Recoveries of Ru(III) and Co(II) by Solvent Extraction and Ion Exchange from Tungsten Carbide-Cobalt Scrap through a HCl Leaching Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Solvent Extraction and Ion Exchange Experimental Procedures
3. Results and Discussion
3.1. Leaching of W, Co, Ru with HCl Solution
3.2. Separation of Ru(III) and Co(II) by Solvent Extraction
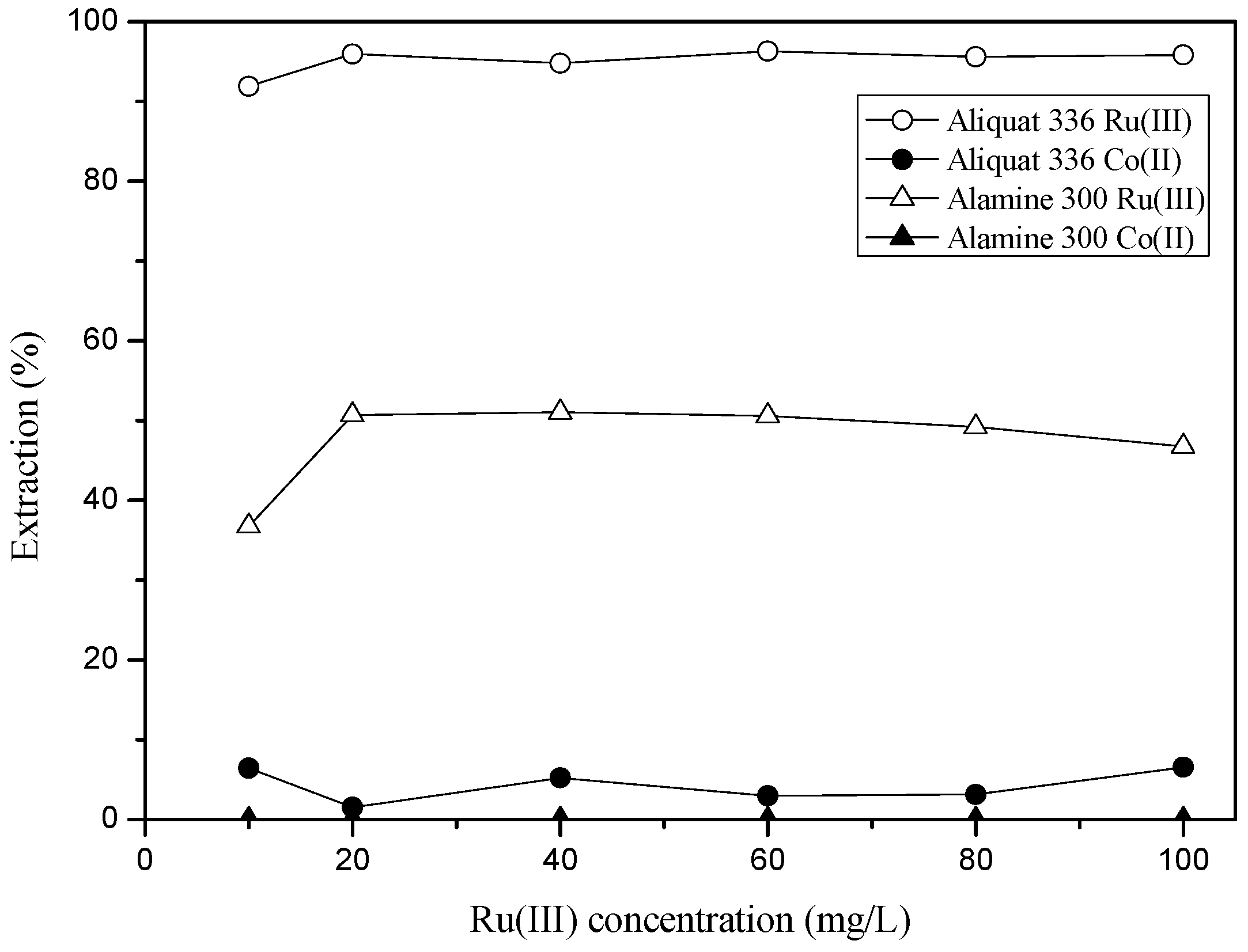
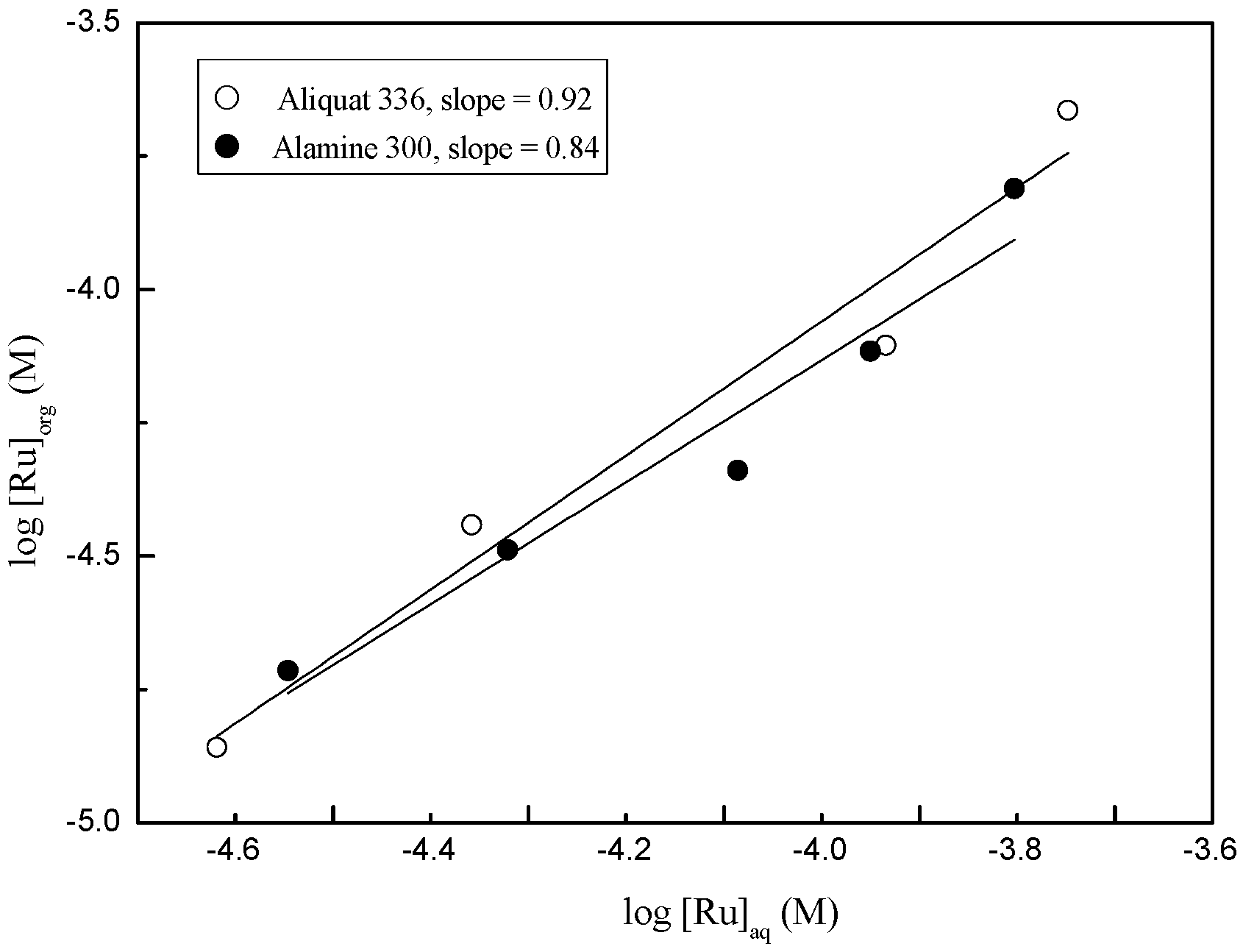
3.2.1. Extraction of Ru(III) and Co(II) by Amine Extractants
3.2.2. Stripping of Ru(III) from the Loaded Aliquat 336
3.3. Ru(III) and Co(II) Separation by Ion Exchange
3.3.1. Adsorption of Ru(III) and Co(II) into Anionic Exchange Resins
3.3.2. Loading Behavior of Ru(III) and Co(II) into the Resins at 3M HCl
3.3.3. Elution of Ru(III) from the Loaded Resin
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lisovskii, A.F. Cemented carbides alloyed with ruthenium, osmium and rhenuim. Powder Metall. Met. Ceram. 2000, 39, 9–10. [Google Scholar] [CrossRef]
- Luyckx, S. High temperature hardness of WC-Co-Ru. J. Mater. Sci. Lett. 2002, 21, 1681–1682. [Google Scholar] [CrossRef]
- García, J.; Collado Ciprés, V.; Blomqvist, A.; Kaplan, B. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mat. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Shing, T.L.; Luyckx, S.; Northrop, I.T.; Wol, I. The effect of ruthenium additions on the hardness, toughness and grain size of Wc-Co. Int. J. Refract. Met. Hard Mat. 2001, 19, 41–44. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Thanjekwayo, N.; Olubambi, P.; Maledi, N.; Potgieter-Vermaak, S.S. Influence of Ru additions on the corrosion behaviour of WC–Co cemented carbide alloys in sulphuric acid. Int. J. Refract. Met. Hard Mat. 2011, 29, 478–487. