Age-Induced Precipitation and Hardening Behavior of Ni3Al Intermetallic Alloys Containing Vanadium
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
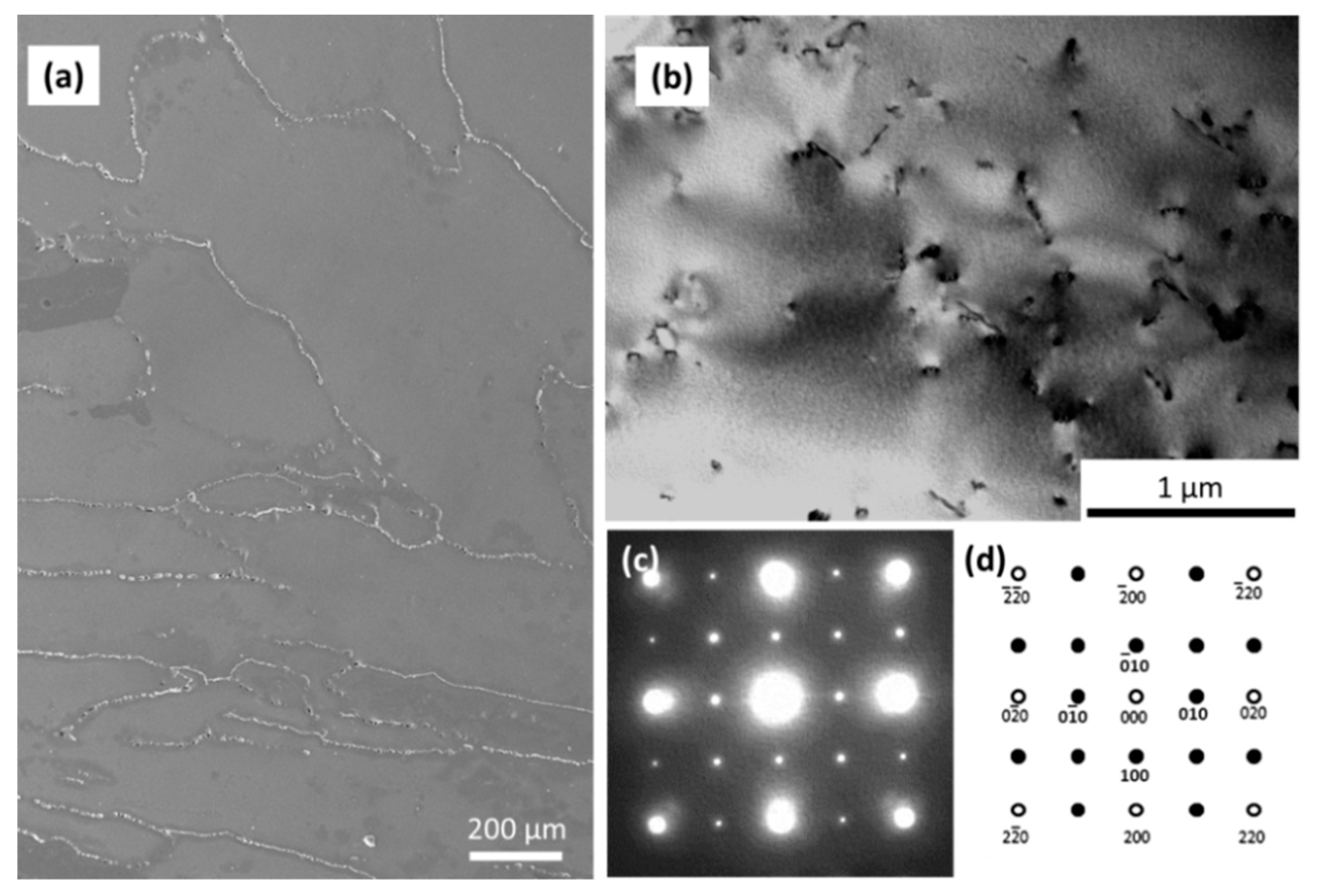
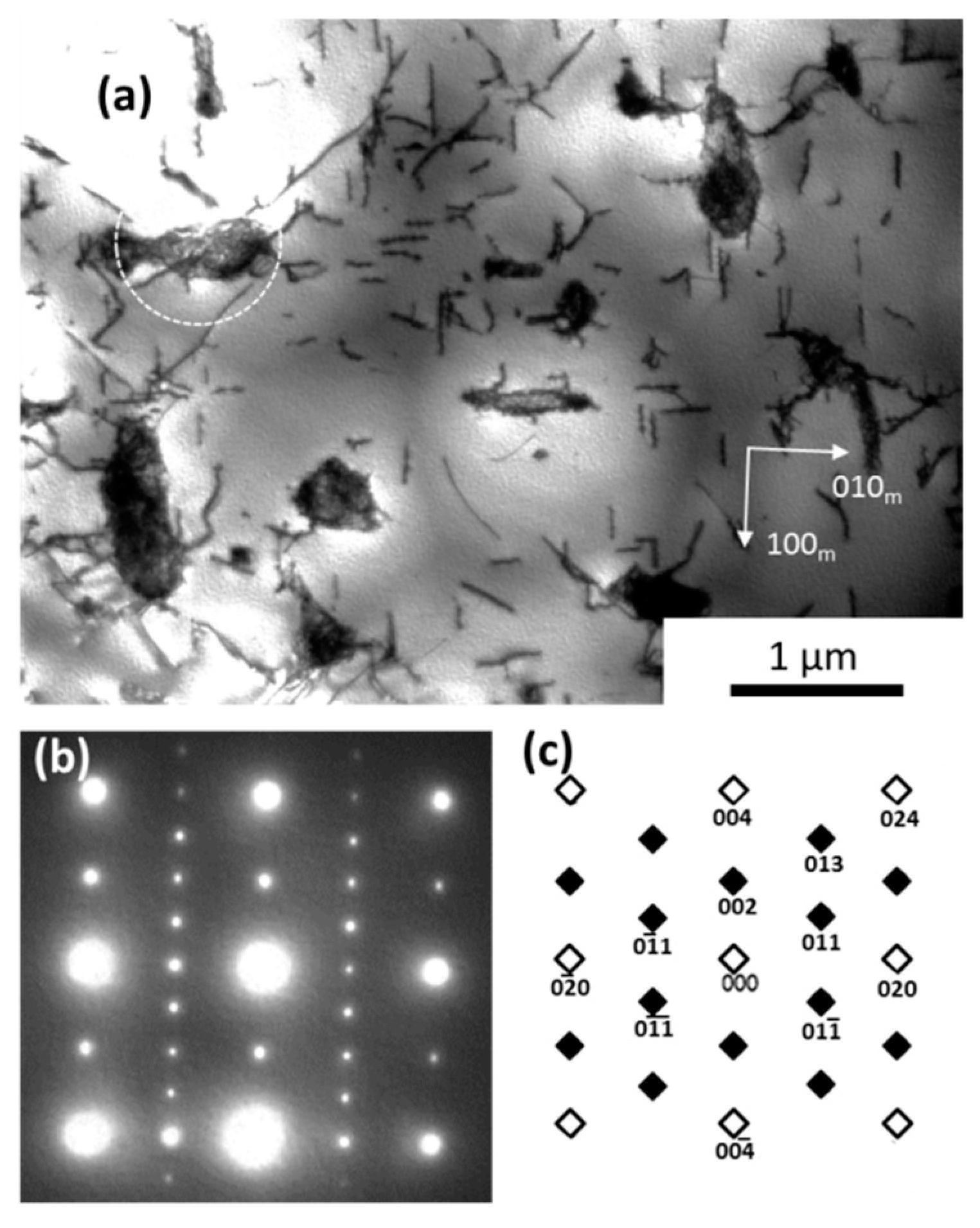
3.1. Microstructure
3.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noguchi, O.; Oya, Y.; Suzuki, T. The effect of non-stoichiometry on the positive temperature-dependence of strength of Ni3Al and Ni3Ga. Metall. Trans. A 1981, 12A, 1647–1654. [Google Scholar] [CrossRef]
- Pope, D.P.; Ezz, S.S. Mechanical properties of Ni3Al and nickel-base alloys with high volume fraction of γ′. Int. Metal. Rev. 1984, 29, 136–167. [Google Scholar]
- Suzuki, T.; Mishima, Y.; Miura, S. Evaluation of the data for the plastic behavior of Ni3(Al,X) single-crystals. Mater. Sci. Eng. A 1991, 146, 245–260. [Google Scholar] [CrossRef]
- Webb, G.; Debussac, A.; Antolovich, S.D. An analytical description for the deformation response of Ni3Al alloys in the anomalous regime. Metall. Trans. A 1993, 24A, 397–401. [Google Scholar] [CrossRef]
- Hirsch, P.B. A new theory of the anomalous yield stress in L12 alloys. Philos. Mag. A 1992, 65, 569–612. [Google Scholar] [CrossRef]
- Hemker, K.J.; Mills, M.J. Measurements of antiphase boundary and complex stacking-fault energies in binary and B-doped Ni3Al using TEM. Philos. Mag. A 1993, 68, 305–324. [Google Scholar] [CrossRef]
- Lapin, J. Effect of ageing on the microstructure and mechanical behavior of a directionally solidified Ni3Al-based alloy. Intermetallics 1997, 5, 615–624. [Google Scholar] [CrossRef]
- Demura, M.; Hirano, T. Stress response by the strain-rate change in a binary stoichiometric Ni3Al single crystal. Philos. Mag. Lett. 1997, 75, 143–148. [Google Scholar] [CrossRef]
- Demura, M.; Kishida, K.; Suga, Y.; Hirano, T. Room-temperature mechanical properties of cold-rolled thin foils of binary, stoichiometric Ni3Al. Metall. Mater. Trans. A 2002, 33A, 2607–2613. [Google Scholar] [CrossRef]
