Elastic Properties of FeCr20Ni8Xn (X = Mo, Nb, Ta, Ti, V, W and Zr) Austenitic Stainless Steels: A First Principles Study
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussions
3.1. Elastic Properties of FeCr20Ni8
3.2. Solutes Effects on the Elastic Properties of FeCr20Ni8
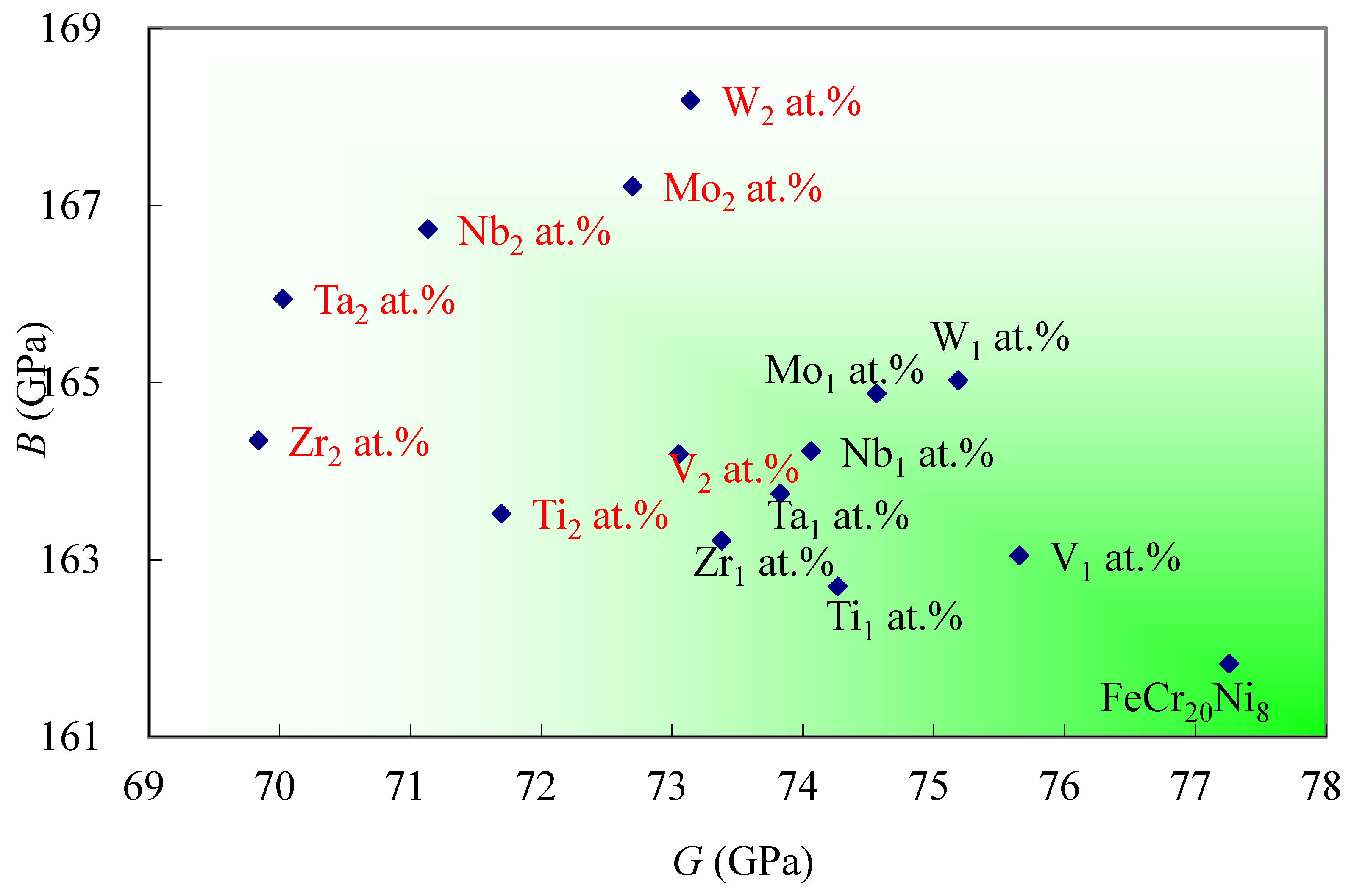
3.3. Design Map of FeCr20Ni8 Based Alloys
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, D.H.; Wang, Q.; Jiang, B.B.; Zhang, C.; Li, X.N.; Chen, G.Q.; Tang, R.; Zhang, R.Q.; Dong, C.; Liaw, P.K. Developing Fuel Cladding Fe-25cr-22ni Stainless Steels with High Microstructural Stabilities Via Mo/Nb/Ti/Ta/W Alloying. Mater. Sci. Eng. A 2018, 719, 27–42. [Google Scholar] [CrossRef]
- Morris, W.Y.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively Manufactured Hierarchical Stainless Steels with High Strength and Ductility. Nat. Mater. 2017, 17, 63–71. [Google Scholar]
- Donghui, W.; Jiang, B.; Wang, Q.; Yu, F.; Li, X.; Tang, R.; Zhang, R.; Chen, G.; Dong, C. Influences of Mo/Zr Minor-Alloying on the Phase Precipitation Behavior in Modified 310s Austenitic Stainless Steels at High Temperatures. Mater. Des. 2017, 128, 34–46. [Google Scholar]
- Hojná, A. Overview of Intergranular Fracture of Neutron Irradiated Austenitic Stainless Steels. Metals 2017, 7, 392. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Carboneras, M.; Arrabal, R. Influence of Ti, C and N Concentration on the Intergranular Corrosion Behaviour of Aisi 316ti and 321 Stainless Steels. Acta Mater. 2007, 55, 2239–2251. [Google Scholar] [CrossRef]
- Perron, A.; Toffolon-Masclet, C.; Ledoux, X.; Buy, F.; Guilbert, T.; Urvoy, S.; Bosonnet, S.; Marini, B.; Cortial, F.; Texier, G.; et al. Understanding Sigma-Phase Precipitation in a Stabilized Austenitic Stainless Steel (316nb) through Complementary Calphad-Based and Experimental Investigations. Acta Mater. 2014, 79, 16–29. [Google Scholar] [CrossRef]
- Kil, K.J.; Kim, Y.H.; Lee, B.H.; Kim, K.Y. New Findings on Intergranular Corrosion Mechanism of Stabilized Stainless Steels. Electrochim. Acta 2011, 56, 1701–1710. [Google Scholar]
- Yabuuchi, A.; Maekawa, M.; Kawasuso, A. Influence of Oversized Elements (Hf, Zr, Ti and Nb) on the Thermal Stability of Vacancies in Type 316l Stainless Steels. J. Nucl. Mater. 2012, 430, 190–193. [Google Scholar] [CrossRef]
- Bogdan, P. Effect of Nb and Ti Additions on Microstructure, and Identification of Precipitates in Stabilized Ni-Cr Cast Austenitic Steels. Mater. Charact. 2001, 47, 181–186. [Google Scholar]
