Microstructure and Martensitic Transformation Behavior in Thermal Cycled Equiatomic CuZr Shape Memory Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. The Crystal Structure and Microstructural Feature of the Water-Quenched Equiatomic CuZr Alloy
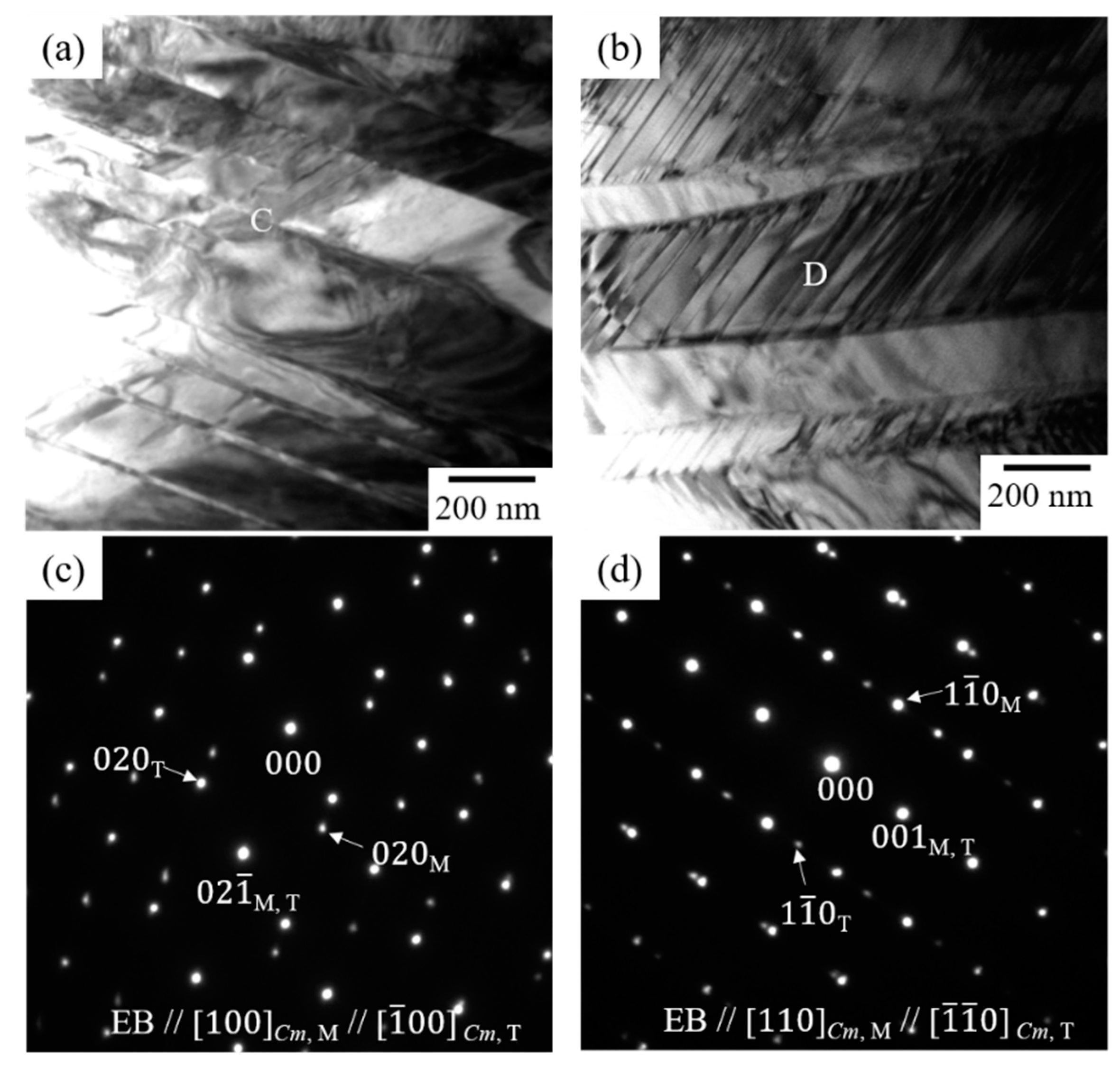