- Kruml, T.; Conforto, E.; Lo Piccolo, B.; Caillard, D.; Martin, J.L. From dislocation cores to strength and work-hardening: a study of binary Ni3Al. Acta Metall. 2002, 50, 5091–5101. [Google Scholar] [CrossRef]
- Wang, G.T.; Ding, H.S.; Chen, R.R.; Guo, J.J.; Fu, H.Z. Effect of current intensity on microstructure of Ni3Al intermetallics prepared by directional solidification electromagnetic cold crucible technique. Acta Metall. Sin. 2017, 53, 1461–1468. [Google Scholar]
- Nunomura, Y.; Kaneno, Y.; Tsuda, H.; Takasugi, T. Phase relation and microstructure in multi-phase intermetallic alloys based on Ni3Al-Ni3Ti-Ni3V pseudo-ternary alloy system. Intermetallics 2004, 12, 389–399. [Google Scholar] [CrossRef]
- Shibuya, S.; Kaneno, Y.; Yoshida, M.; Takasugi, T. Dual multi-phase intermetallic alloys composed of geometrically close packed Ni3X (X: Al, Ti and V) type structures-II. Mechanical properties. Acta Mater. 2006, 54, 861–870. [Google Scholar] [CrossRef]
- Kawahara, K.; Kaneno, Y.; Kakitsuji, A.; Takasugi, T. Microstructural factors affecting hardness property of dual two-phase intermetallic alloys based on Ni3Al-Ni3V pseudo-binary alloy system. Intermetallics 2009, 17, 938–944. [Google Scholar] [CrossRef]
- Edatsugi, D.; Kaneno, Y.; Semboshi, S.; Takasugi, T. Fine precipitation in channel region of two-phase Ni3Al and Ni3V intermetallic alloys containing Mo and W. Metall. Mater. Trans. A 2016, 47A, 998–1008. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Gopinath, K.; Gogia, A.K.; Kamat, S.V.; Balamuralikrishnan, R.; Ramamurty, U. Tensile properties of Ni-based superalloy 720Li: Temperature and strain rate effects. Metall. Mater. Trans. A 2008, 39A, 2340–2350. [Google Scholar] [CrossRef]
- Burns, D.E.; Zhang, Y.; Teutsh, M.; Bade, K.; Aktaa, J.; Hemker, K.J. Development of Ni-based superalloys for microelectromechanical systems. Scripta Mater. 2012, 67, 459–462. [Google Scholar] [CrossRef]