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Olubambi, P.; Potgieter-Vermaak, S.S. The corrosion behaviour of WC-Co-Ru alloys in aggressive chloride media. Int. J. Electrochem. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Shemi, A.; Magumisea, A.; Ndlovua, S.; Sacks, N. Recycling of tungsten carbide scrap metal: A review of recycling methods and future prospects. Miner. Eng. 2018, 122, 195–205. [Google Scholar] [CrossRef]
- Martins, J.I. Leaching systems of wolframite and scheelite: A thermodynamic approach. Miner. Process Extr. Metall. Rev. 2013, 35, 23–43. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. Recovery of gold(iii) from the stripping solution containing palladium(ii) by ion exchange and synthesis of gold particles. J. Ind. Eng. Chem. 2019, 69, 255–262. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Separation of Co(II) and Ni(II) from chloride leach solution of nickel laterite ore by solvent extraction with cyanex 301. Int. J. Miner. Process. 2017, 166, 45–52. [Google Scholar] [CrossRef]
- Katiyara, P.K.; Randhawa, N.S.; Hait, J.; Jana, R.K.; Singh, K.K.; Mankhand, T.R. An overview on different processes for recovery of valuable metals from tungsten carbide scrap. In Proceedings of the 18th International Conference on Nonferrous Minerals and Metals, Nagpur, India, 11–12 July 2014. [Google Scholar]
- Farrell, G.; Anderson, D.M.; Walton, M.E. Tungsten Recovery from Carbides. US Patent 4533527, 6 August 1985. [Google Scholar]
- Reilly, K.T. Recovery of Refractory Metal Values from Scrap Cemented Carbide. US Patent 4406866, 27 September 1983. [Google Scholar]
- Wellens, S.; Thijs, B.; Binnemans, K. An environmentally friendlier approach to hydrometallurgy: Highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef]