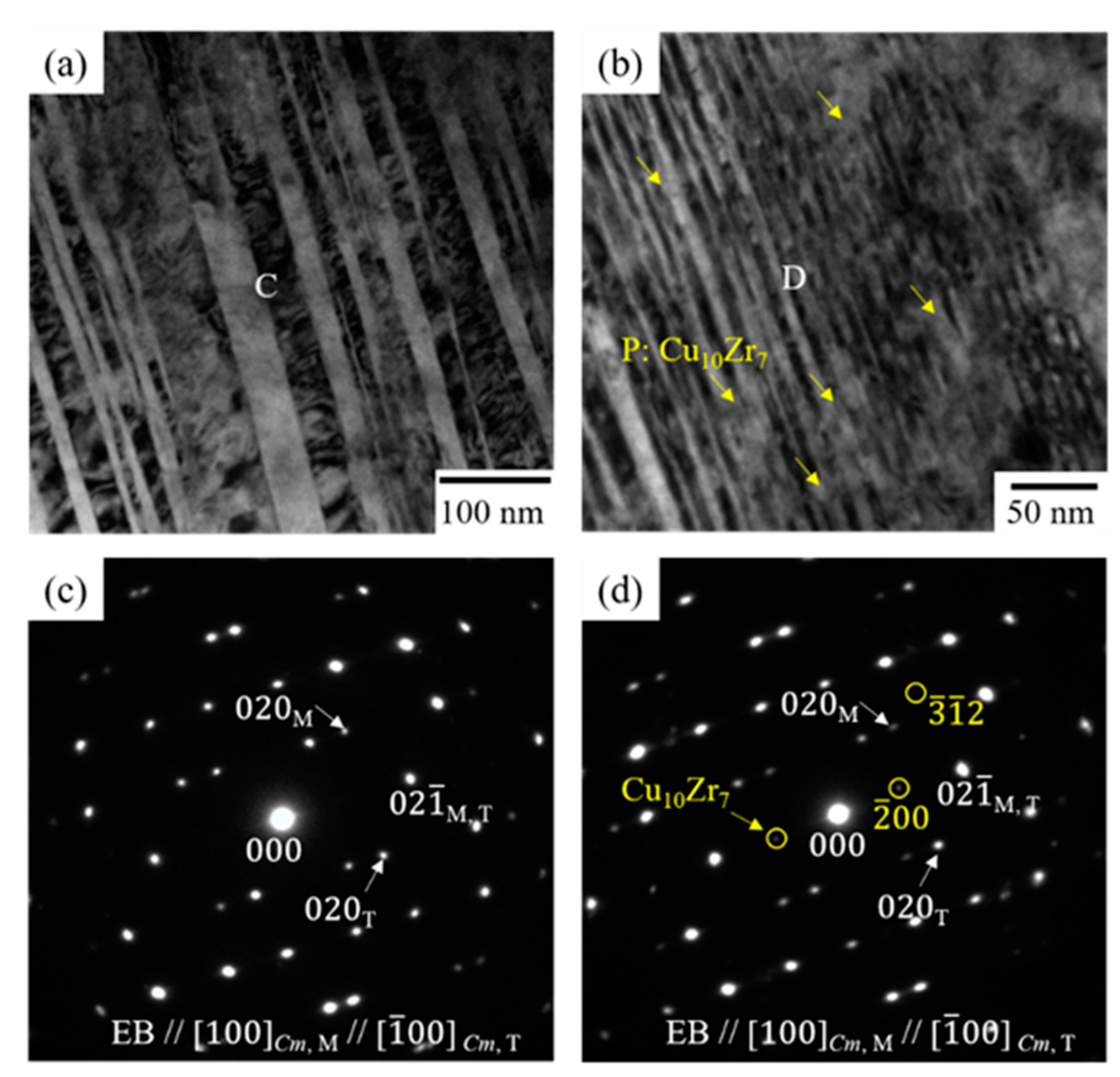
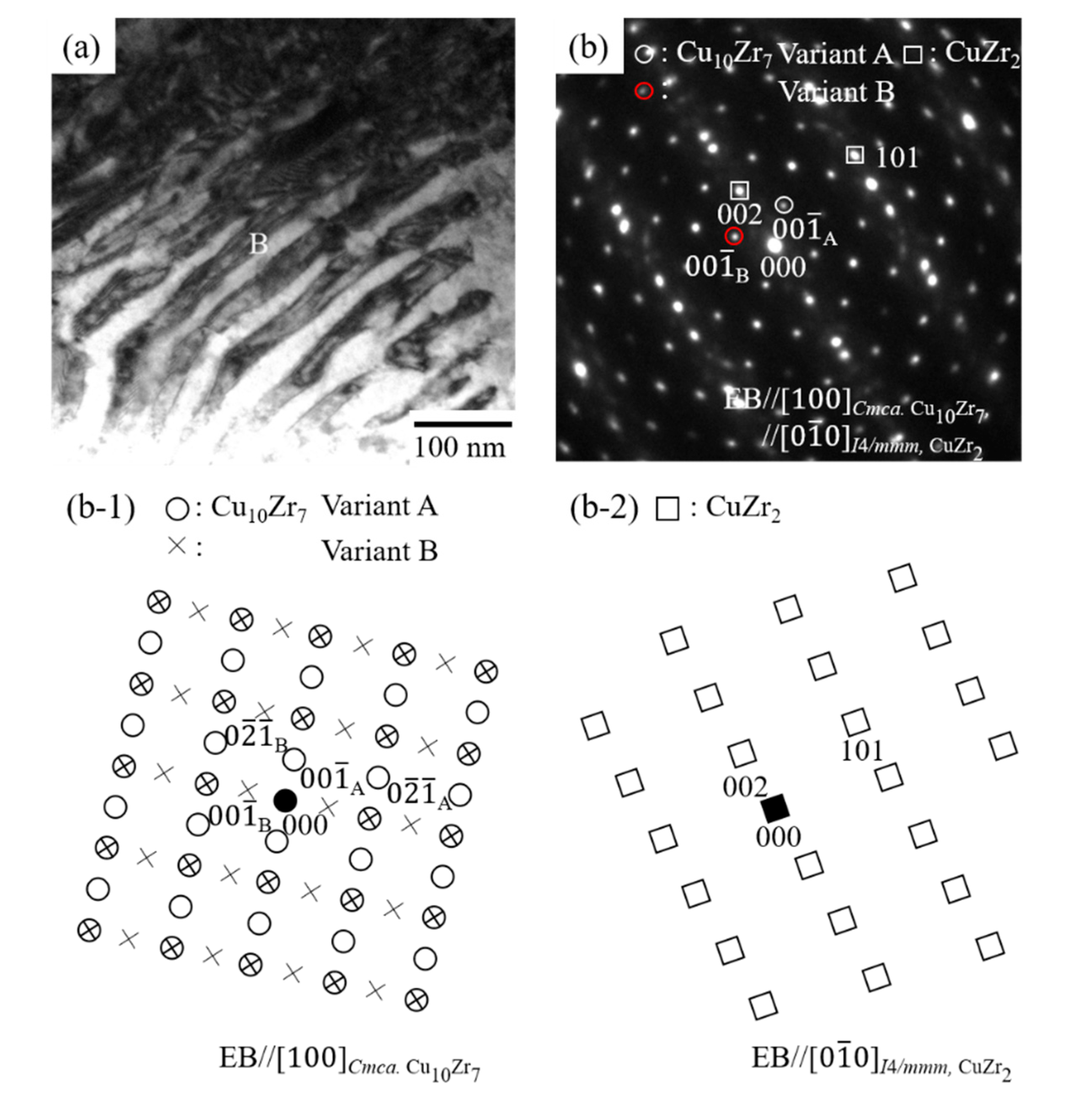
3.2. Effect of Thermal Cycling on the Microstructure in Equiatomic CuZr Alloy
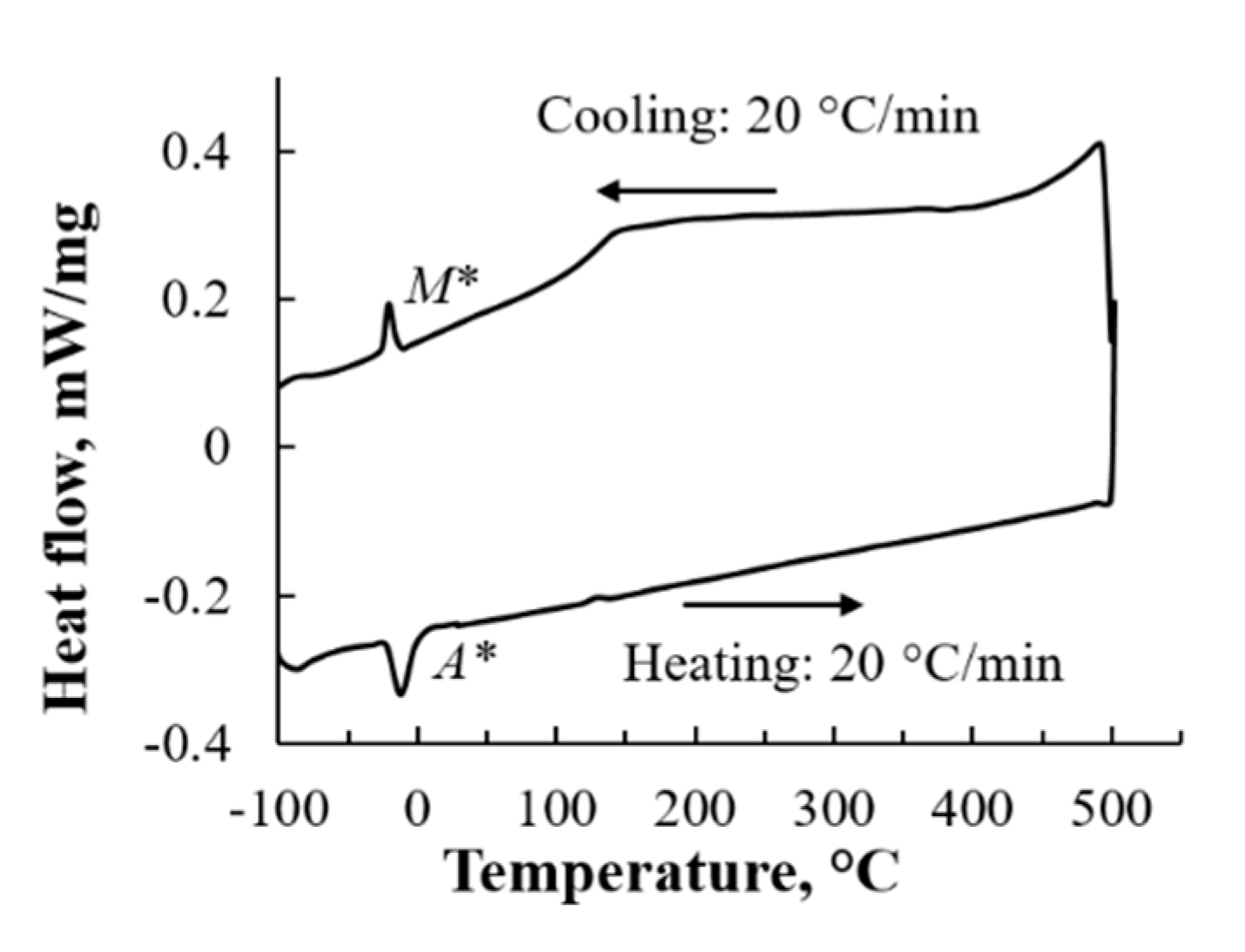
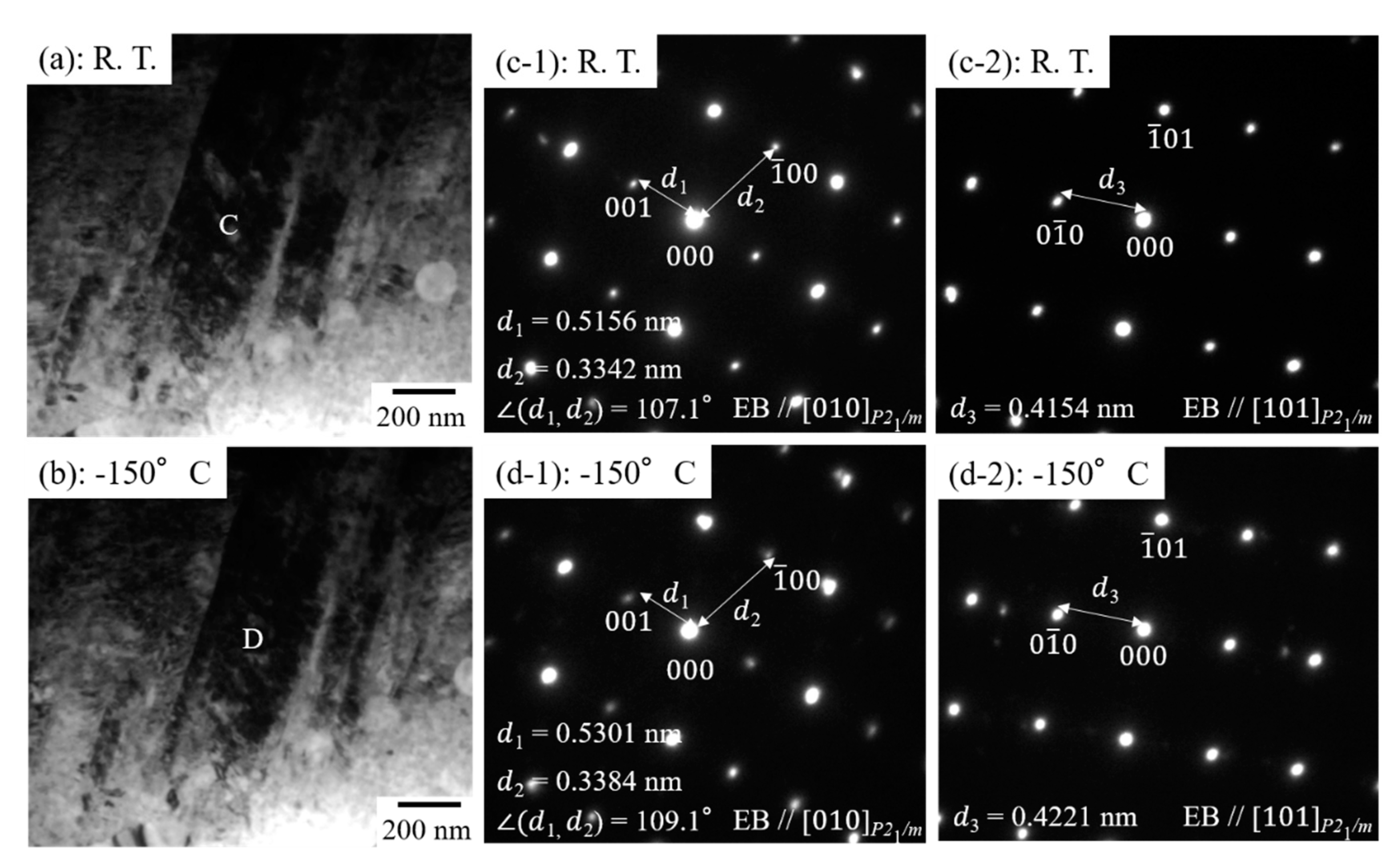
3.3. The Martensitic Transformation Behavior in Equiatomic CuZr Alloy Subjected to Thermal Cycling
4. Conclusions
- In the 10th cycled equiatomic CuZr alloy (the temperature range of DSC measurement: −100 °C to 400 °C), three structures were observed by TEM; I: The martensitic variant, II: The structure in which Cu10Zr7 precipitates exist inside the martensitic variant, III: The structure in which the lamellar consisting of Cu10Zr7 and CuZr2 phases and martensitic variants are mixed. TEM observation of the 10th cycled equiatomic CuZr alloy (the temperature range of DSC measurement: −100 °C to 500 °C) showed two structures; I: The martensitic variant, II: The lamellar structure in which the CuZr martensitic phase was completely eutectoid transformed. This fact indicated that the eutectoid decomposition occurred in equiatomic CuZr alloy during the thermal cycling.