- Smith, T.M.; Bonacuse, P.; Sosa, J.; Kulis, M.; Evans, L. A quantifiable and automated volume fraction characterization technique for secondary and tertiary gamma’ precipitates in Ni-based superalloys. Mater. Charact. 2018, 140, 86–94. [Google Scholar] [CrossRef]
- Knowles, A.J.; Reynolds, L.; Vorontsov, V.A.; Dye, D. A nickel based superalloy reinforced by both Ni3Al and Ni3V ordered-fcc precipitates. Scripta Mater. 2019, 162, 472–476. [Google Scholar] [CrossRef]
- Aoki, K. Ductilization of L12 intermetallic compound Ni3Al by microalloying with boron. Mater. Trans. JIM 1990, 31, 443–448. [Google Scholar] [CrossRef]
- Liu, C.T.; White, C.L.; Horton, J.A. Effect of boron on grain-boundaries in Ni3Al. Acta Metall. 1985, 33, 213–229. [Google Scholar] [CrossRef]
- Taub, A.I.; Briant, C.L. Composition dependence of ductility in boron-doped, nickel-base L12 alloys. Acta Metall. 1987, 35, 1597–1603. [Google Scholar] [CrossRef]
- Varin, R.A.; Winnicka, M.B. Plasticity of structural intermetallic compounds. Mater. Sci. Eng. A 1991, 137, 93–103. [Google Scholar] [CrossRef]
- Ochiai, S.; Oya, Y.; Suzuki, T. Alloying behavior of Ni3Al, Ni3Ga, Ni3Si and Ni3Ge. Acta Metall. 1984, 32, 289–298. [Google Scholar] [CrossRef]
- Mishima, Y.; Ochiai, S.; Suzuki, T. Lattice-parameters of Ni(γ), Ni3Al(γ’) and Ni3Ga(γ’) solid-solutions with additions of transition and B-subgroup elements. Acta Metall. 1985, 33, 1161–1169. [Google Scholar] [CrossRef]
- Mishima, Y.; Ochiai, S.; Hamao, N.; Yodogawa, M.; Suzuki, T. Mechanical-properties of Ni3Al with ternary addition of transition-metal elements. Trans. JPN. Inst. Met. 1986, 27, 648–655. [Google Scholar] [CrossRef]
- Tain, W.H.; Sano, T.; Nemoto, M. Hardening of ordered γ′-Ni3(Al,Ti) by precipitation of ordered γ. Scripta Met. 1986, 20, 933–936. [Google Scholar]
- Ma, Y.; Ardell, J.A. Coarsening of γ (Ni–Al solid solution) precipitates in a γ′ (Ni3Al) matrix. Acta Mater. 2007, 55, 4419–4427. [Google Scholar] [CrossRef]
