Evaluation of the Aging Effect on the Microstructure of Co-28Cr-6Mo-0.3C Alloy: Experimental Characterization and Computational Thermodynamics
Abstract
:1. Introduction
2. Calculations and Experimental Procedure
2.1. Thermodynamic Calculations
2.2. Experimental Procedure
3. Results and Discussion
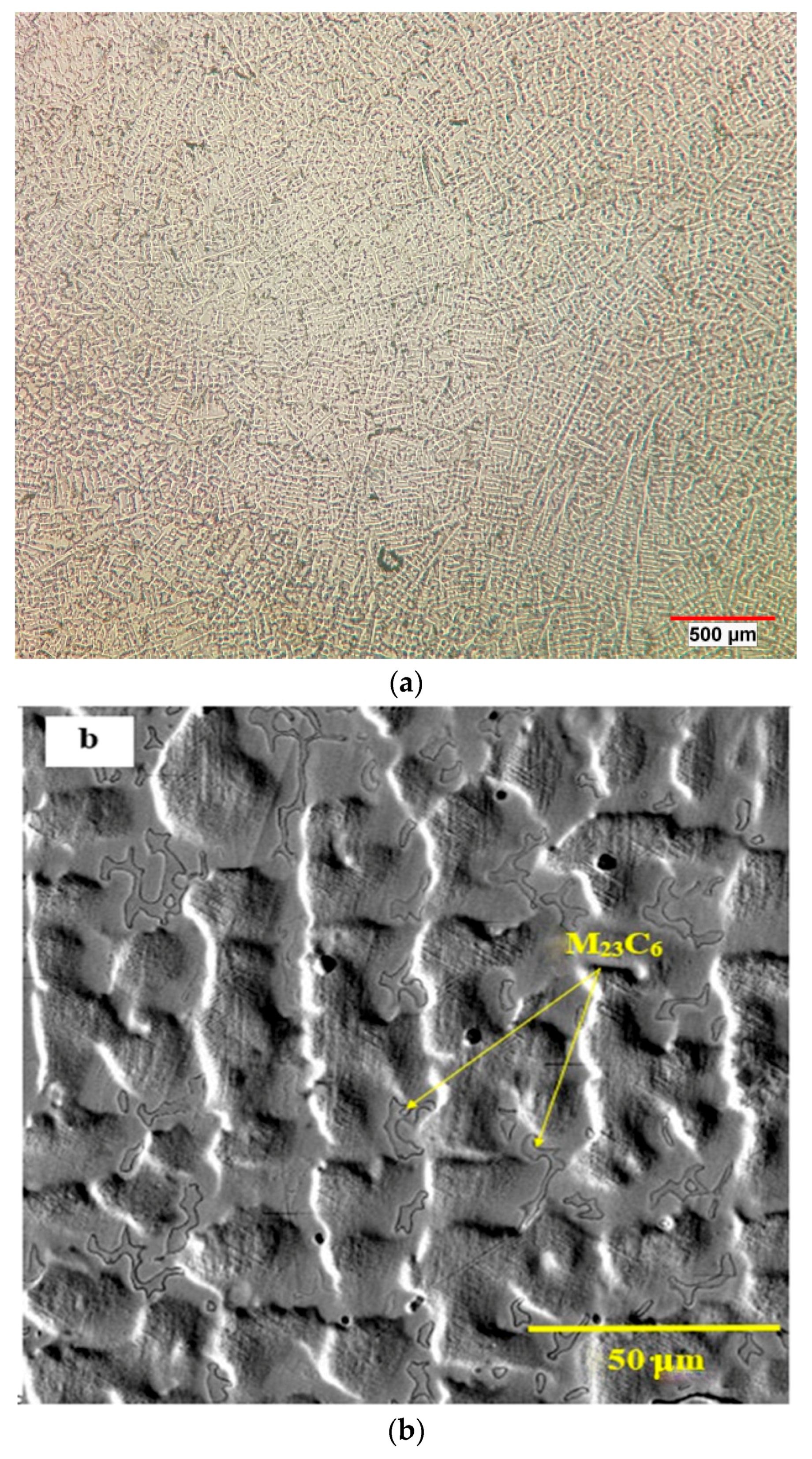
3.1. As-Received Alloy
3.2. Thermodynamical Calculations
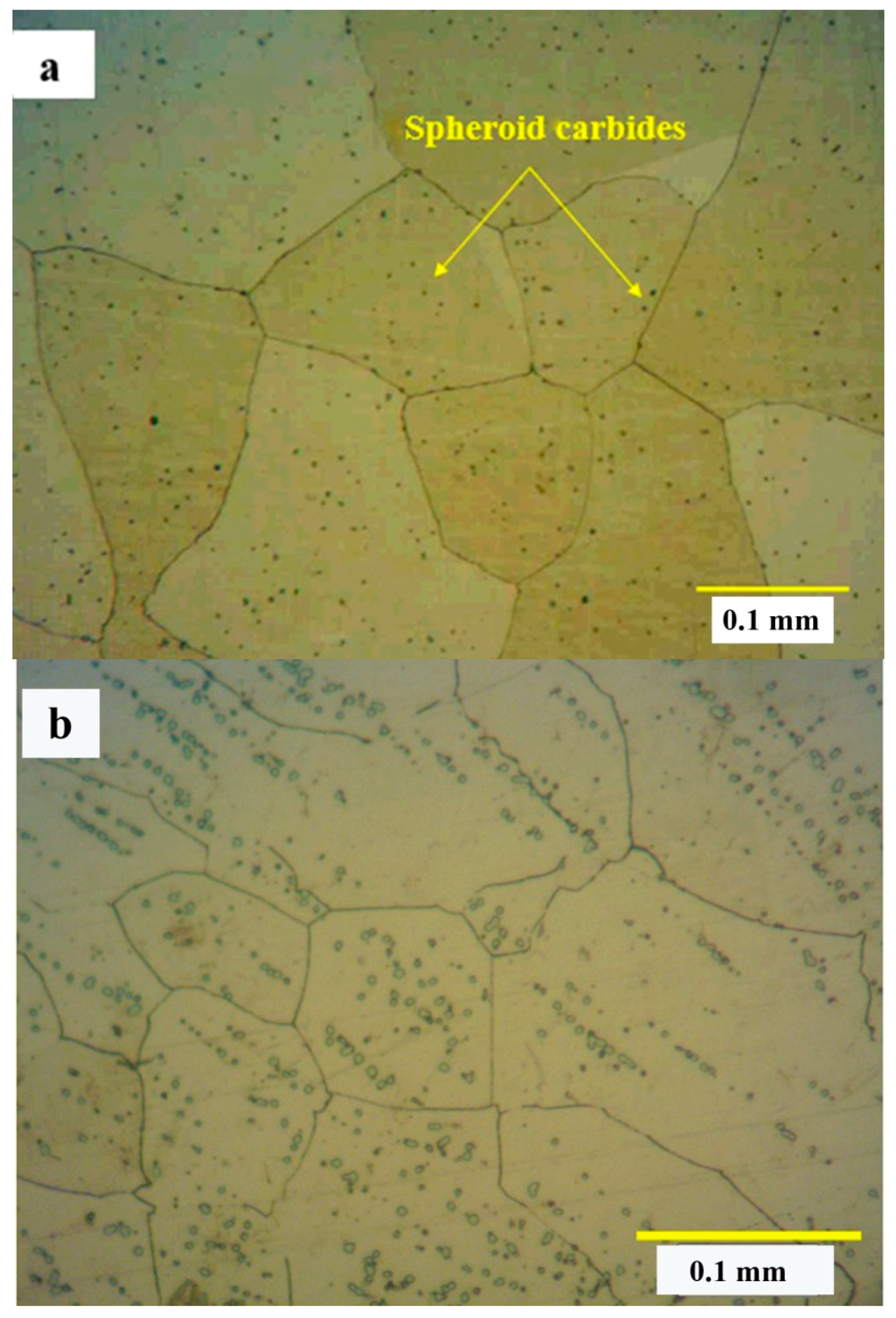
3.3. Solution Treatment and Quenching
