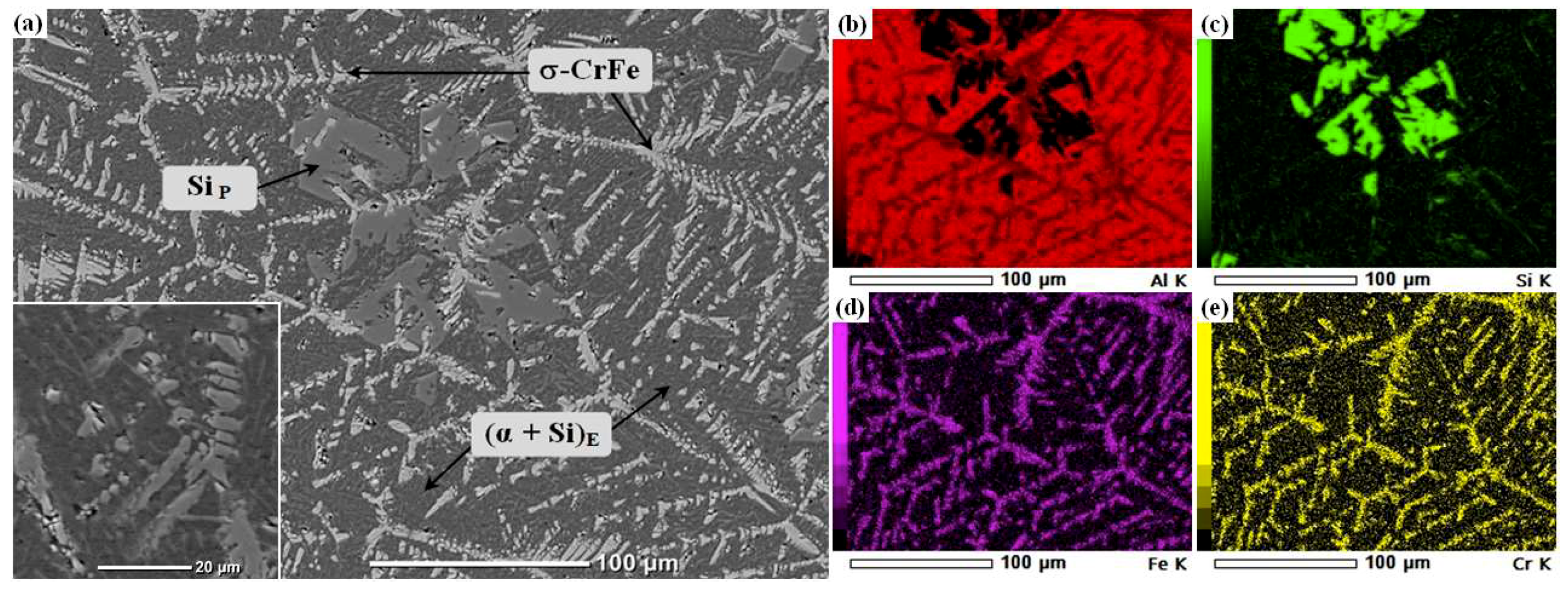
3.1. Analysis of the Al–20Si–5Fe Master Alloy
3.2. Analysis of Al–20S–5Fe Base Alloy with Chromium Additions
3.3. Analysis of Al–20Si–5Fe Base Alloy with Titanium Additions
3.4. Analysis of Al–20Si–5Fe Base Alloy with Manganese Additions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Efffect of Cr, Ti, V and Zr Micro-additions on Microstructure and Mechanical Properties of the Al–Si–Cu–Mg cast alloy. Metall. Mater. Trans. A 2016, 47, 2396–2409. [Google Scholar] [CrossRef]
- Gustafsson, G.; Thorvaldsson, T.; Dunlop, G.L. The Influence of Fe and Cr on the Microstructure of Cast Al–Si–Mg Alloys. Metall. Mater. Trans. A 1986, 17, 45–52. [Google Scholar] [CrossRef]
- Dám, K.; Prus, F.; Vojtech, D. Structural and mechanical characteristics of the Al–23Si–8Fe–5Mn alloy prepared by combination of centrifugal spraying and hot die forging. Mat. Sci. Eng. A-Struct. 2014, 610, 197–202. [Google Scholar] [CrossRef]
- Gorny, A.; Manickaraj, J.; Cai, Z.; Shankar, S. Evolution of Fe based intermetallic phases in Al–Si hypoeutectic casting alloys: Influence of the Si and Fe concentrations, and solidification rate. J. Alloy. Compd. 2013, 577, 103–124. [Google Scholar] [CrossRef]
- Khalif, W.; Samuel, F.H.; Gruzleski, J.E.; Doty, H.W.; Valtierr, S. Nucleation of Fe-Intermetalic Phases in the Al–Si–Fe Alloys. Metall. Mater. Trans. A 2005, 36, 1017–1032. [Google Scholar] [CrossRef]
- Khalif, W.; Samuel, F.H.; Gruzleski, J.E. Iron Intermetallic Phases in the Al Corner of the Al–Si–Fe. Metall. Mater. Trans. A 2003, 34, 807–825. [Google Scholar] [CrossRef]
- Pontevichi, S.; Bosselet, F.; Barbeau, F.; Peronnet, M.; Vial, J.C. Solid-Liquid Phase Equilibria in the Al–Fe–Si System at 727 °C. J. Phase Equilib. Diffus. 2004, 25, 528–537. [Google Scholar] [CrossRef]
- Lu, L.; Dahle, A.K. Iron-Rich Intermetallic Phases and Their Role in Casting Defect Formation in Hypoeutectic Al–Si Alloys. Metall. Mater. Trans. A 2005, 36, 819–835. [Google Scholar]
- Narayanan, L.A.; Samuel, F.H.; Gruzleski, J.E. Dissolution of Iron Intermetallics in Al-Si Alloys through Nonequilibrium Heat Treatment. Metall. Mater. Trans. A 1995, 26, 2161–2174. [Google Scholar] [CrossRef]
- Ferraro, S.; Bjurenstedt, A.; Seifeddine, S. On the Formation of Sludge Intermetallic Particles in Secondary Aluminum Alloys. Metall. Mater. Trans. A 2015, 46, 3713–3722. [Google Scholar] [CrossRef]
- Wang, B.; Xue, S.; Wang, J.; Lin, Z. Effect of combinative addition of mischmetal and Titanium on the microstructure and mechanical properties of hypoeutectic Al–Si alloys used for brazing and/or welding consumables. J Rare Earths 2017, 35, 193–202. [Google Scholar] [CrossRef]
- Guan, K.S.; Xu, X.D.; Zhang, Y.Y.; Wang, Z.W. Cracks and precipitate phases in 321 stainless steel weld of flue gas pipe. Eng. Fail. Anal. 2005, 12, 623–633. [Google Scholar] [CrossRef]
