Devolatilization Kinetics of Different Types of Bio-Coals Using Thermogravimetric Analysis
Abstract
:1. Introduction
2. Kinetic Theory
3. Materials and Methods
3.1. Materials and Characterization
3.2. Experimental Method
4. Results and Discussion
4.1. Thermogravimetric Analysis
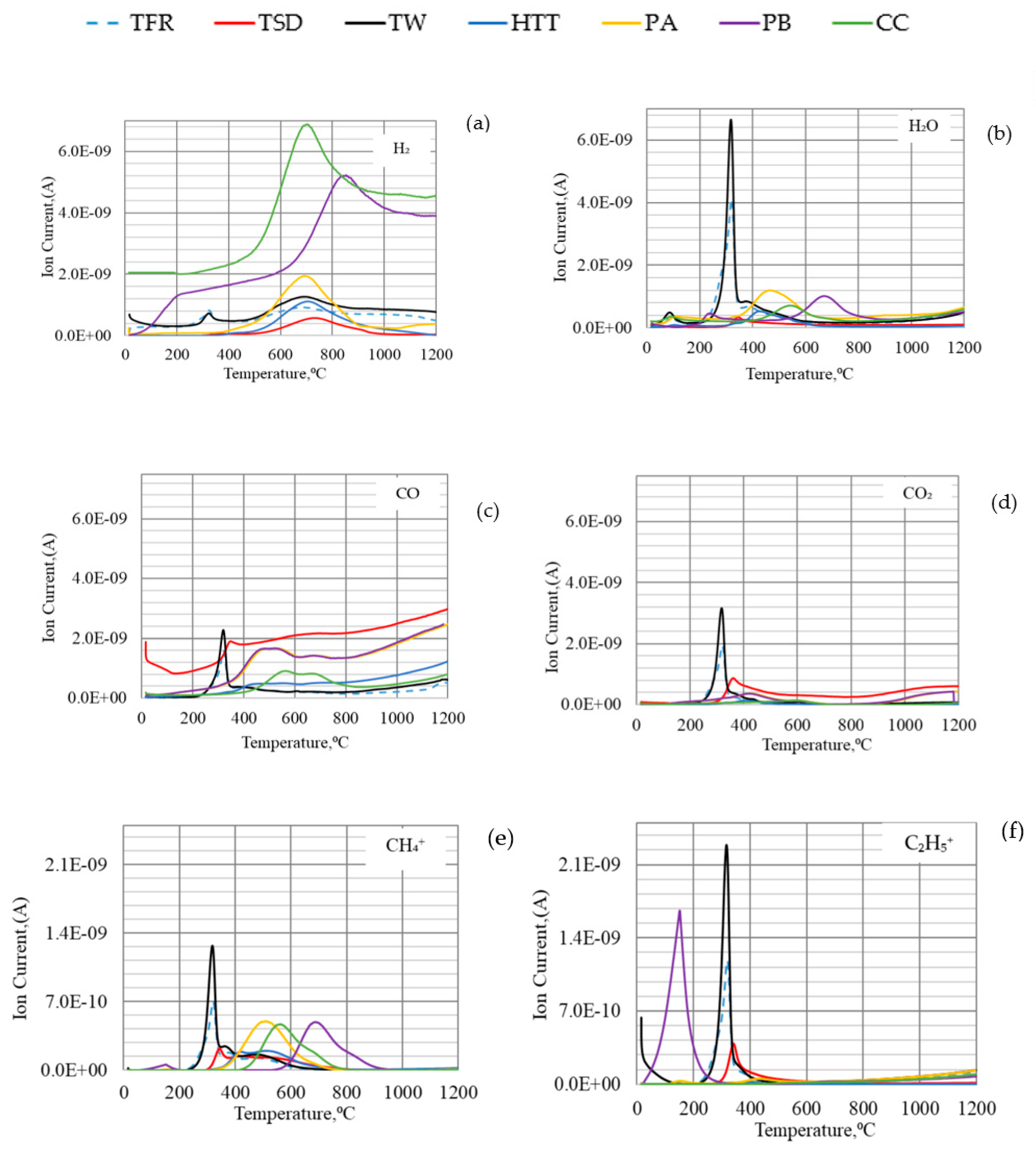
4.2. Off-Gas Analysis during the Thermal Decomposition of Bio-Coals
5. Kinetic Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geerdes, M.; Chaigneau, R.; Kurunov, I. Modern Blast Furnace Ironmaking: An Introduction, 3rd ed.; IOS Press: Amsterdam, The Netherlands, 2015; pp. 1–215. [Google Scholar]
- Xu, C.; Cang, D. A Brief Overview of Low CO2 Emission Technologies for Iron and Steel Making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar] [CrossRef]
- Hannu, S.; Timo, F. Towards More Sustainable Ironmaking—An Analysis of Energy Wood Availability in Finland and the Economics of Charcoal Production. Sustainability 2013, 5, 1188–1207. [Google Scholar]
- Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies. Available online: https://www.worldsteel.org/publications/bookshop/product-details~Steel-s-Contribution-to-a-Low-Carbon-Future--2018-update-~PRODUCT~Steel-s-Contribution-to-a-Low-Carbon-Future~.html (accessed on 14 April 2018).