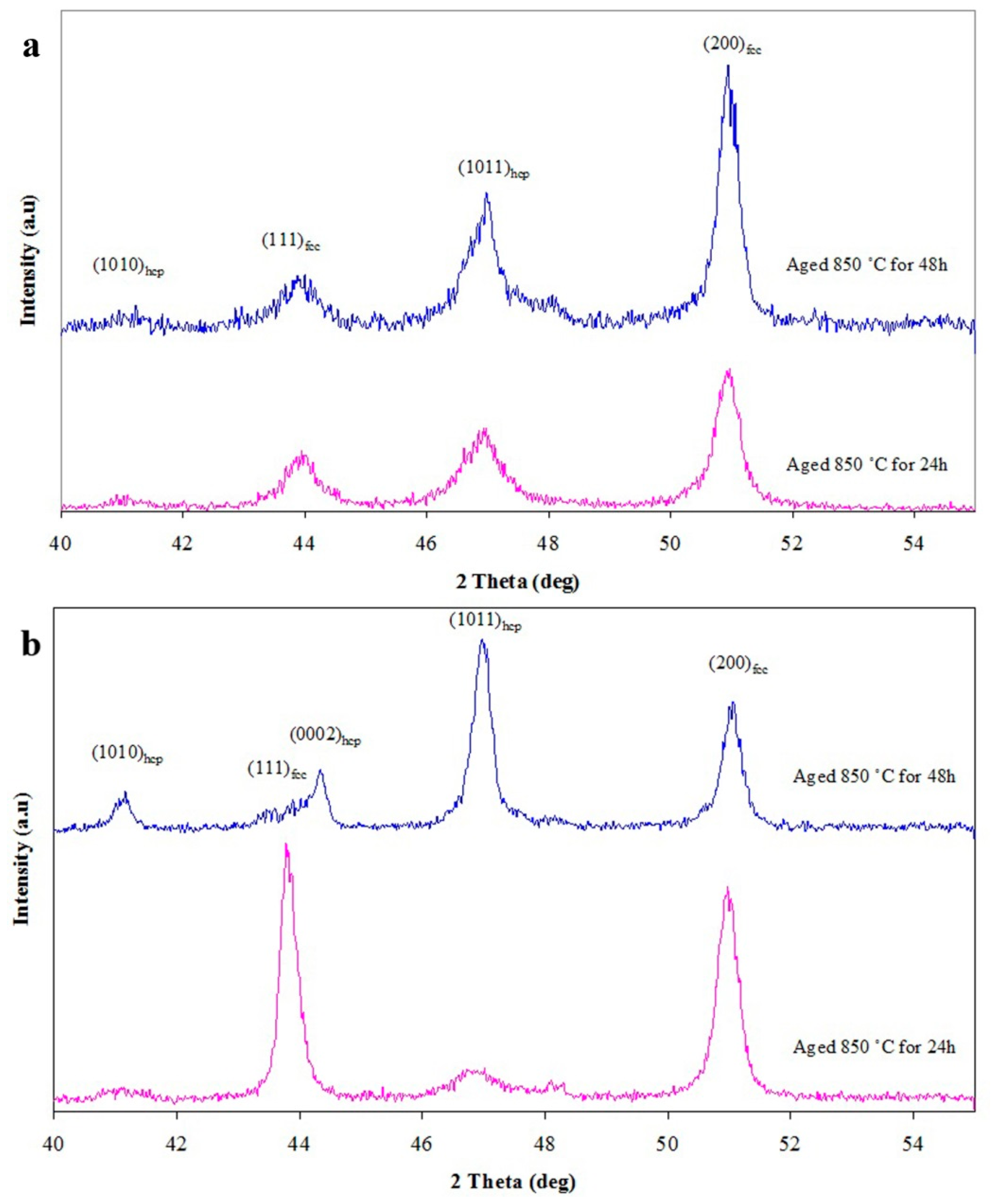
3.4. Aging of the Nearly Fully Solution Treated Sample
3.5. Aging of the Partially Solution Treated Sample
3.6. Microhardness Evolution
4. Conclusions
- (1).
- We used the thermodynamic databases, TCFE8 and TCNI8, for calculations with the ThermoCalc software. Despite observing similarities for the phase transformations at similar temperatures, we also observed some differences, such as the appearance of the sigma phase formation according to the TCNI8 database. The FCC phase also calculated the FCC_L12 and FCC_A1 structures.
- (2).
- Solution treatment at 1230 °C for 3 h resulted in the nearly fully solution of carbides, whilst solution treatment at 1200 °C for 1 h caused most of the primary carbides to remain in the matrix in an undissolved condition. The thermodynamic calculations were also consistent with the experimental results. Based on the calculations, ~1.7–4.3% of the carbides should be present at 1200 °C and 1230 °C.
- (3).