- Vitos, L.; Korzhavyi, P.A.; Johansson, B. Elastic Property Maps of Austenitic Stainless Steels. Phys. Rev. Lett. 2002, 88, 155501. [Google Scholar] [CrossRef]
- Levente, V.; Korzhavyi, P.A.; Johansson, B. Modeling of Alloy Steels. Mater. Today 2002, 5, 14–23. [Google Scholar]
- Vijh, A.K. The Pitting Corrosion Potentials of Metals and Surface Alloys in Relation to Their Solid State Cohesion. Mater. Chem. Phys. 1988, 20, 371–380. [Google Scholar] [CrossRef]
- Gschneidner, K.A. Physical Properties and Interrelationships of Metallic and Semimetallic Elements. In Solid State Physics; Seitz, F., Turnbull, D., Eds.; Academic Press: Cambridge, MA, USA, 1964; pp. 275–426. [Google Scholar]
- Li, X.Q.; Zhao, J.J.; Xu, J.C.; Liu, X. Mechanical Properties and Defective Effects of 316ln Stainless Steel by First-Principles Simulations. J. Mater. Sci. Technol. 2011, 27, 1029–1033. [Google Scholar] [CrossRef]
- Clerc, D.G. Mechanical Hardness: Atomic-Level Calculations for Diamond-Like Materials. J. Mater. Sci. Lett. 1998, 17, 1461–1462. [Google Scholar] [CrossRef]
- Levente, V.; Korzhavyi, P.A.; Johansson, B. Stainless Steel Optimization from Quantum Mechanical Calculations. Nat. Mater. 2002, 2, 25–28. [Google Scholar]
- Reeh, S.; Music, D.; Ekholm, M.; Abrikosov, I.A.; Schneider, J.M. Elastic Properties of Fcc Fe-Mn-X (X = Cr, Co, Ni, Cu) Alloys from First-Principles Calculations. Phys. Rev. B 2013, 87, 224103. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Korzhavyi, P.; Johansson, B. Ab Initio Calculations of Elastic Properties of Fe-Cr-W Alloys. Comput. Mater. Sci. 2014, 84, 301–305. [Google Scholar] [CrossRef]
- Vitos, L. Total-Energy Method Based on the Exact Muffin-Tin Orbitals Theory. Phys. Rev. B 2001, 64, 014107. [Google Scholar] [CrossRef]
- Vitos, L. Computational Quantum Mechanics for Materials Engineers the Emto Method and Applications; Springer: London, UK, 2007. [Google Scholar]
- Vitos, L.; Abrikosov, I.A.; Johansson, B. Anisotropic Lattice Distortions in Random Alloys from First-Principles Theory. Phys. Rev. Lett. 2001, 87, 156401. [Google Scholar] [CrossRef]
- Soven, P. Coherent-Potential Model of Substitutional Disordered Alloys. Phys. Rev. 1967, 156, 809–813. [Google Scholar] [CrossRef]