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as blast furnace injectant—Considering availability, pretreatment and deployment in the Swedish steel industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef]
- Feliciano-Bruzual, C. Charcoal injection in blast furnaces (Bio-PCI): CO2 reduction potential and economic prospects. J. Mater. Res. Technol. 2014, 3, 233–243. [Google Scholar] [CrossRef]
- Alexander, B.; Dieter, S.; Miguel, F. Charcoal Behaviour by Its Injection into the Modern Blast Furnace. ISIJ Int. 2010, 50, 81–88. [Google Scholar]
- Skogsdata 2018. Available online: https://www.slu.se/en/Collaborative-Centres-and-Projects/the-swedish-national-forest-inventory/forest-statistics/skogsdata/ (accessed on 12 December 2018).
- Fick, G.; Mirgaux, O.; Neau, P.; Patisson, F. Using biomass for pig iron production: A technical, environmental and economical assessment. Waste Biomass Valoris 2014, 5, 43–55. [Google Scholar] [CrossRef]
- Chen, W.; Du, S.; Tsai, C.; Wang, Z. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, A. The production of biofuels and renewable chemicals by fast pyrolysis of biomass. Int. J. Glob. Energy Issues 2007, 27, 160–203. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Keown, D.M.; Favas, G.; Hayashi, J.I.; Li, C.Z. Volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass: Differences between sugar cane bagasse and cane trash. Bioresour. Technol. 2005, 96, 1570–1577. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Besler, S. Inorganic compounds in biomass feedstocks. 1. Effect on the quality of fast pyrolysis oils. Energy Fuels 1996, 10, 293–298. [Google Scholar] [CrossRef]
- Jensen, P.A.; Frandsen, F.J.; Dam-Johansen, K.; Sander, B. Experimental Investigation of the Transformation and Release to Gas Phase of Potassium and Chlorine during Straw Pyrolysis. Energy Fuels 2000, 14, 1280–1285. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J.M.; Brydson, R.M.D.; Ross, A.B. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Darvell, L.I.; Jones, J.M.; Yates, N.; Thain, S.; Donnison, I.S. The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Kinetics in solids. Annu. Rev. Phys. Chem. 1997, 48, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Galwey, A.K.; Brown, M.E. Kinetic Background to Thermal Analysis and Calorimetry. Princ. Pract. 1998, 1, 147–223. [Google Scholar]
- Cai, J.M.; Bi, L.S. Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J. Therm. Anal. Calorim. 2009, 98, 325–330. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Miccio, M.; Ruoppolo, G. Isoconversional kinetic analysis of olive pomace decomposition under torrefaction operating conditions. Fuel Process. Technol. 2015, 130, 147–154. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Görgens, J.F. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Kongkaew, N.; Pruksakit, W.; Patumsawad, S. Thermogravimetric Kinetic Analysis of the Pyrolysis of Rice Straw. Energy Procedia 2015, 79, 663–670. [Google Scholar] [CrossRef]
