Persistence of Antibody Responses to the SARS-CoV-2 in Dialysis Patients and Renal Transplant Recipients Recovered from COVID-19
Abstract
:1. Introduction
2. Results
2.1. Patients
2.2. SARS-CoV-2 S1/S2 IgG
2.3. Neutralizing Antibodies
2.4. Factors Associated to Antibody Responses
3. Discussion
4. Materials and Methods
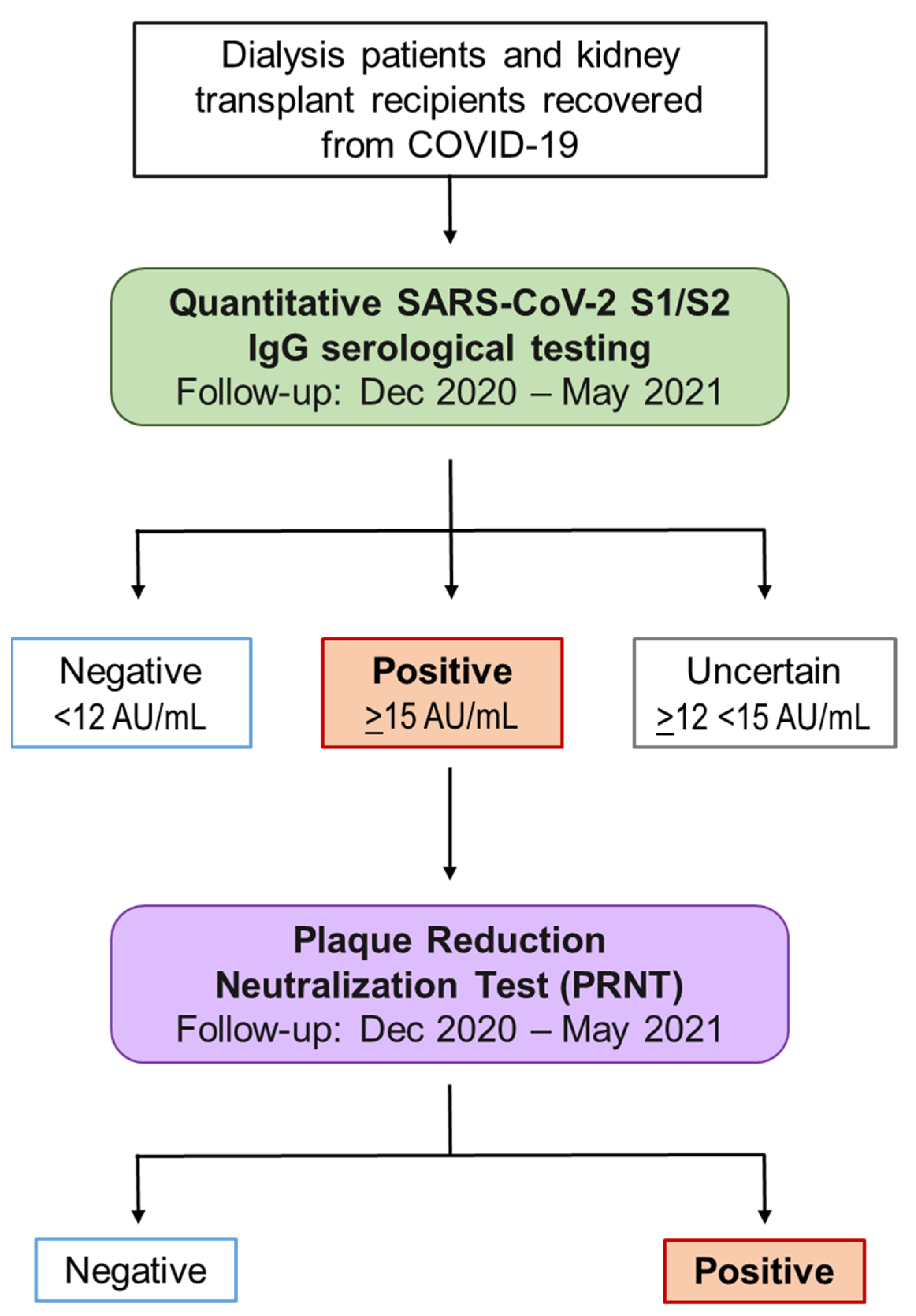
4.1. Study Design and Participants
4.2. Laboratory Assays
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlson, N.; Nelveg-Kristensen, K.; Ballegaard, E.F.; Feldt-Rasmussen, B.; Hornum, M.; Kamper, A.; Gislason, G.; Torp-Pedersen, C. Increased vulnerability to COVID-19 in chronic kidney disease. J. Intern. Med. 2021, 290, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Ajaimy, M.; Melamed, M.L. COVID-19 in Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, A. The COVID-19 pandemic: Consequences for nephrology. Nat. Rev. Nephrol. 2020, 17, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Gibertoni, D.; Reno, C.; Rucci, P.; Fantini, M.P.; Buscaroli, A.; Mosconi, G.; Rigotti, A.; Giudicissi, A.; Mambelli, E.; Righini, M.; et al. COVID-19 incidence and mortality in non-dialysis chronic kidney disease patients. PLoS ONE 2021, 16, e0254525. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Miskulin, D.C.; Manley, H.J.; Stewart, C.; Ladik, V.; Hosford, J.; Lacson, E.C.; Johnson, D.S.; et al. COVID-19 Among US Dialysis Patients: Risk Factors and Outcomes From a National Dialysis Provider. Am. J. Kidney Dis. 2021, 77, 748–756.e1. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Jaladanki, S.K.; Somani, S.; Paranjpe, I.; Kumar, A.; Zhao, S.; Kaufman, L.; Leisman, S.; Sharma, S.; He, J.C.; et al. Outcomes of Patients on Maintenance Dialysis Hospitalized with COVID-19. Clin. J. Am. Soc. Nephrol. 2020, 16, 452–455. [Google Scholar] [CrossRef]
- Nair, V.; Jandovitz, N.; Hirsch, J.S.; Nair, G.; Abate, M.; Bhaskaran, M.; Grodstein, E.; Berlinrut, I.; Hirschwerk, D.; Cohen, S.L.; et al. COVID-19 in kidney transplant recipients. Arab. Archaeol. Epigr. 2020, 20, 1819–1825. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, J.; Zhu, Y.; Liu, L.; Liu, Y.; He, Q. Mortality in chronic kidney disease patients with COVID-19: A systematic review and meta-analysis. Int. Urol. Nephrol. 2021, 53, 1623–1629. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Q.; Zhang, W.; Yu, S.; Cheng, X.; Wang, L.; Chen, X.; Chen, Y. The Involvement of Chronic Kidney Disease and Acute Kidney Injury in Disease Severity and Mortality in Patients with COVID-19: A Meta-Analysis. Kidney Blood Press. Res. 2021, 46, 17–30. [Google Scholar] [CrossRef]
- De Meester, J.; De Bacquer, D.; Naesens, M.; Meijers, B.; Couttenye, M.M.; De Vriese, A.S.; for the NBVN Kidney Registry Group. Incidence, Characteristics, and Outcome of COVID-19 in Adults on Kidney Replacement Therapy: A Regionwide Registry Study. J. Am. Soc. Nephrol. 2020, 32, 385–396. [Google Scholar] [CrossRef]