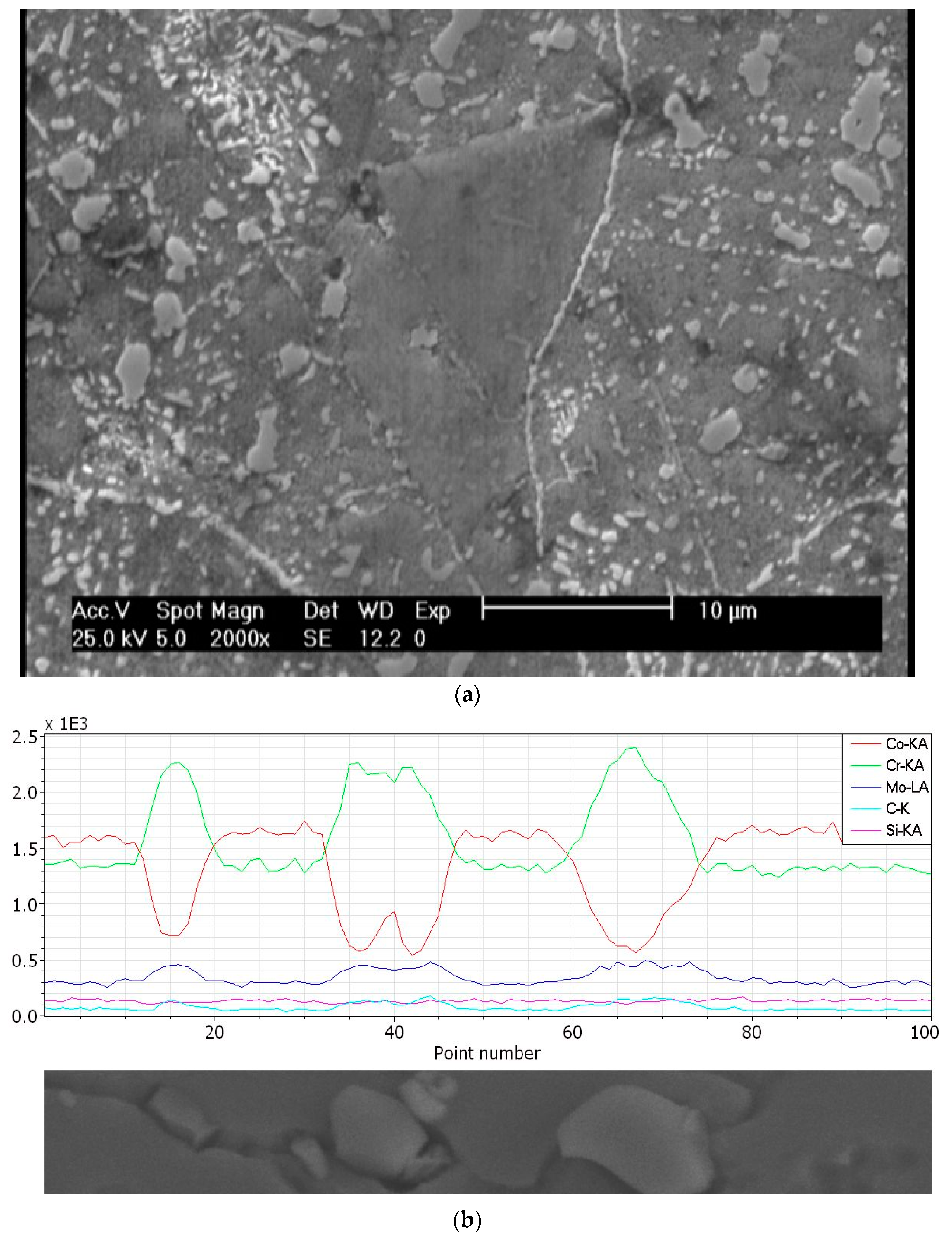
- The undissolved carbides of Cr, Mo, and Co have significant effects on the microstructural and mechanical characteristics of the solution treated and aged sample.
- (4).
- Aging of the nearly fully solution treated sample resulted in the development of HCP1 (straight bands) and HCP2 (lamellar-type constitution) phases, but aging of the partially solution treated sample resulted in formation of the HCP2 phase (lamellar-type constitution) at the grain boundaries.
- (5).
- The presence of undissolved carbides of Cr, Mo, and Co depletes the matrix from the HCP stabilizer elements, and it consequently enhances the FCC peak intensity of the substance after quenching. However, the FCC matrix changes more intensively compared to the HCP during aging. Thermodynamical calculations also indicated a decrease of the Cr and C amount in the FCC phase, with increasing solution temperatures.
- (6).
- The hardness enhancement as a result of aging for the sample solution treated at 1230 °C for 3 h was lower than that for the sample solution treated at 1200 °C for 1 h.
