Effect of Ni Addition on Catalytic Performance of Fe87Si5B2P3Nb2Cu1 Amorphous Alloys for Degrading Methylene Blue Dyes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Ball milling
2.2.2. Degrading MB dyes
2.3. Characterizations
3. Results
3.1. Subsection
Bulleted lists
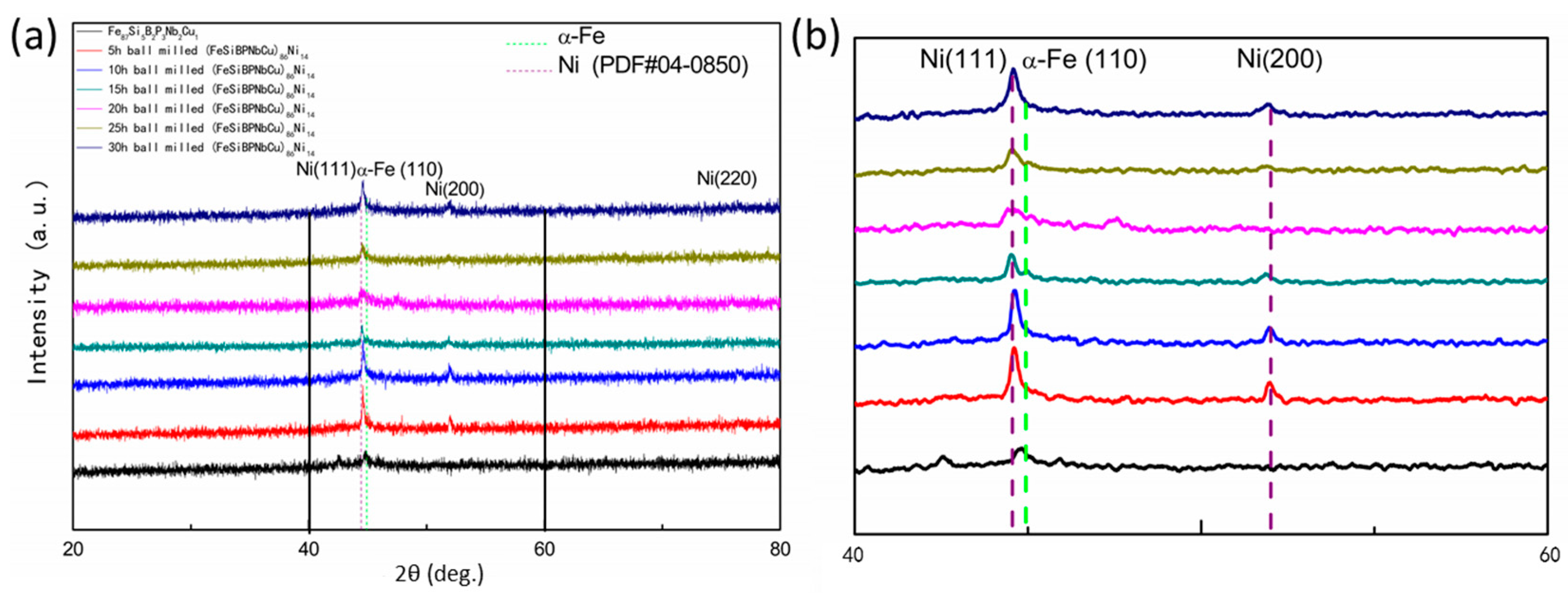
- XRD
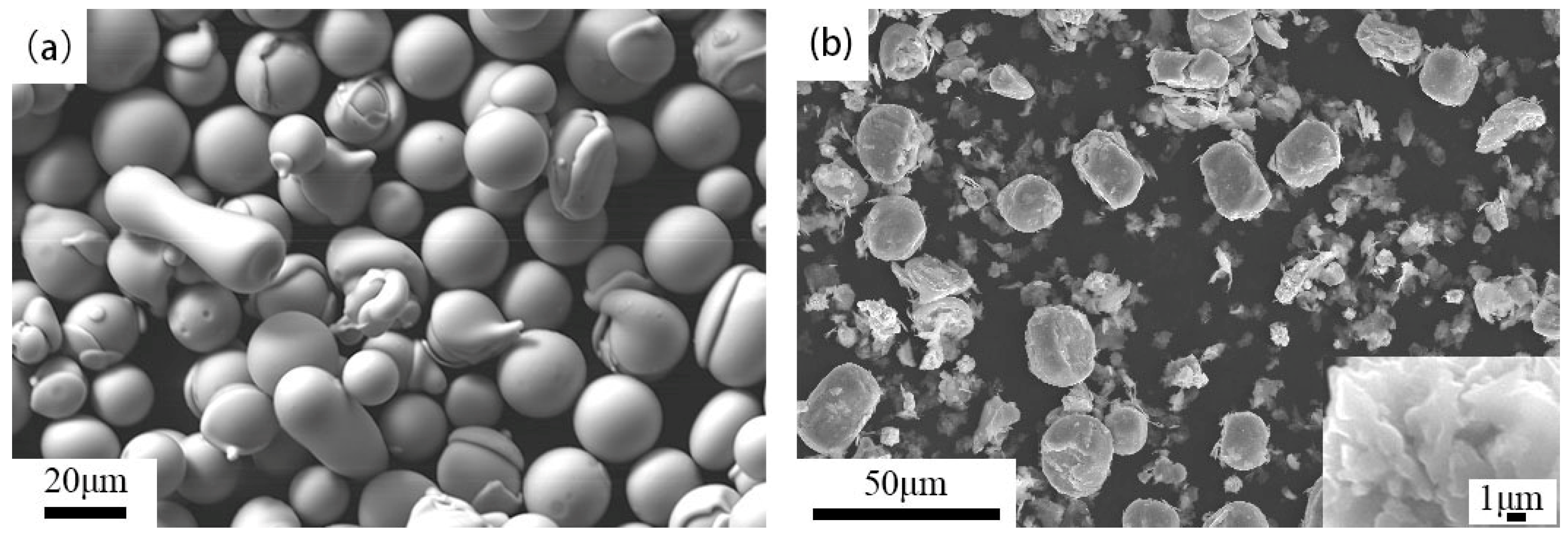
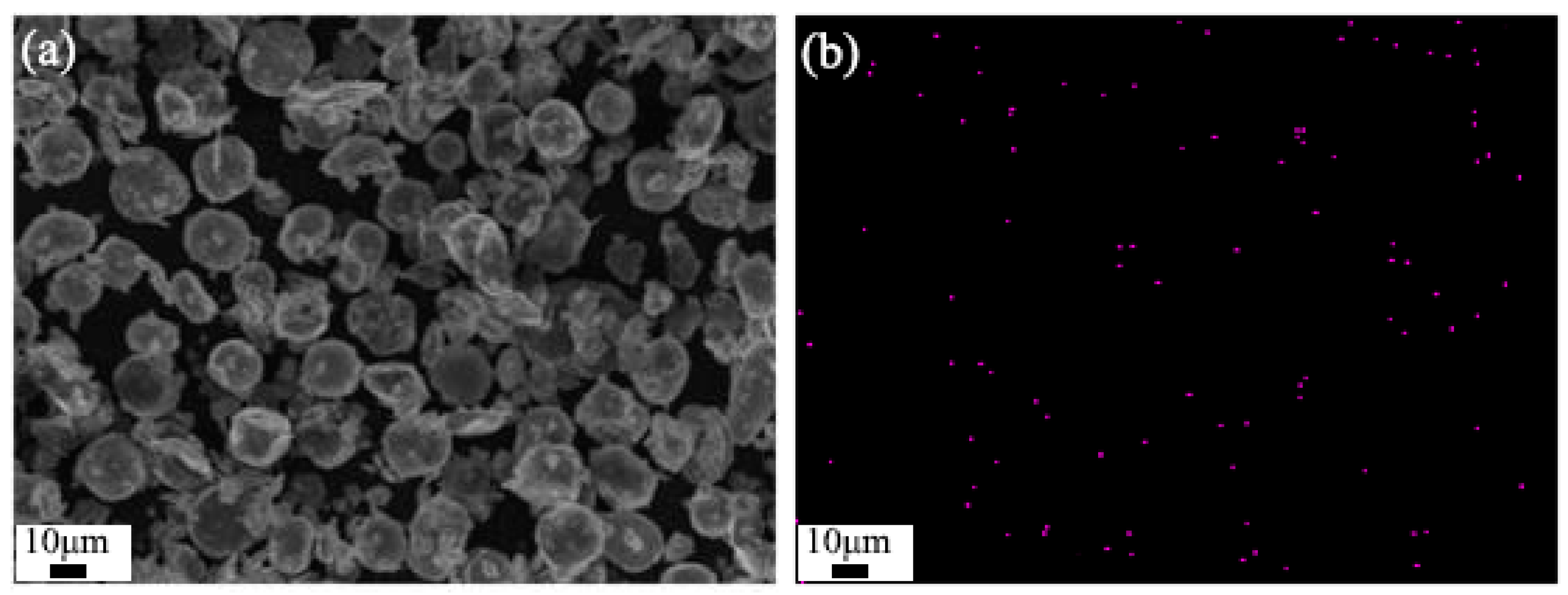
- SEM
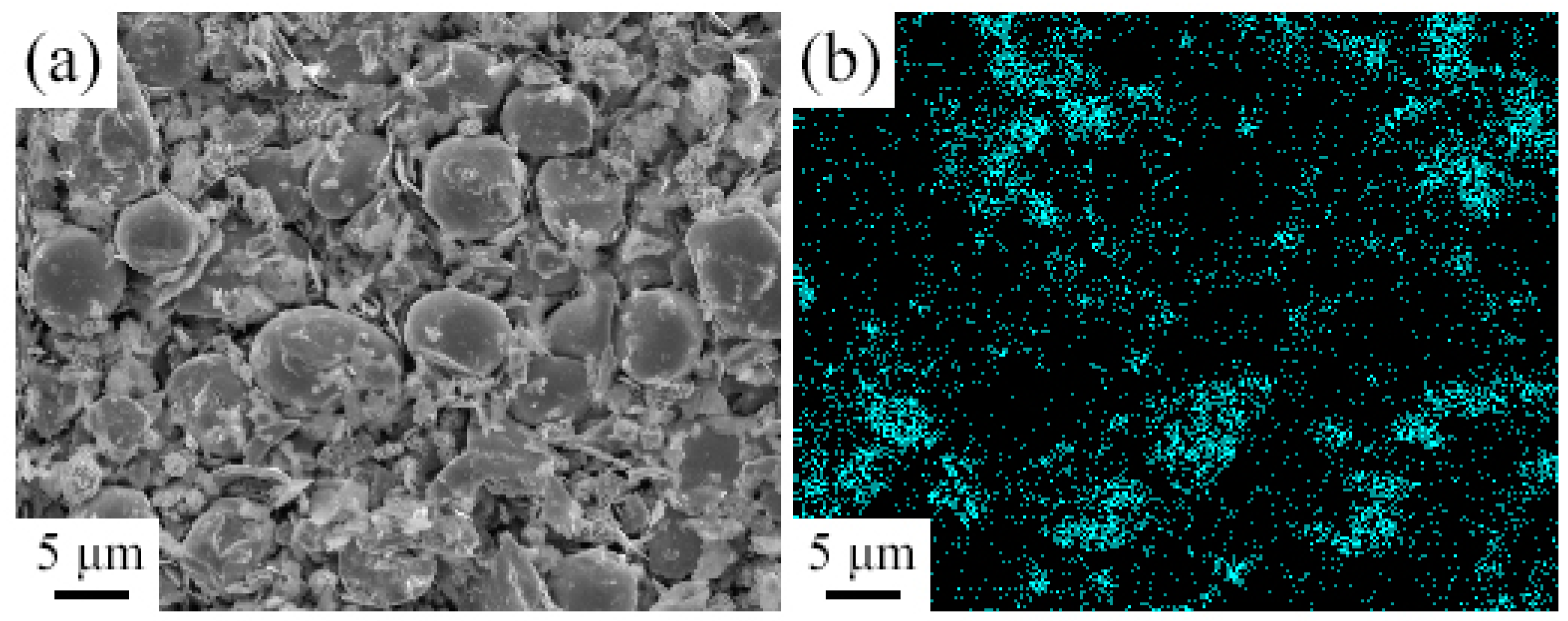
- EDS
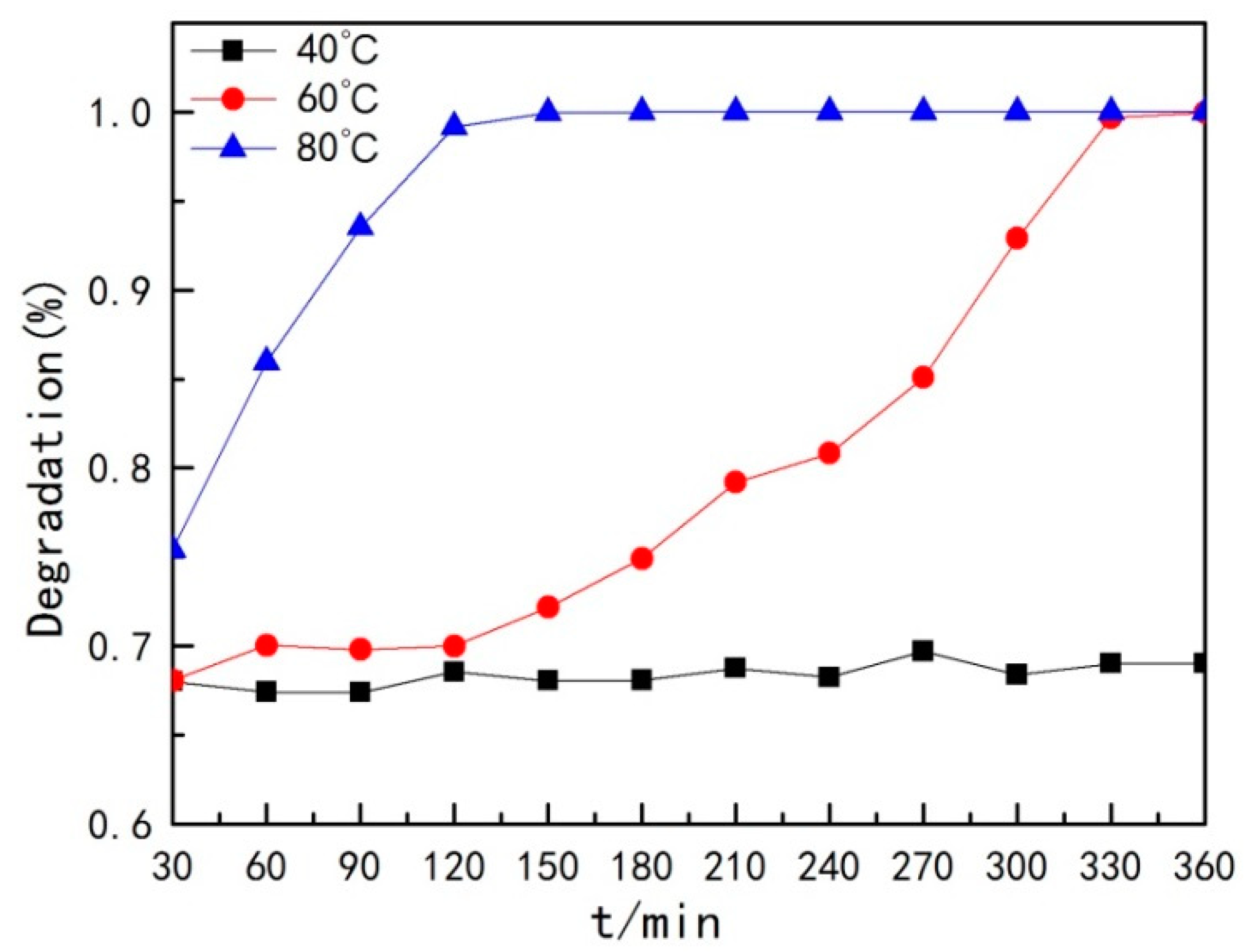
- Degradation rate
- UV-VIS
3.2. Formation of Mathematical Components
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miao, J.; Zhang, L.C.; Lin, H.A. Novel kind of thin film composite nanofiltration membrane with sulfated chitosan as the active layer material. Chem. Eng. Sci. 2013, 87, 152–159. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Gonçalves, F.; Órfão, J.J.M.; Fabregat, A.; Fortuny, A.; Font, J.; Bengoa, C.; Stuber, F. Tailored activated carbons as catalysts in biodecolourisation of textile azo dyes. Appl. Catal. B 2010, 94, 179–185. [Google Scholar] [CrossRef]
- Guibal, E.; Roussy, J. Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan). React. Funct. Polym. 2007, 67, 33–42. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res. 2002, 36, 4717–4724. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwara, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Jia, Z.; Duan, X.; Zhang, W.; Wang, W.; Sun, H.; Wang, S.; Zhang, L.-C. Ultra-sustainable Fe78Si9B13 metallic glass as a catalyst for activation of persulfate on methylene blue degradation under UV-Vis light. Sci. Rep. 2016, 6, 38520. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Liang, S.X.; Zhang, W.C.; Wang, W.M.; Yang, C.; Zhang, C.L. Heterogeneous photo Fenton-like degradation of cibacron brilliant red 3B-A dye using amorphous Fe78Si9B13, and Fe73.5Si13.5B9Cu1Nb3, alloys: The influence of adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 128–136. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Lv, M.; Hu, Z. Decolorization of azo dye solution by Fe-Mo-Si-B amorphous alloy. J. Non-Cryst. Solids 2010, 356, 1703–1706. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid Degradation of Azo Dye by Fe-Based Metallic Glass Powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H.; Hu, Z. Rapid decolorization of acid orange II aqueous solution by amorphous zero-valent iron. J. Environ. Sci. 2012, 24, 1021–1026. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H.; Hu, Z. On the decolorization property of Fe-Mo-Si-B alloys with different structures. J. Non-Cryst. Solids 2012, 358, 61–64. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, J.L.; Zha, M.Q.; Shek, C.H. Synthesis of an Fe rich amorphous structure with a catalytic effect to rapidly decolorize Azo dye at room temperature. ACS Appl. Mater. Interfaces 2016, 6, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, Y.; Zhu, Z.; Wu, J.L. Efficient degradation of rhodamine B using Fe-based metallic glass catalyst by Fenton-like process. Chemosphere 2014, 117, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Shen, K.C.; Pan, S.P.; Zhao, J.; Qin, J.Y.; Kim, K.B.; Wang, W.M. Role of tri-capped triangular prism (TTP) polyhedra in formation and destabilization of Fe-Y-B glassy alloys. J. Non-Cryst. Solids 2015, 425, 67–73. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Yao, K.-F. Insight into the high reactivity of commercial Fe-Si-B amorphous zero-valent iron in degrading azo dye solutions. RSC Adv. 2015, 5, 34032–34039. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Yao, K.-F. Rapid decomposition of Direct Blue 6 in neutral solution by Fe-B amorphous alloys. RSC Adv. 2014, 5, 6215–6221. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, W.C.; Wang, W.M.; Habibi, D.; Zhang, L.Z. Amorphous Fe78Si9B13, alloy: An efficient and reusable photo-enhanced Fenton-like catalyst in degradation of cibacron brilliant red 3B-A dye under UV-vis light. Appl. Catal. B 2016, 192, 46–56. [Google Scholar] [CrossRef]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Excellent capability in degrading azo dyes by MgZn-based metallic glass powders. Sci. Rep. 2012, 2, 418. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Li, R.; Zong, J.; Zhang, Y.; Li, H.; Zhang, T. Enhanced degradation of azo dye by nanoporous-copper-decorated Mg-Cu-Y metallic glass powder through dealloying pretreatment. Appl. Surf. Sci. 2014, 305, 314–320. [Google Scholar] [CrossRef]