- Contou, D.; Fraissé, M.; Pajot, O.; Tirolien, J.-A.; Mentec, H.; Plantefève, G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: No prognostic improvement during the second wave? Crit. Care 2021, 25, 1–4. [Google Scholar] [CrossRef]
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; de Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef]
- Ozturk, S.; Turgutalp, K.; Arici, M.; Odabas, A.R.; Altiparmak, M.R.; Aydin, Z.; Cebeci, E.; Basturk, T.; Soypacaci, Z.; Sahin, G.; et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey. Nephrol. Dial. Transplant. 2020, 35, 2083–2095. [Google Scholar] [CrossRef]
- Alfishawy, M.; Elbendary, A.; Mohamed, M.; Nassar, M. COVID-19 Mortality in Transplant Recipients. Int. J. Organ. Transplant. Med. 2020, 11, 145–162. [Google Scholar]
- Mamode, N.; Ahmed, Z.; Jones, G.; Banga, N.; Motallebzadeh, R.; Tolley, H.; Marks, S.; Stojanovic, J.; Khurram, M.A.; Thuraisingham, R.; et al. Mortality Rates in Transplant Recipients and Transplantation Candidates in a High-prevalence COVID-19 Environment. Transplantation 2020, 105, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Finer, N.; Garnett, S.P.; Bruun, J.M. COVID-19 and obesity. Clin. Obes. 2020, 10. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Catalano, M.; Colgan, M.-P.; Pecsvarady, Z.; Wautrecht, J.C.; Fazeli, B.; Olinic, D.-M.; Farkas, K.; Elalamy, I.; Falanga, A.; et al. Guidance for the Management of Patients with Vascular Disease or Cardiovascular Risk Factors and COVID-19: Position Paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb. Haemost. 2020, 120, 1597–1628. [Google Scholar] [CrossRef]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef]
- Hartzell, S.; Bin, S.; Benedetti, C.; Haverly, M.; Gallon, L.; Zaza, G.; Riella, L.V.; Menon, M.C.; Florman, S.; Rahman, A.H.; et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am. J. Transplant. 2020, 20, 3149–3161. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; McKinnon, T.; Lightstone, L.; Pickering, M.C.; Thomas, D.C.; McAdoo, S.P.; Willicombe, M. SARS-CoV-2 Antibody Point-of-Care Testing in Dialysis and Kidney Transplant Patients With COVID-19. Kidney Med. 2020, 3, 54–59. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.-H.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Eucher, C.; Elsen, M.; Gillot, C.; Van Eeckhoudt, S.; Dogné, J.-M.; Douxfils, J. Persistence of Anti-SARS-CoV-2 Antibodies Depends on the Analytical Kit: A Report for Up to 10 Months after Infection. Microorganisms 2021, 9, 556. [Google Scholar] [CrossRef]
- Bruno, P.F.; Cappuccilli, M.; Spazzoli, A.; De Liberali, M.; Sejdiu, B.; Napoli, M.; Minerva, V.; Semprini, S.; Dirani, G.; Sambri, V.; et al. COVID-19 Infection: Viral Clearance and Antibody Response in Dialysis Patients and Renal Transplant Recipients. Nephron 2021, 145, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, S.; Resmini, B.; Merlino, M.; Licciardello, C.; Aloardi, R.; Palmetti, D.; Danelli, L. Inferno, disruption, concern, sense of community, teamwork, tears: Reflections by renal healthcare team members on the front lines of the COVID-19 pandemic. J. Nephrol. 2021, 34, 7–10. [Google Scholar] [CrossRef]