- Viljoen, K. The Interconversion of the Hexachlororuthenate(iii) and Aquapentachlororuthenate(iii) Species. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2003. [Google Scholar]
- Filiz, M.; Sayar, A.A.; Sayar, A.A. Extraction of cobalt(ii) from aqueous hydrochloric acid solutions into alamine 336–m-xylene mixtures. Hydrometallurgy 2006, 81, 167–173. [Google Scholar] [CrossRef]
- Banda, R.; Sohn, S.H.; Lee, M.S. Solvent extraction separation of Mo and Co from chloride solution containing Al. Mater. Trans. 2013, 54, 61–65. [Google Scholar] [CrossRef]
- Shen, Y.F.; Xue, W.Y.; Niu, W.Y. Recovery of Co(II) and Ni(II) from hydrochloric acid solution of alloy scrap. Trans. Nonferrous Met. Soc. 2008, 18, 1262–1268. [Google Scholar] [CrossRef]
- Goralska, E.; Coll, M.T.; Fortuny, A.; Kedari, C.S.; Sastre, A.M. Studies on the selective separation of Ir(IV), Ru(III) and Rh(III) from chloride solutions using alamine 336 in kerosene. Solvent Extr. Ion Exch. 2007, 25, 65–77. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dash, T.; Nathsarma, K.C.; Sarangi, K. Extraction of ruthenium using both tertiary and quaternary amine from chloride media. Sep. Sci. Technol. 2014, 49, 545–552. [Google Scholar] [CrossRef]
- Kedari, S.; Col, M.T.; Fortuny, A.; Goralska, E.; Sastre, A.M. Liquid-liquid extraction of ir, ru and rh from chloride solutions and their separation using different commercially available solvent extraction reagents. Sep. Sci. Technol. 2005, 40, 1927–1946. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, J.H.; Kim, J.H. Ion exchange characteristics of rhodium and ruthenium from a simulated radioactive liquid waste. Korean J. Chem. Eng. 2004, 5, 1038–1043. [Google Scholar] [CrossRef]