- Arzt, E.; Grahle, P. Dispersion strengthening of disordered and ordered metallic materials: From dislocation mechanisms to new alloys. Z. Metallkd. 1996, 87, 874–884. [Google Scholar]
- Arzt, E.; Behr, R.; Gohring, E.; Grahle, P.; Mason, R.P. Dispersion strengthening of intermetallics. Mater. Sci. Eng. A 1997, 234–236, 22–29. [Google Scholar] [CrossRef]
- Meng, J.; Jia, C.C.; He, Q. Fabrication of oxide-reinforced Ni3Al composites by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2006, 434, 246–249. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sato, K.; Hayashi, E.; Osaka, T.; Konno, T.J.; Kaneno, Y.; Takasugi, T. Alloying effects on the phase equilibria among Ni(A1), Ni3Al(L1(2)) and Ni3V(D0(22)) phases. Intermetallics 2012, 23, 68–75. [Google Scholar] [CrossRef]
- Soffa, W.A.; Laughlin, D.E. High-strength age hardening copper-titanium alloys: redivivus. Prog. Mater. Sci. 2004, 49, 347–366. [Google Scholar] [CrossRef]
- Semboshi, S.; Tsuda, H.; Kanano, Y.; Iwase, A.; Takasugi, T. Thermal conductivity of Ni3V–Ni3Al pseudo-binary alloys. Intermetallics 2015, 59, 1–7. [Google Scholar] [CrossRef]
- Ioroi, K.; Kaneno, Y.; Semboshi, S.; Takasugi, T. Effect of transition metal addition on microstructure and hardening behavior of two-phase Ni3Al-Ni3V intermetallic alloys. Materialia. in press. [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 110–184. [Google Scholar]
- Lumley, R.N.; Polmear, I.J.; Morton, A.J. Interrupted aging and secondary precipitation in aluminium alloys. Mater. Sci. Technol. 2003, 19, 1483–1490. [Google Scholar] [CrossRef]
- Khan, I.N.; Starink, M.J.; Yan, J.L. A model for precipitation kinetics and strengthening in Al-Cu-Mg alloys. Mater. Sci. Eng. A 2008, 472, 66–74. [Google Scholar] [CrossRef]
- Semboshi, S.; Amano, S.; Fu, J.; Iwase, A.; Takasugi, T. Kinetics and equilibrium of age-induced precipitation in Cu−Ti binary alloys. Metall. Mater. Trans. A 2017, 48, 1501–1511. [Google Scholar] [CrossRef]