- Gyorffy, B.L.; Pindor, A.J.; Staunton, J.; Stocks, G.M.; Winter, H. A First-Principles Theory of Ferromagnetic Phase Transitions in Metals. J. Phys. F Met. Phys. 1985, 15, 1337. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Vitos, L.; Kollár, J.; Skriver, H.L. Full Charge-Density Scheme with a Kinetic-Energy Correction: Application to Ground-State Properties of the 4d Metals. Phys. Rev. B 1997, 55, 13521–13527. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.L.; Ho, K.M. First-Principles Calculation of the Equilibrium Ground-State Properties of Transition Metals Applications to Nb and Mo. Phys. Rev. B 1983, 28, 5480–5486. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Hill, R. Elastic Properties of Reinforced Solids: Some Theoretical Principles. J. Mech. Phys. Solids 1963, 11, 357–372. [Google Scholar] [CrossRef]
- Voigt, W. Ueber Die Beziehung Zwischen Den Beiden Elasticitätsconstanten Isotroper Körper. Ann. Phys. 1889, 274, 573–587. [Google Scholar] [CrossRef]
- Reuss, A. Berechnung Der Fließgrenze Von Mischkristallen Auf Grund Der Plastizitätsbedingung Für Einkristalle. PMM J. Appl. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Ledbetter, H.M. Sound Velocities and Elastic Constants of Steels 304, 310, and 316. Met. Sci. 1980, 14, 595–596. [Google Scholar] [CrossRef]
- Geng, Y.J.; Wang, D.H.; Chen, B.Q.; Cui, F.Z. Orientation Relationships of Crn and Fe3O4 with Gamma—Phase in the High-Dose Nitrogen-Implanted 304 Stainless Steel. J. Phys. D Appl. Phys. 1995, 28, 226. [Google Scholar] [CrossRef]
- Ledbetter, H.M.; Kim, S.A. Molybdenum Effect on Fe-Cr-Ni-Alloy Elastic Constants. J. Mater. Res. 1988, 3, 40–44. [Google Scholar] [CrossRef]
- Wills, J.M.; Eriksson, O.; Söderlind, P.; Boring, A.M. Trends of the Elastic Constants of Cubic Transition Metals. Phys. Rev. Lett. 1992, 68, 2802–2805. [Google Scholar] [CrossRef] [PubMed]
- Hull, F.C. Delta Ferrite and Martensite Formation in Stainless Steels. Weld. J. 1973, 52, 193–203. [Google Scholar]
- Medvedeva, N.I.; van Aken, D.C.; Medvedeva, J.E. Stability of Binary and Ternary M23c6 Carbides from First Principles. Comput. Mater. Sci. 2015, 96, 159–164. [Google Scholar] [CrossRef]
- Moura, V.; Kina, A.Y.; Tavares, S.S.M.; Lima, L.D.; Mainier, F.B. Influence of Stabilization Heat Treatments on Microstructure, Hardness and Intergranular Corrosion Resistance of the Aisi 321 Stainless Steel. J. Mater. Sci. 2008, 43, 536–540. [Google Scholar] [CrossRef]
- Fullman, R.L. A Thermodynamic Model of the Effects of Composition on the Susceptibility of Austenitic Stainless Steels to Intergranular Stress Corrosion Cracking. Acta Metall. 1982, 7, 1407–1415. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent Developments in Stainless Steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Seo, W.-G.; Park, J.Y.; Lee, T.-H.; Kim, S. Influences of Mo on Stress Corrosion Cracking Susceptibility of Newly Developed Fecrmnninc-Based Lean Austenitic Stainless Steels. Mater. Charact. 2016, 119, 200–208. [Google Scholar] [CrossRef]