- Mani, T.; Murugan, P.; Abedi, J.; Mahinpey, N. Pyrolysis of wheat straw in a thermogravimetric analyzer: Effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chem. Eng. Res. Des. 2010, 88, 952–958. [Google Scholar] [CrossRef]
- Biagini, E.; Fantei, A.; Tognotti, L. Effect of the heating rate on the devolatilization of biomass residues. Thermochim. Acta 2008, 472, 55–63. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Konttinen, J.; Hukka, T.I.; Moilanen, A. Influence of torrefaction pretreatment on the pyrolysis of Eucalyptus clone: A study on kinetics, reaction mechanism and heat flow. Ind. Crop. Prod. 2016, 92, 244–254. [Google Scholar] [CrossRef]
- Tran, K.Q.; Bach, Q.V.; Trinh, T.T.; Seisenbaeva, G. Non-isothermal pyrolysis of torrefied stump—A comparative kinetic evaluation. Appl. Energy 2014, 136, 759–766. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of pre-exponential factors and of the mathematical functions f (a) or G (a) that describe the reaction mechanism in a model-free way. Thermochim. Acta 2013, 564, 59–69. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Persson, A.; Okvist, L.S.; Bjorkman, B. Reduction Behaviour of Self-reducing Blends of In-plant Fines in Inert Atmosphere. ISIJ Int. 2015, 55, 2082–2089. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, A.; Knoetze, J.H. Using Apparent Activation Energy as a Reactivity Criterion for Biomass Pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Thermal behaviour and kinetic study for woody biomass torrefaction and torrefied biomass pyrolysis by TGA. Biosyst. Eng. 2013, 116, 420–426. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Liang, D.T.; Zheng, C. Mechanism of Palm Oil Waste Pyrolysis in a Packed Bed. Energy Fuels 2006, 20, 1321–1328. [Google Scholar] [CrossRef]
- Konishi, H.; Ichikawa, K.; Usui, T. Effect of residual volatile matter on reduction of iron oxide in semi-charcoal composite pellets. ISIJ Int. 2010, 50, 386–389. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, Y.W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking; Cootha Publishing House: Brisbane, Australia, 1981; pp. 1–512. [Google Scholar]





| Bio-Coal Type | Bio-Coal | Origin | Temperature, °C | Time, min | Abbreviation |
|---|---|---|---|---|---|
| Highly volatile bio-coals | Torrefied forest residue | Top and branches pine /spruce | 286 | 6 | TFR |
| Torrefied saw dust | Spruce | 297 | 6 | TSD | |
| Torrefied willow | Willow | 330 | 6 | TW | |
| Lowly volatile bio-coals | High-temperature torrefied | 50 % Pine/50% spruce | 350 | 8 | HTT |
| Pine A | Pine | 350 | 14 | PA | |
| Pine B | Pine | 400 | 14 | PB | |
| Charcoal | Mixture of pine, birch, alder, aspen | 550 | - | CC |
| Bio-Coals | Proximate Analysis (wt %) | Ultimate Analysis (wt %) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cfix | VM | Ash | Ctot | H | N | S | O | |
| TFR | 23.6 | 73.2 | 3.2 | 52.0 | 5.9 | 0.57 | 0.035 | 35.2 |
| TSD | 24.0 | 75.6 | 0.45 | 57.1 | 5.9 | 0.12 | 0.004 | 36.4 |