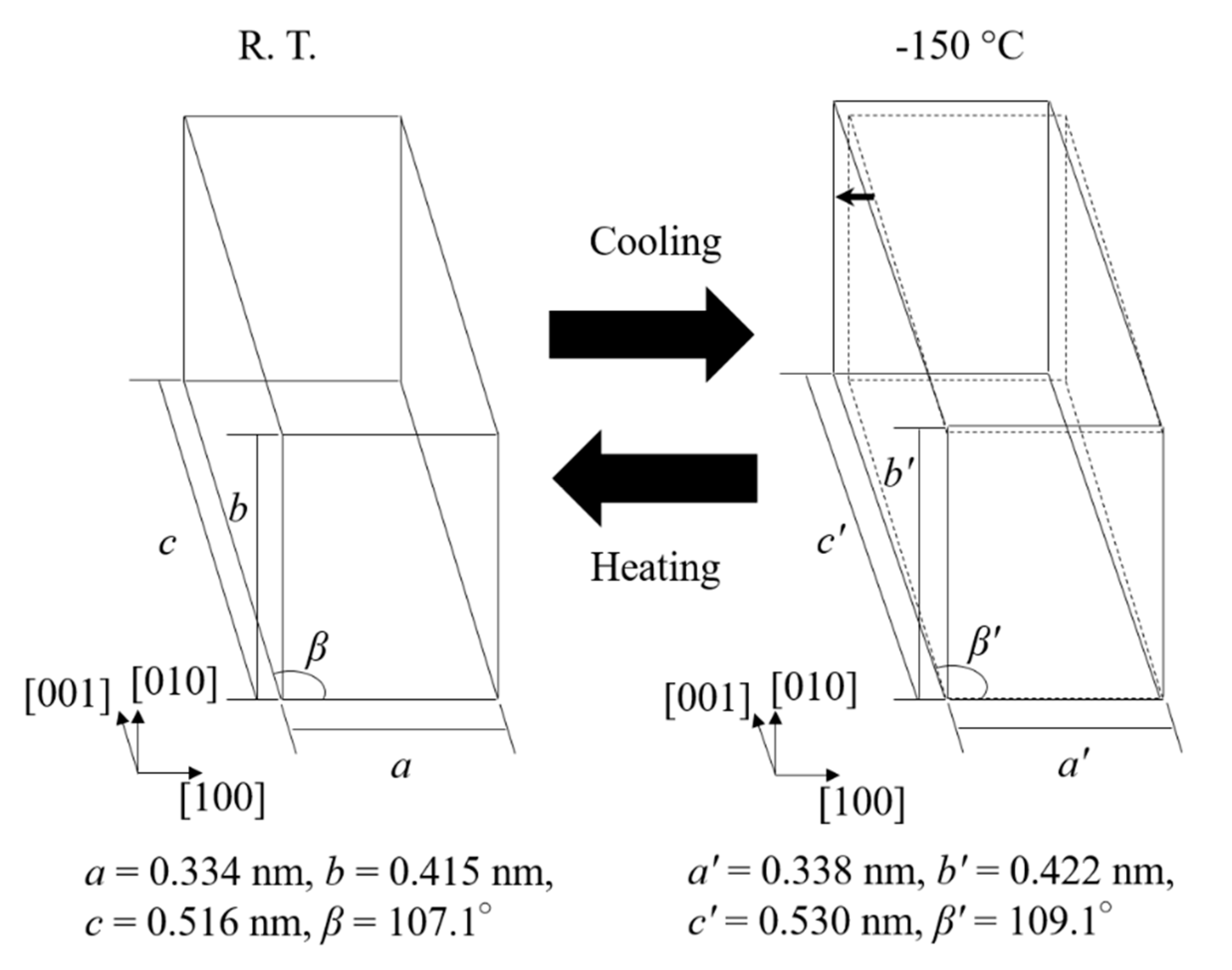
- When the martensitic variant having P21/m (lattice constant: a = 0.334 nm, b = 0.415 nm, c = 0.516 nm, β = 107.1°) of 10th cycled equiatomic CuZr alloy was cooled from room temperature to −150 °C, the change in the crystal structure corresponding to the new peak M* occurred, and the lattice constant was a’ = 0.338 nm, b′ = 0.422 nm, c’ = 0.530 nm, β′ = 109.1°. This new martensitic transformation at low temperature is considered to be closely related to the strain and stress caused by thermal cycling.
Author Contributions
Conflicts of Interest
References
- Carvalho, E.M.; Harris, I.R. Constitutional and structural studies of the intermetallic phase, ZrCu. J. Mater. Sci. 1980, 15, 1224–1230. [Google Scholar] [CrossRef]
- Koval, Y.N.; Firstov, G.S.; Kotko, A.V. Martensitic transformation and shape memory effect in ZrCu intermetallic compound. Scr. Metall. Mater. 1992, 27, 1611–1616. [Google Scholar] [CrossRef]
- Schryvers, D.; Firstov, G.S.; Seo, J.W.; Van Humbeeck, J.; Koval, Y.N. Unit cell determination in CuZr martensite by electron microscopy and X-ray diffraction. Scr. Mater. 1997, 36, 1119–1125. [Google Scholar] [CrossRef]
- Seo, J.W.; Schryvers, D. TEM investigation of the microstructure and defects of CuZr martensite. Part I: Morphology and twin systems. Acta Mater. 1998, 46, 1165–1175. [Google Scholar] [CrossRef]
- Seo, J.W.; Schryvers, D. TEM investigation of the microstructure and defects of CuZr martensite. Part II: Planar defects. Acta Mater. 1998, 46, 1177–1183. [Google Scholar] [CrossRef]
- Firstov, G.S.; Van Humbeeck, J.; Koval, Y.N. High-temperature shape memory alloys some recent developments. Mater. Sci. Eng. A 2004, 378, 2–10. [Google Scholar] [CrossRef]