- Mosconi, G.; Spazzoli, A.; Bruno, P.F.; Angelini, M.L.; Cristino, S.; Lifrieri, M.F.; Americo, C.; De Fabritiis, M.; Ambri, K.; Dirani, G.; et al. Resilience in COVID-19 times: General considerations on the recovery of a 93-year-old patient on haemodialysis treatment. G. Ital. Nefrol. 2020, 37. [Google Scholar]
- Wei, J.; Zhao, J.; Han, M.; Meng, F.; Zhou, J. SARS-CoV-2 infection in immunocompromised patients: Humoral versus cell-mediated immunity. J. Immunother. Cancer 2020, 8, e000862. [Google Scholar] [CrossRef]
- Silvano, J.; Ferreira, F.; Bustorff, M.; Nunes, A.T.; Tavares, I.; Simões, J.S.; Ramos, A.; Cardoso, M.J.; Sampaio, S.; Pestana, M. Viral Clearance and Serological Response to SARS-CoV-2 in Kidney Transplant Recipients. Transplant. Proc. 2020, 53, 1180–1186. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of Immune Dysfunction in End-stage Renal Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, B.; Yee, J. Eradicating the Viral Triad in Hemodialysis Units. Adv. Chronic Kidney Dis. 2019, 26, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Del Bello, A.; Abravanel, F.; Marion, O.; Couat, C.; Esposito, L.; Lavayssière, L.; Izopet, J.; Kamar, N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Arab. Archaeol. Epigr. 2021. [Google Scholar] [CrossRef]
- Vaccari, J.C.D.R.; Dietrich, W.D.; Keane, R.W. The Inflammasome in Times of COVID-19. Front. Immunol. 2020, 11, 583373. [Google Scholar] [CrossRef] [PubMed]
- La Manna, G.; Cappuccilli, M.; Cianciolo, G.; Conte, D.; Comai, G.; Carretta, E.; Scolari, M.P.; Stefoni, S. Cardiovascular Disease in Kidney Transplant Recipients: The Prognostic Value of Inflammatory Cytokine Genotypes. Transplantation 2010, 89, 1001–1008. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Soraya, G.V. Anti-IL-6 receptor antibody treatment for severe COVID-19 and the potential implication of IL-6 gene polymorphisms in novel coronavirus pneumonia. Med. Clin. 2020, 155, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Nordio, M.; Reboldi, G.; Di Napoli, A.; Quintaliani, G.; Alberici, F.; Postorino, M.; Aucella, F.; Messa, P.; Brunori, G. Risk factors and action thresholds for the novel coronavirus pandemic. Insights from the Italian Society of Nephrology COVID-19 Survey. J. Nephrol. 2021, 34, 325–335. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Oliaei, S.; Kianzad, S.; Afsahi, A.M.; Mohssenipour, M.; Barzegary, A.; Mirzapour, P.; Behnezhad, F.; Noori, T.; Mehraeen, E.; et al. Reinfection risk of novel coronavirus (CoVID-19): A systematic review of current evidence. World J. Virol. 2020, 9, 79–90. [Google Scholar] [CrossRef]
- Harrington, W.E.; Trakhimets, O.; Andrade, D.V.; Dambrauskas, N.; Raappana, A.; Jiang, Y.; Houck, J.; Selman, W.; Yang, A.; Vigdorovich, V.; et al. Rapid decline of neutralizing antibodies is associated with decay of IgM in adults recovered from mild COVID-19 disease. Cell Rep. Med. 2021, 2, 100253. [Google Scholar] [CrossRef]