- Ramya, M.; Karthika, M.; Selvakumar, R.; Ravi, K.R. A Facile and Efficient Single Step Ball Milling Process for Synthesis of Partially Amorphous Mg-Zn-Ca alloy Powders for Dye Degradation. J. Alloys Compd. 2016, 696, 185–192. [Google Scholar] [CrossRef]
- Qin, X.D.; Zhu, Z.W.; Liu, G.; Fu, H.M.; Zhang, H.M.; Zhang, A.M.; Li, H.; Zhang, H.F. Ultrafast degradation of azo dyes catalyzed by cobalt-based metallic glass. Sci. Rep. 2015, 5, 18226. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, J.Q.; Li, H.; Yang, H.; Huo, J.T.; Wang, J.G.; Chang, C.T.; Wang, X.M.; Li, R.-W.; Wang, G.; et al. Fast decolorization of azo dyes in both alkaline and acidic solutions by Al-based metallic glasses. J. Alloys Compd. 2017, 701, 759–767. [Google Scholar] [CrossRef]
- Nam, S.; Tratnyek, P.G. Reduction of azo dyes with zero-valent iron. Water Res. 2000, 34, 1837–1845. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H. Effects of the addition of Co, Ni or Cr on the decolorization performance of Fe-Si-B amorphous alloys. J. Phys. Chem. Solids 2017, 110, 152–160. [Google Scholar] [CrossRef]
- Yao, K.F.; Shi, L.X.; Chen, S.Q.; Shao, Y.; Chen, N.; Jia, J.-L. Iron-based soft magnetic amorphous/nanocrystalline alloy Progress and prospects. J. Phys. 2018, 67, 016101–016108. [Google Scholar]
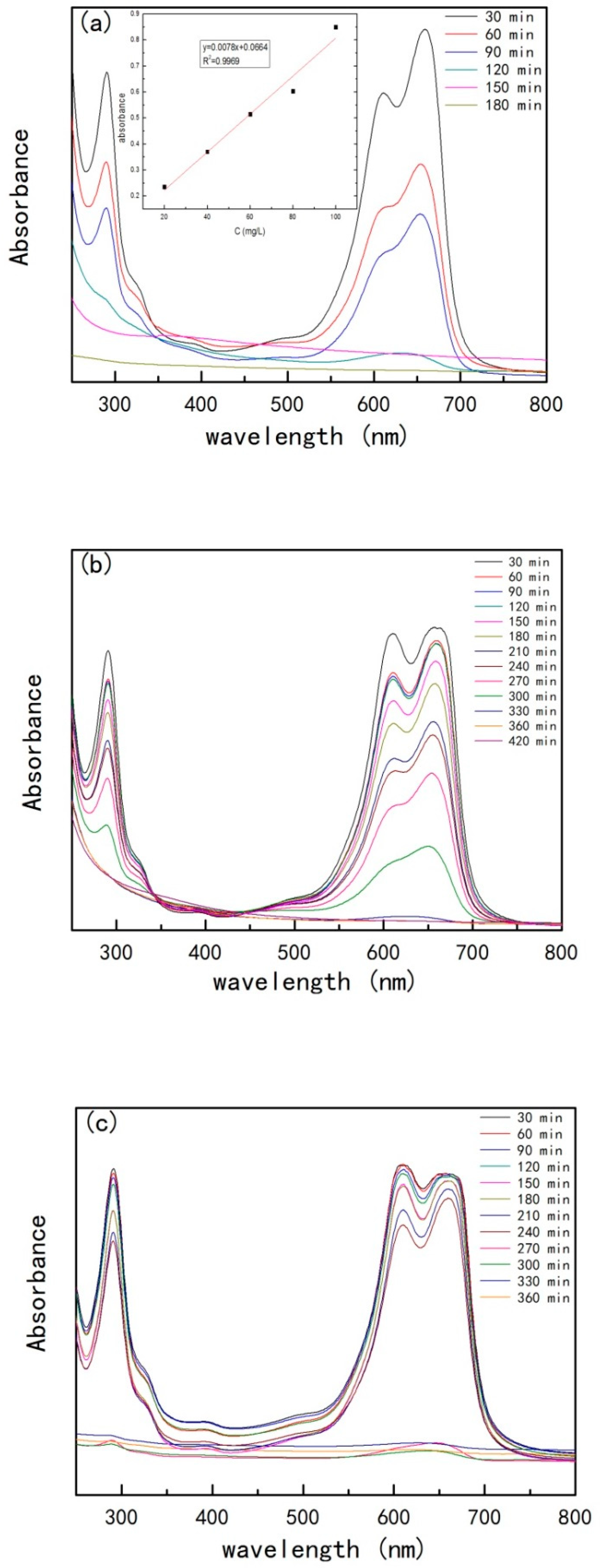
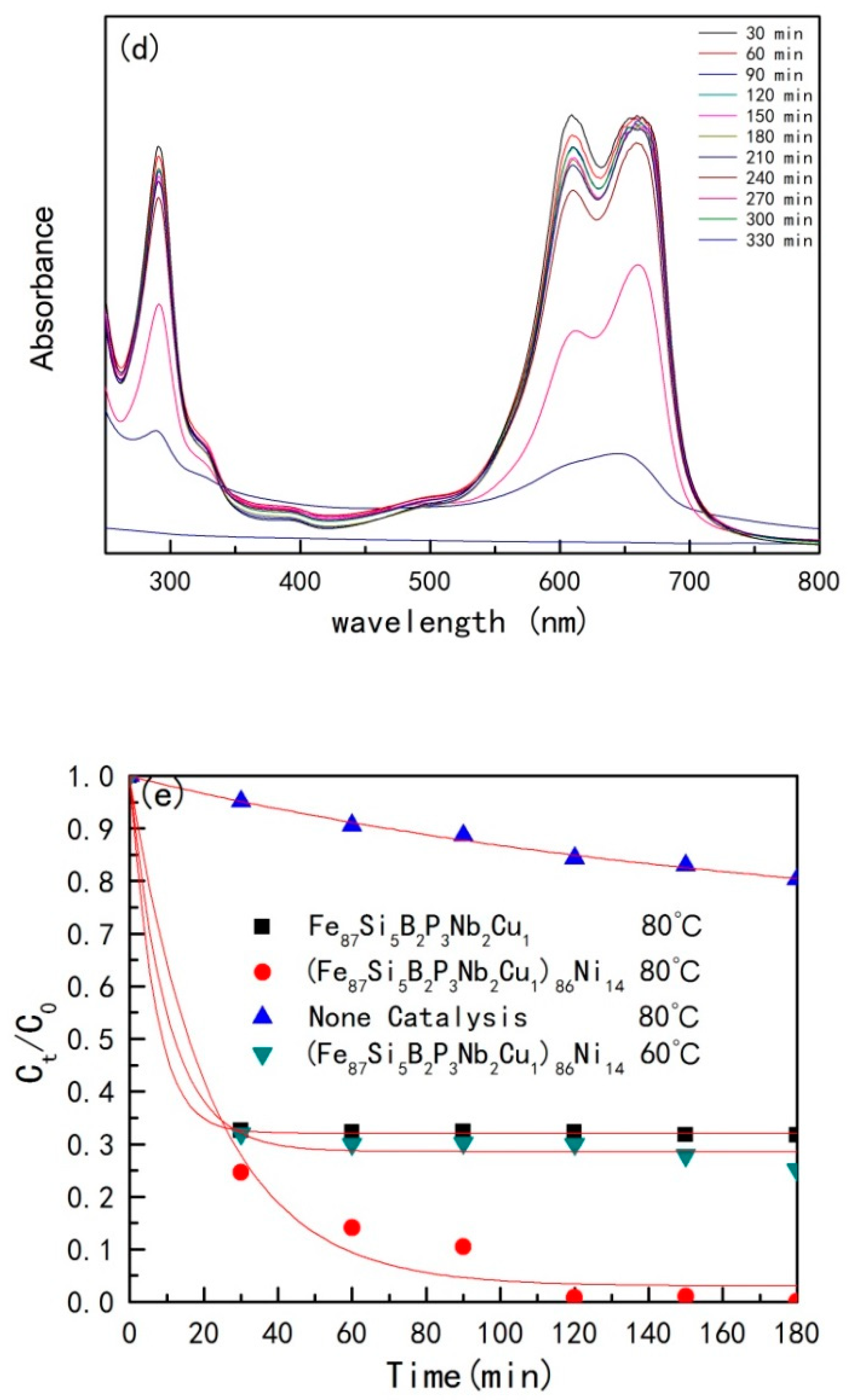
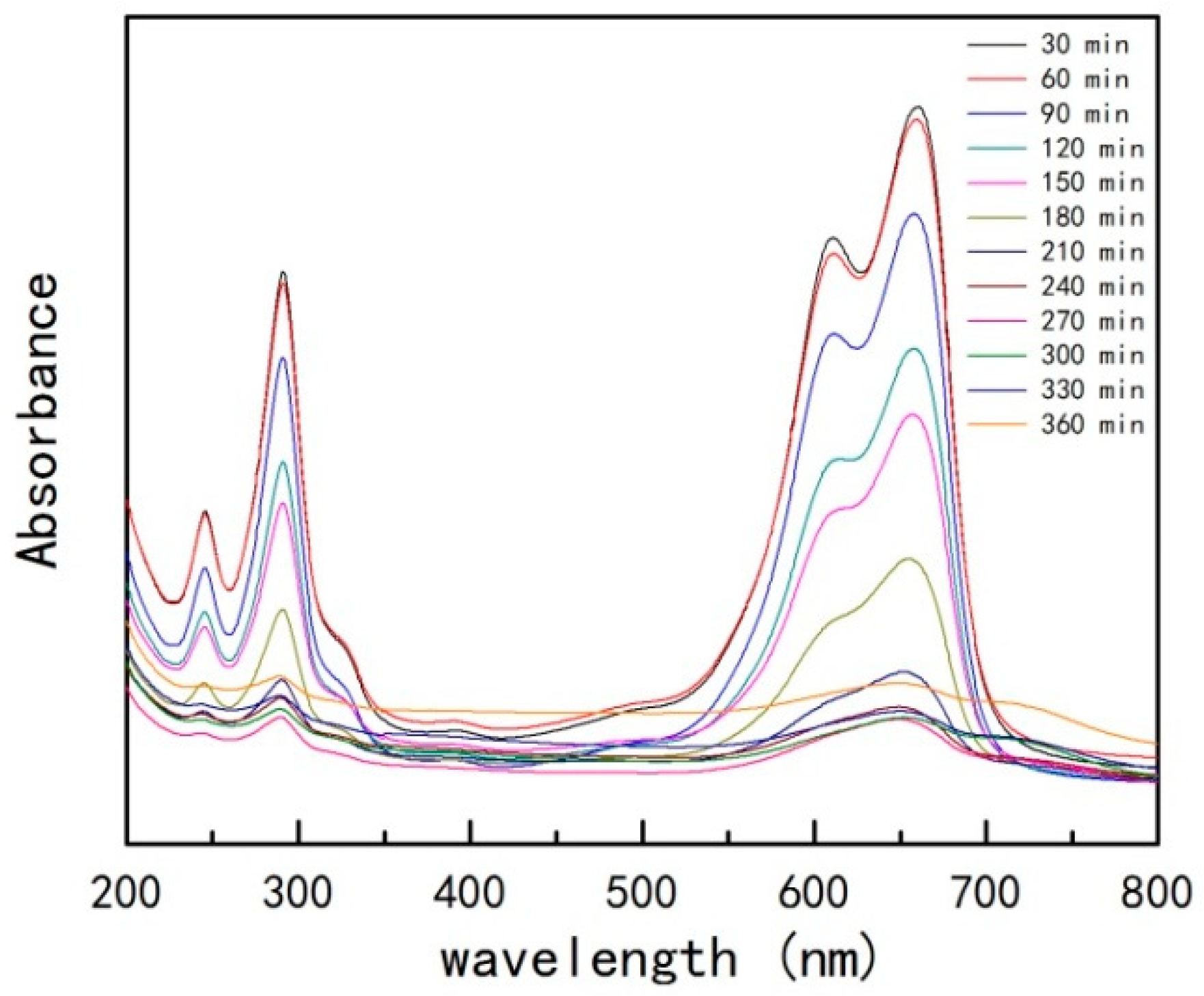
| Material | Temperature (°C) | k | t1/2 (min) | Degradation Rate (%) |
|---|---|---|---|---|
| None catalysis | 80 | 0.001 | 693.15 | 19.68 |
| Fe87Si5B2P3Nb2Cu1 | 80 | 0.018 | 38.51 | 67.76 |
| (Fe87Si5B2P3Nb2Cu1)86Ni14 | 80 | 0.065 | 10.66 | 99.99 |