- Zhou, S.H.; Napolitano, R.E. Identification of the B33 martensite phase in Cu-Zr using first-principles and X-ray diffraction. Scr. Mater. 2008, 59, 1143–1146. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Zhang, Y.; Gargarella, P.; Li, R.; Barekar, N.S.; Kühn, U.; Eckert, J. Strategy for pinpointing the formation of B2 CuZr in metastable CuZr-based shape memory alloys. Acta Mater. 2011, 59, 6620–6630. [Google Scholar] [CrossRef]
- Meng, X.; Gao, W.; Gao, Z.; Cai, W.; Zhao, L. Substructure and interface of the superstructure martensite in Zr50Cu50 High temperature shape memory alloy. Mater. Lett. 2014, 117, 221–224. [Google Scholar] [CrossRef]
- Biffi, C.A.; Figini, A.; Tuissi, A. Influence of compositional ratio on microstructure and martensitic transformation of CuZr shape memory alloys. Intermetallics 2014, 46, 4–11. [Google Scholar] [CrossRef]
- Gao, W.; Yi, X.; Sun, B.; Meng, X.; Cai, W.; Zhao, L. Microstructural evolution of martensite during deformation in Zr50Cu50 shape memory alloy. Acta Mater. 2017, 132, 405–415. [Google Scholar] [CrossRef]
- Biffi, C.A.; Coduri, M.; Yoshida, H.; Soejima, Y.; Nishida, M.; Tuissi, A. The effect of thermal cycling on the martensitic transformation in equiatomic CuZr shape memory alloy. J. Alloy. Compd. 2015, 653, 591–595. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Evans, E.H.; Smith, D.K. The reference intensity ratio, I/Ic, for computer simulated powder patterns. J. Appl. Cryst. 2002, 9, 169–174. [Google Scholar] [CrossRef]
- Kosorukova, T.; Firstov, G.; Koval, Y.; Verhovlyuk, P.; Van Humbeeck, J.; Noel, H. Structural phase transformations and shape memory effect in ZrCu along with Ni and Hf additions. MATEC Web Conf. 2015, 33, 06005. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Glimois, J.L.; Forey, P.; Feron, J.; Becle, C. Structural investigations of the pseudo-binary compounds Ni10−xCuxZr7. J. Less Common Met. 1981, 78, 45–50. [Google Scholar] [CrossRef]
- Biffi, C.A.; Figini, A.; Tuissi, A. Synthesis and structural analysis of Cu10Zr7. J. Alloy. Compd. 2012, 544, 42–45. [Google Scholar]