| TW | 24.7 | 73.3 | 2.0 | 52.7 | 5.8 | 0.30 | 0.021 | 39.2 |
| HTT | 60.8 | 38.2 | 1.0 | 75.3 | 4.9 | 0.10 | 0.008 | 18.8 |
| PA | 70.3 | 28.7 | 1.0 | 78.6 | 4.4 | 0.23 | <0.01 | 15.8 |
| PB | 79.1 | 19.7 | 1.2 | 85.0 | 3.8 | 0.30 | <0.01 | 9.70 |
| CC | 80.7 | 18.6 | 0.70 | 87.0 | 3.4 | 0.25 | <0.004 | 8.30 |
| Bio-coals | Al2O3 | CaO | SiO2 | Fe2O3 | K2O | MgO | MnO | Na2O | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| TFR | 0.047 | 0.872 | 0.618 | 0.044 | 0.238 | 0.124 | 0.062 | 0.014 | 0.151 | 0.004 |
| TSD | 0.003 | 0.122 | 0.033 | 0.011 | 0.051 | 0.046 | 0.013 | 0.003 | 0.008 | 0.000 |
| TW | 0.004 | 0.494 | 0.019 | 0.003 | 0.230 | 0.076 | 0.004 | 0.005 | 0.142 | 0.000 |
| HTT | 0.024 | 0.310 | 0.305 | 0.079 | 0.145 | 0.061 | 0.040 | 0.023 | 0.032 | 0.001 |
| PA | 0.032 | 0.270 | 0.235 | 0.007 | 0.148 | 0.074 | 0.035 | 0.010 | 0.021 | 0.001 |
| PB | 0.013 | 0.339 | 0.067 | 0.006 | 0.167 | 0.096 | 0.043 | 0.004 | 0.024 | 0.000 |
| CC | 0.006 | 0.317 | 0.028 | 0.009 | <0.002 | 0.112 | 0.044 | <0.009 | 0.006 | 0.001 |
| Conversion, α | TFR | TSD | TW | HTT | PA | PB | CC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 | |
| 0.1 | 169 | 0.999 | 149 | 0.998 | 111 | 1.000 | 361 | 0.981 | 145 | 1.000 | 449 | 0.996 | 297 | 0.999 |
| 0.2 | 179 | 1.000 | 158 | 0.997 | 120 | 0.999 | 278 | 1.000 | 189 | 1.000 | 411 | 0.996 | 271 | 1.000 |
| 0.3 | 185 | 0.998 | 162 | 0.997 | 123 | 0.999 | 246 | 0.999 | 222 | 0.997 | 272 | 1.000 | 271 | 1.000 |
| 0.4 | 191 | 0.997 | 158 | 0.999 | 124 | 1.00 | 238 | 0.997 | 232 | 1.000 | 225 | 0.999 | 249 | 1.000 |
| 0.5 | 185 | 0.998 | 159 | 0.997 | 124 | 0.999 | 274 | 1.000 | 269 | 1.000 | 215 | 1.000 | 260 | 0.997 |
| 0.6 | 186 | 0.999 | 191 | 1.000 | 123 | 0.999 | 263 | 1.000 | 235 | 0.999 | 204 | 1.000 | 215 | 0.998 |
| 0.7 | 192 | 0.998 | 269 | 1.000 | 124 | 0.999 | 161 | 1.000 | 250 | 0.999 | 202 | 0.999 | 195 | 0.998 |
| 0.8 | 194 | 0.997 | 385 | 0.999 | 124 | 0.999 | 135 | 1.000 | 243 | 1.000 | 158 | 1.000 | 194 | 0.997 |
| 0.9 | 143 | 1.000 | 252 | 1.000 | 90 | 0.999 | 99 | 0.999 | 221 | 0.997 | 139 | 1.000 | 139 | 0.999 |
| 0,99 | 61 | 0.999 | 285 | 0.997 | 78 | 0.999 | 58 | 1.000 | 122 | 0.996 | 77 | 1.000 | 38 | 1.000 |
| Average value | 169 | - | 217 | - | 114 | - | 211 | - | 213 | - | 235 | - | 213 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tawil, A.A.; Ahmed, H.M.; Ökvist, L.S.; Björkman, B. Devolatilization Kinetics of Different Types of Bio-Coals Using Thermogravimetric Analysis. Metals 2019, 9, 168. https://doi.org/10.3390/met9020168
El-Tawil AA, Ahmed HM, Ökvist LS, Björkman B. Devolatilization Kinetics of Different Types of Bio-Coals Using Thermogravimetric Analysis. Metals. 2019; 9(2):168. https://doi.org/10.3390/met9020168
Chicago/Turabian StyleEl-Tawil, Asmaa A., Hesham M. Ahmed, Lena Sundqvist Ökvist, and Bo Björkman. 2019. "Devolatilization Kinetics of Different Types of Bio-Coals Using Thermogravimetric Analysis" Metals 9, no. 2: 168. https://doi.org/10.3390/met9020168