| (Fe87Si5B2P3Nb2Cu1)86Ni14 | 60 | 0.022 | 31.51 | 99.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Ni, B.; Zhang, J.; Wu, C.; Cheng, D.; Chi, Y.; Wang, H.; Wang, M.; Zhao, Z. Effect of Ni Addition on Catalytic Performance of Fe87Si5B2P3Nb2Cu1 Amorphous Alloys for Degrading Methylene Blue Dyes. Metals 2019, 9, 341. https://doi.org/10.3390/met9030341
Shi J, Ni B, Zhang J, Wu C, Cheng D, Chi Y, Wang H, Wang M, Zhao Z. Effect of Ni Addition on Catalytic Performance of Fe87Si5B2P3Nb2Cu1 Amorphous Alloys for Degrading Methylene Blue Dyes. Metals. 2019; 9(3):341. https://doi.org/10.3390/met9030341
Chicago/Turabian StyleShi, Jinfang, Bingying Ni, Jingjing Zhang, Chen Wu, Daowen Cheng, Yue Chi, Hongli Wang, Minggang Wang, and Zhankui Zhao. 2019. "Effect of Ni Addition on Catalytic Performance of Fe87Si5B2P3Nb2Cu1 Amorphous Alloys for Degrading Methylene Blue Dyes" Metals 9, no. 3: 341. https://doi.org/10.3390/met9030341