- Du, J.; Wen, B.; Melnik, R.; Kawazoe, Y. Phase stability, elastic and electronic properties of Cu-Zr binary system intermetallic compounds: A first-principles study. J. Alloy. Compd. 2014, 588, 96–102. [Google Scholar] [CrossRef]
- Bykov, V.A.; Kulikova, T.V.; Yagodin, D.A.; Filippov, V.V.; Shunyaev, K.Y. Thermophysical and electrical properties of equiatomic CuZr alloy. Phys. Met. Metallogr. 2015, 116, 1067–1072. [Google Scholar] [CrossRef]
- Zhao, Y.; Pang, T.; He, J.; Tao, X.; Chen, H.; Ouyang, Y.; Du, Y. Interdiffusion behaviors and mechanical properties of Cu-Zr system. Calphad 2018, 61, 92–97. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Recent developments in the research of shape memory alloys. Intermetallics 1999, 7, 511–528. [Google Scholar] [CrossRef]
- Rotaru, G.M.; Tirry, W.; Sittner, P.; Van Humbeeck, J.; Schryvers, D. Microstructural study of equiatomic PtTi martensite and the discovery of a new long-period structure. Acta Mater. 2007, 55, 4447–4454. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisada, S.; Matsuda, M.; Nishida, M.; Biffi, C.A.; Tuissi, A. Microstructure and Martensitic Transformation Behavior in Thermal Cycled Equiatomic CuZr Shape Memory Alloy. Metals 2019, 9, 580. https://doi.org/10.3390/met9050580
Hisada S, Matsuda M, Nishida M, Biffi CA, Tuissi A. Microstructure and Martensitic Transformation Behavior in Thermal Cycled Equiatomic CuZr Shape Memory Alloy. Metals. 2019; 9(5):580. https://doi.org/10.3390/met9050580
Chicago/Turabian StyleHisada, Shota, Mitsuhiro Matsuda, Minoru Nishida, Carlo Alberto Biffi, and Ausonio Tuissi. 2019. "Microstructure and Martensitic Transformation Behavior in Thermal Cycled Equiatomic CuZr Shape Memory Alloy" Metals 9, no. 5: 580. https://doi.org/10.3390/met9050580
APA StyleHisada, S., Matsuda, M., Nishida, M., Biffi, C. A., & Tuissi, A. (2019). Microstructure and Martensitic Transformation Behavior in Thermal Cycled Equiatomic CuZr Shape Memory Alloy. Metals, 9(5), 580. https://doi.org/10.3390/met9050580