- Ma, J.; Hornbuckle, B.C.; Karaman, I.; Thompson, G.B.; Luo, Z.P.; Chumlyakov, Y.I. The effect of nanoprecipitates on the superelastic properties of FeNiCoAlTa shape memory alloy single crystals. Acta Mater. 1972, 61, 3445–3455. [Google Scholar]
- Kaneno, Y.; Myoki, T.; Takasugi, T. Tensile properties of L12 intermetallic foils fabricated by cold rolling. Int. J. Mater. Res. 2008, 99, 1229–1236. [Google Scholar] [CrossRef]
- Suzuki, T.; Mishima, Y.; Miura, S. Plastic Behavior in Ni3(Al,X) single-crystal-temperature, strain-rate, orientation and composition. ISIJ Int. 1989, 29, l–23. [Google Scholar] [CrossRef]
- Masahashi, N.; Takasugi, T.; Izumi, O. High-temperature strength and ductility of L12-type Ni3Al-Ni3Mn intermetallic compound. J. Mater. Sci. 1987, 22, 2599–2608. [Google Scholar] [CrossRef]
- Masahashi, N.; Takasugi, T.; Izumi, O. High-temperature strength and ductility of recrystallized Ni3Al-Ni3Mn alloys. Metall. Trans. A 1988, 19, 345–352. [Google Scholar] [CrossRef]
- Masahashi, N.; Kawazoe, H.; Takasugi, T.; Izumi, O. Phase-relations in the section Ni3Al-Ni3Fe of the Al-Fe-Ni system. Z. Metallkd. 1987, 78, 788–794. [Google Scholar]
- Takasugi, T.; Nagashima, M.; Izumi, O. Strengthening and ductilization of Ni3Si by the addition of Ti elements. Acta Metall. 1990, 38, 747–755. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semboshi, S.; Sasaki, R.; Kaneno, Y.; Takasugi, T. Age-Induced Precipitation and Hardening Behavior of Ni3Al Intermetallic Alloys Containing Vanadium. Metals 2019, 9, 160. https://doi.org/10.3390/met9020160
Semboshi S, Sasaki R, Kaneno Y, Takasugi T. Age-Induced Precipitation and Hardening Behavior of Ni3Al Intermetallic Alloys Containing Vanadium. Metals. 2019; 9(2):160. https://doi.org/10.3390/met9020160
Chicago/Turabian StyleSemboshi, Satoshi, Ryosuke Sasaki, Yasuyuki Kaneno, and Takayuki Takasugi. 2019. "Age-Induced Precipitation and Hardening Behavior of Ni3Al Intermetallic Alloys Containing Vanadium" Metals 9, no. 2: 160. https://doi.org/10.3390/met9020160