| Composition at.% | V | EBCC-EFCC | C11 | C12 | C44 | B | G | B/G | E | v |
|---|---|---|---|---|---|---|---|---|---|---|
| FeCr20Ni8 | 11.75 | −14.01 | 206.8 | 139.3 | 133.1 | 161.8 | 77.3 | 2.09 | 199.9 | 0.29 |
| FeCr20Ni8 | 11.75 a | - | 208.6 a | 143.5 a | 132.8 a | 165.2 a | 77.3 a | 2.13 a | - | - |
| FeCr19.7Ni8.9 | 11.83 b | - | - | - | - | 158.2 c | 77.4 c | 2.04 c | 199.6 | 0.29 |
| FeCr20Ni8Mo1 | 11.85 | −17.28 | 207.4 | 143.6 | 130.2 | 164.9 | 74.6 | 2.21 | 194.3 | 0.30 |
| FeCr20Ni8Mo2 | 11.95 | −20.68 | 208.6 | 146.5 | 126.9 | 167.2 | 72.7 | 2.30 | 190.4 | 0.31 |
| FeCr20Ni8Nb1 | 11.88 | −18.31 | 206.7 | 143.0 | 128.9 | 164.2 | 74.1 | 2.22 | 193.1 | 0.30 |
| FeCr20Ni8Nb2 | 12.01 | −22.99 | 206.5 | 146.9 | 125.7 | 166.7 | 71.1 | 2.34 | 186.8 | 0.31 |
| FeCr20Ni8Ta1 | 11.88 | −18.78 | 206.2 | 142.5 | 128.1 | 163.7 | 73.8 | 2.22 | 192.5 | 0.30 |
| FeCr20Ni8Ta2 | 12.01 | −23.13 | 204.3 | 146.8 | 125.1 | 165.9 | 70.0 | 2.37 | 184.1 | 0.32 |
| FeCr20Ni8Ti1 | 11.81 | −17.82 | 205.0 | 141.6 | 129.7 | 162.7 | 74.3 | 2.19 | 193.3 | 0.30 |
| FeCr20Ni8Ti2 | 11.88 | −21.77 | 204.4 | 143.1 | 125.2 | 163.5 | 71.7 | 2.28 | 187.6 | 0.31 |
| FeCr20Ni8V1 | 11.77 | −16.60 | 207.1 | 141.0 | 130.4 | 163.0 | 75.7 | 2.16 | 196.5 | 0.30 |
| FeCr20Ni8V2 | 11.80 | −19.32 | 205.5 | 143.5 | 128.1 | 164.2 | 73.1 | 2.25 | 190.8 | 0.31 |
| FeCr20Ni8W1 | 11.91 | −17.28 | 208.0 | 143.6 | 131.1 | 165.0 | 75.2 | 2.19 | 195.8 | 0.30 |
| FeCr20Ni8W2 | 12.02 | −20.54 | 209.2 | 147.7 | 128.9 | 168.2 | 73.1 | 2.30 | 191.6 | 0.31 |
| FeCr20Ni8Zr1 | 11.93 | −19.86 | 205.1 | 142.3 | 127.9 | 163.2 | 73.4 | 2.22 | 191.4 | 0.30 |
| FeCr20Ni8Zr2 | 12.10 | −25.03 | 203.5 | 144.8 | 123.1 | 164.3 | 69.8 | 2.35 | 183.5 | 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Luo, H.; Zhang, J. Elastic Properties of FeCr20Ni8Xn (X = Mo, Nb, Ta, Ti, V, W and Zr) Austenitic Stainless Steels: A First Principles Study. Metals 2019, 9, 145. https://doi.org/10.3390/met9020145
Dou Y, Luo H, Zhang J. Elastic Properties of FeCr20Ni8Xn (X = Mo, Nb, Ta, Ti, V, W and Zr) Austenitic Stainless Steels: A First Principles Study. Metals. 2019; 9(2):145. https://doi.org/10.3390/met9020145
Chicago/Turabian StyleDou, Yuchen, Hong Luo, and Jing Zhang. 2019. "Elastic Properties of FeCr20Ni8Xn (X = Mo, Nb, Ta, Ti, V, W and Zr) Austenitic Stainless Steels: A First Principles Study" Metals 9, no. 2: 145. https://doi.org/10.3390/met9020145