- Chen, M.; Lan, L.; Shi, X.; Yang, H.; Zhang, M.; Quiao, J. The tribological properties of Al0.6CoCrFeNi high-entropy alloy with the σ phase precipitation at elevated temperature. J. Alloy. Compd. 2019, 777, 180–189. [Google Scholar] [CrossRef]
- Tang, Z.; Williams, J.J.; Tho, A.J.; Akinc, M. High temperature oxidation behavior of Ti5Si3-based intermetallics. Intermetallics 2008, 16, 1118–1124. [Google Scholar] [CrossRef]
- Swanson, T. Standard X-ray Diffraction Powder Patterns; Dept. of Commerce, National Bureau of Standards: Washington, DC, USA, 1953; Volume I, p. 11.
- Bond, W.L.; Kaiser, W. Calculated from ICSD using POWD-12++ (1997). J. Phys. Chem. Solids 1960, 16, 44. [Google Scholar] [CrossRef]
- Zeng, L. ICDD Grant-in-Aid (2001); Schubert, K., Burkhardt, K., Gold, W., Panday, P., Naturwissenschaften, Eds.; Guangxi Univ., Inst. of Materials Science: Nanning, Guangxi, China, 1965; Volume 52, p. 301. [Google Scholar]
- Yakel, H.L. Atom Distributions in Sigma Phases: I. Fe and Cr Atom Distributions in a Binary Sigma Phase Equilibrated at 1063, 1013 and 923 K. Acta Crystallogr. Sec. B Struct. Sci. 1983, 39, 20. [Google Scholar] [CrossRef]
- Kajitani, T.; Kawase, T.; Yamad, K.; Hirabayashi, M. Site Occupation and Local Vibration of Hydrogen Isotopes in Hexagonal Ti5Si3 H(D)1-x. Trans. Jpn. Inst. Met. 1986, 27, 639. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Sumiyam, K.; Ohshim, N.; Nakamur, Y. Magnetic Properties of Metastable α-Mn Type Mn1-yFey Alloys Produced by Vapor Quenching. Phys. Status Solidi A 1986, 98, 229. [Google Scholar] [CrossRef]
















| Nominal Composition (wt. %) | Experimental Composition (wt. %) | |||||
|---|---|---|---|---|---|---|
| Alloy | Al | Si | Fe | Cr | Ti | Mn |
| Al 20Si 5Fe | 79.0 | 16.0 | 4.9 | - | - | - |
| Al 20Si 5Fe 1Cr | 79.1 | 15.5 | 4.4 | 1.2 | - | - |
| Al 20Si 5Fe 3Cr | 76.3 | 17.4 | 3.7 | 2.6 | - | - |
| Al 20Si 5Fe 5Cr | 75.1 | 16.1 | 3.0 | 5.9 | - | - |
| Al 20Si 5Fe 1Ti | 78.9 | 13.1 | 6.7 | - | 1.3 | - |
| Al 20Si 5Fe 3Ti | 78.1 | 14.4 | 4.4 | - | 3.0 | - |
| Al 20Si 5Fe 5Ti | 74.2 | 13.1 | 5.7 | - | 7.3 | - |
| Al 20Si 5Fe 1Mn | 79.8 | 15.1 | 4.4 | - | - | 0.8 |
| Al 20Si 5Fe 3Mn | 78.3 | 14.8 | 4.3 | - | - | 2.6 |
| Al 20Si 5Fe 5Mn | 74.3 | 17.8 | 3.7 | - | - | 4.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda, V.A.; Figueroa, I.A.; González, G.; García-Hinojosa, J.A.; Lara-Rodríguez, G.A. Effect of Small Additions of Cr, Ti, and Mn on the Microstructure and Hardness of Al–Si–Fe–X Alloys. Metals 2019, 9, 136. https://doi.org/10.3390/met9020136
Aranda VA, Figueroa IA, González G, García-Hinojosa JA, Lara-Rodríguez GA. Effect of Small Additions of Cr, Ti, and Mn on the Microstructure and Hardness of Al–Si–Fe–X Alloys. Metals. 2019; 9(2):136. https://doi.org/10.3390/met9020136
Chicago/Turabian StyleAranda, Víctor A., Ignacio A. Figueroa, Gonzalo González, J. Alejandro García-Hinojosa, and Gabriel A. Lara-Rodríguez. 2019. "Effect of Small Additions of Cr, Ti, and Mn on the Microstructure and Hardness of Al–Si–Fe–X Alloys" Metals 9, no. 2: 136. https://doi.org/10.3390/met9020136