Author Contributions
Funding
Conflicts of Interest
References
- Saldívar, A.J.; López, H.F. Role of aging on the martensitic transformation in a cast cobalt alloy. Scr. Mater. 2001, 45, 427–433. [Google Scholar] [CrossRef]
- Julián, L.C.; Muñoz, A.L. Influence of microstructure of HC CoCrMo biomedical alloys on the corrosion and wear behaviour in simulated body fluids. Tribol. Int. 2011, 44, 318–329. [Google Scholar] [CrossRef]
- Huang, P.; López, H.F. Athermal ε-martensite in a Co–Cr–Mo alloy: Grain size effects. Mater. Lett. 1999, 39, 249–253. [Google Scholar] [CrossRef]
- Montero-Ocampo, C.; Lopez, H.; Talavera, M. Effect of alloy preheating on the mechanical properties of as-cast Co-Cr-Mo-C alloys. Metall. Mater. Trans. A 1999, 30, 611–620. [Google Scholar] [CrossRef]
- Zangeneh, S.; Lashgari, H.R.; Saghafi, M.; Karshenas, M. Effect of isothermal aging on the microstructural evolution of Co–Cr–Mo–C alloy. Mater. Sci. Eng. A 2010, 527, 6494–6500. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Zangeneh, S.; Hasanabadi, F.; Saghafi, M. Microstructural evolution during isothermal aging and strain-induced transformation followed by isothermal aging in Co-Cr-Mo-C alloy: A comparative study. Mater. Sci. Eng. A 2010, 527, 4082–4091. [Google Scholar] [CrossRef]
- López, H.F.; Saldivar-Garcia, A.J. Martensitic Transformation in a Cast Co-Cr-Mo-C Alloy. Metall. Mater. Trans. A 2008, 39, 8–18. [Google Scholar] [CrossRef]
- Thermo-Calc TCC Software Users Guide, Version S. Available online: https://www.thermocalc.com/media/8136/tcc_usersguide.pdf (accessed on 17 May 2019).
- Yamanaka, K.; Mori, M.; Chiba, A. Effects of carbon concentration on microstructure and mechanical properties of as-cast nickel-free Co–28Cr–9W-based dental alloys. Mater. Sci. Eng. C 2014, 40, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Mori, M.; Sato, K.; Chiba, A. Characterisation of nanoscale carbide precipitation in as-cast Co–Cr–W-based dental alloys. J. Mater. Chem. B 2016, 4, 1778–1786. [Google Scholar] [CrossRef]
- Kurosu, S.; Matsumoto, H.; Chiba, A. Isothermal phase transformation in biomedical Co-29Cr-6Mo alloy without addition of carbon or nitrogen. Metall. Mater. Trans. A 2010, 41, 2613–2625. [Google Scholar] [CrossRef]
- Koßmann, J.; Zenk, C.H.; Lopez-Galilea, I.; Neumeier, S.; Kostka, A.; Huth, S.; Hammerschmidt, T. Microsegregation and precipitates of an as-cast Co-based superalloy—microstructural characterization and phase stability modelling. J. Mater. Sci. 2015, 50, 6329–6338. [Google Scholar] [CrossRef]
- Salam, S.; Hou, P.Y.; Zhang, Y.D.; Zhang, X.H.; Wang, H.F.; Zhang, C.; Yang, Z.G. Microstructural modelling solution for complex Co based alloys and coatings. Surf. Coat. Technol. 2013, 236, 510–517. [Google Scholar] [CrossRef]
- Thermodynamic database TCFE8-TCS Steels/Fe-alloys database (v.8.0) for Thermo-Calc. Available online: www.thermocalc.com (accessed on 17 May 2019).
- Thermodynamic database TCNI8-TCS Ni-based Superalloys Database (v.8.0) for Thermo-Calc. Available online: www.thermocalc.com (accessed on 17 May 2019).