- Gao, N.; Liu, D.; Zhang, G.; Zhang, J.; Jia, S.; Yu, C.; Jiang, K.; Gao, N. The role of lactic acid adsorption by ion exchange chromatography. PLoS ONE 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miessler, G.L.; Fischer, P.J.; Tarr, D.A. Inorganic Chemistry; Pearson Education: London, UK, 2014; pp. 205–214. [Google Scholar]








| Resin | Ionic Forms | Functional Group | Bead Size (µm) | Capacity (meq/mL) | Density (g/mL) | Matrix |
|---|---|---|---|---|---|---|
| Amberlite® XAD7HP | nonionic | Nonionic | 560–710 | - | 1.05 | Macroreticular aliphatic cross-linked polymer |
| Amberlite® IRA402 | chloride | Trimethyl ammonium | 600–750 | 1.2 | 0.67 | Styrene divinylbenzene copolymer |
| AG®1-X8 | chloride | R-CH2N+ (CH3)3 | 45–106 | 1.2 | 0.75 | - |
| Bonlite BA304 | chloride | R-N+(CH3)3-X− | 450–700 | ≥1.3 | 1.06–1.10 | Polystyrene–DVB |
| Lewatit®MP-64 | chloride | Tertiary/quarternary amine | 300–1250 | 1.3 | 1.04 | Macroporous |
| Eluants | AG 1-X8 Elution (%) | Lewatit MP-64 Elution (%) |
|---|---|---|
| 3 M HCl | 37 | 22 |
| 1 M Thiourea | 83 | 80 |
| 0.2 M Thiourea + 0.2 M HCl | 94 | 93 |
| Aqua regia (Distilled 10 times) | 59 | 39 |
| 1 M HCl + 10% acetone | 64 | 32 |
| 1 M NaOH | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.H.; Lee, M.S. Recoveries of Ru(III) and Co(II) by Solvent Extraction and Ion Exchange from Tungsten Carbide-Cobalt Scrap through a HCl Leaching Solution. Metals 2019, 9, 858. https://doi.org/10.3390/met9080858
Ahn HH, Lee MS. Recoveries of Ru(III) and Co(II) by Solvent Extraction and Ion Exchange from Tungsten Carbide-Cobalt Scrap through a HCl Leaching Solution. Metals. 2019; 9(8):858. https://doi.org/10.3390/met9080858
Chicago/Turabian StyleAhn, Hyeong Hun, and Man Seung Lee. 2019. "Recoveries of Ru(III) and Co(II) by Solvent Extraction and Ion Exchange from Tungsten Carbide-Cobalt Scrap through a HCl Leaching Solution" Metals 9, no. 8: 858. https://doi.org/10.3390/met9080858