- Zangeneh, S.; Farhangi, H. Influence of service-induced microstructural changes on the failure of a cobalt-based superalloy first stage nozzle. Mater. Des. 2010, 31, 3504–3511. [Google Scholar] [CrossRef]
- Sage, M.; Gillaud, C. Méthode d’analyse quantitative des variétés allotropiques du cobalt par les rayons X. Rev. Met. 1950, 49, 139–145. [Google Scholar] [CrossRef]
- Bleck, W. Material Science of Steel: Textbook for RWTH Students; Mainz: Aachen, Germany, 2007. [Google Scholar]
- Koizumi, Y.; Suzuki, S.; Yamanaka, K.; Lee, B.-S.; Sato, K.; Li, Y.; Kurosu, S.; Matsumoto, S.; Chiba, A. Strain-induced martensitic transformation near twin boundaries in a biomedical Co–Cr–Mo alloy with negative stacking fault energy. Acta Mater. 2013, 61, 1648–1661. [Google Scholar] [CrossRef]
- Ramírez, L.E.; Castro, M.; Méndez, M.; Lacaze, J.; Herrera, M.; Lesoult, G. Precipitation path of secondary phases during solidification of the Co–25.5%Cr–5.5%Mo–0.26%C alloy. Scr. Mater. 2002, 47, 811–816. [Google Scholar] [CrossRef]
- Zangeneh, S.; Ketabchi, M. Grain refinement by pearlitic-type constituents in Co–28Cr–5Mo–0.3C alloy. Mater. Lett. 2013, 94, 206–209. [Google Scholar] [CrossRef]
- Sims, C.T.; Stoloff, N.S.; Hagel, W.C. Superalloys II; Wiley: New York, NY, USA, 1987. [Google Scholar]





| %Cr | %Mo | %C | %Si | %Co |
|---|---|---|---|---|
| 28 | 5 | 0.3 | 1.5 | Balance |
| Database | Temperature, °C | ||||
|---|---|---|---|---|---|
| Liquidus | Solidus | M23C6 | HCP | Sigma | |
| TCFE8 | 1399 | 1255 | 1260 | 921 °C | - |
| TCNI8 | 1380 | 1248 | 1255 | 942 °C | 950 °C |
| Database | HCP | M23C6 | ||||||
|---|---|---|---|---|---|---|---|---|
| Phase vol.-% | wt.-% in HCP | Phase vol.-% | wt.-% in M23C6 | |||||
| Cr | Mo | C | Cr | Mo | C | |||
| TCFE8 | 94.36 | 25.84 | 4.28 | 0.037 | 5.64 | 67.85 | 18.32 | 5.237 |
| TCNI8 | 93.98 | 25.68 | 4.32 | 0.017 | 6.02 | 67.99 | 16.64 | 5.182 |
| Temperature, °C | Database | FCC | M23C6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase vol.-% | wt.-% in FCC | Phase vol.-% | wt.-% in M23C6 | ||||||
| Cr | Mo | C | Cr | Mo | C | ||||
| 1200 | TCFE8 | 97.75 | 27.13 | 4.86 | 0.193 | 2.25 | 68.39 | 11.65 | 5.282 |
| 1200 | TCNI8 | 95.67 | 26.37 | 4.67 | 0.091 | 4.33 | 66.61 | 15.42 | 5.249 |
| 1230 | TCFE8 | 98.31 | 27.35 | 4.91 | 0.220 | 1.69 | 69.09 | 10.79 | 5.299 |
| 1230 | TCNI8 | 96.07 | 26.54 | 4.72 | 0.111 | 3.92 | 66.45 | 12.38 | 5.255 |
| Solution Treatment | Solutionized and Quenched | Aged at 850 °C for 24 h | Aged at 850 °C for 48 h |
|---|---|---|---|
| 1230 °C for 3 h | 380 ± 10 | 520 ± 15 | 530 ± 25 |
| 1200 °C for 1 h | 410 ± 15 | 647 ± 23 | 705 ± 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangeneh, S.; Erisir, E.; Abbasi, M.; Ramazani, A. Evaluation of the Aging Effect on the Microstructure of Co-28Cr-6Mo-0.3C Alloy: Experimental Characterization and Computational Thermodynamics. Metals 2019, 9, 581. https://doi.org/10.3390/met9050581
Zangeneh S, Erisir E, Abbasi M, Ramazani A. Evaluation of the Aging Effect on the Microstructure of Co-28Cr-6Mo-0.3C Alloy: Experimental Characterization and Computational Thermodynamics. Metals. 2019; 9(5):581. https://doi.org/10.3390/met9050581
Chicago/Turabian StyleZangeneh, Shahab, Ersoy Erisir, Mahmoud Abbasi, and Ali Ramazani. 2019. "Evaluation of the Aging Effect on the Microstructure of Co-28Cr-6Mo-0.3C Alloy: Experimental Characterization and Computational Thermodynamics" Metals 9, no. 5: 581. https://doi.org/10.3390/met9050581