- Verghese, M.; Jiang, B.; Iwai, N.; Mar, M.; Sahoo, M.K.; Yamamoto, F.; Mfuh, K.O.; Miller, J.; Wang, H.; Zehnder, J.; et al. A SARS-CoV-2 Variant with L452R and E484Q Neutralization Resistance Mutations. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Ocal, S.; Selcuk, H.; Korkmaz, M.; Altun, R.; Yildirim, A.E.; Akbaş, E. Effect of HLA on hepatitis C virus clearance and persistence in anti-HCV-positive end-stage renal disease patients. Saudi J. Gastroenterol. 2014, 20, 175–181. [Google Scholar] [CrossRef]
- Stervbo, U.; Nienen, M.; Weist, B.J.D.; Kuchenbecker, L.; Hecht, J.; Wehler, P.; Westhoff, T.H.; Reinke, P.; Babel, N. BKV Clearance Time Correlates With Exhaustion State and T-Cell Receptor Repertoire Shape of BKV-Specific T-Cells in Renal Transplant Patients. Front. Immunol. 2019, 10, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L’Huillier, A.G.; Meyer, B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: A prospective longitudinal study. Clin. Microbiol. Infect. 2021, 27, 784.e1–784.e8. [Google Scholar] [CrossRef]
- Muir, L.; Jaffer, A.; Rees-Spear, C.; Gopalan, V.; Chang, F.Y.; Fernando, R.; Vaitkute, G.; Roustan, C.; Rosa, A.; Earl, C.; et al. Neutralizing Antibody Responses After SARS-CoV-2 Infection in End-Stage Kidney Disease and Protection Against Reinfection. Kidney Int. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Moradi, G.; Bolbanabad, A.M.; Ahmadi, S.; Aghaei, A.; Bahrami, F.; Veysi, A.; Kalmarzi, R.N.; Shokri, A.; Ghaderi, E.; Mohsenpour, B.; et al. Persistence assessment of SARS-CoV-2-specific IgG antibody in recovered COVID-19 individuals and its association with clinical symptoms and disease severity: A prospective longitudinal cohort study. Int. Immunopharmacol. 2021, 98, 107893. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Rubey, H.; Treipl, A.; Gromann, M.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef]
- Rockstroh, A.; Wolf, J.; Fertey, J.; Kalbitz, S.; Schroth, S.; Lübbert, C.; Ulbert, S.; Borte, S. Correlation of humoral immune responses to different SARS-CoV-2 antigens with virus neutralizing antibodies and symptomatic severity in a German COVID-19 cohort. Emerg. Microbes Infect. 2021, 10, 774–781. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Williams, W.W.; Ingelfinger, J.R. Third Time’s a Charm — COVID-19 Vaccine Hope for Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 1233–1234. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiology 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedeberg′s Arch. Pharmacol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Spearman, C. The Method of “Right and Wrong Cases” (Constant Stimuli) without Gauss’s Formula. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef]
- Amanat, F.; White, K.M.; Miorin, L.; Strohmeier, S.; McMahon, M.; Meade, P.; Liu, W.; Albrecht, R.A.; Simon, V.; Martinez-Sobrido, L.; et al. An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening. Curr. Protoc. Microbiol. 2020, 58, e108. [Google Scholar] [CrossRef] [PubMed]

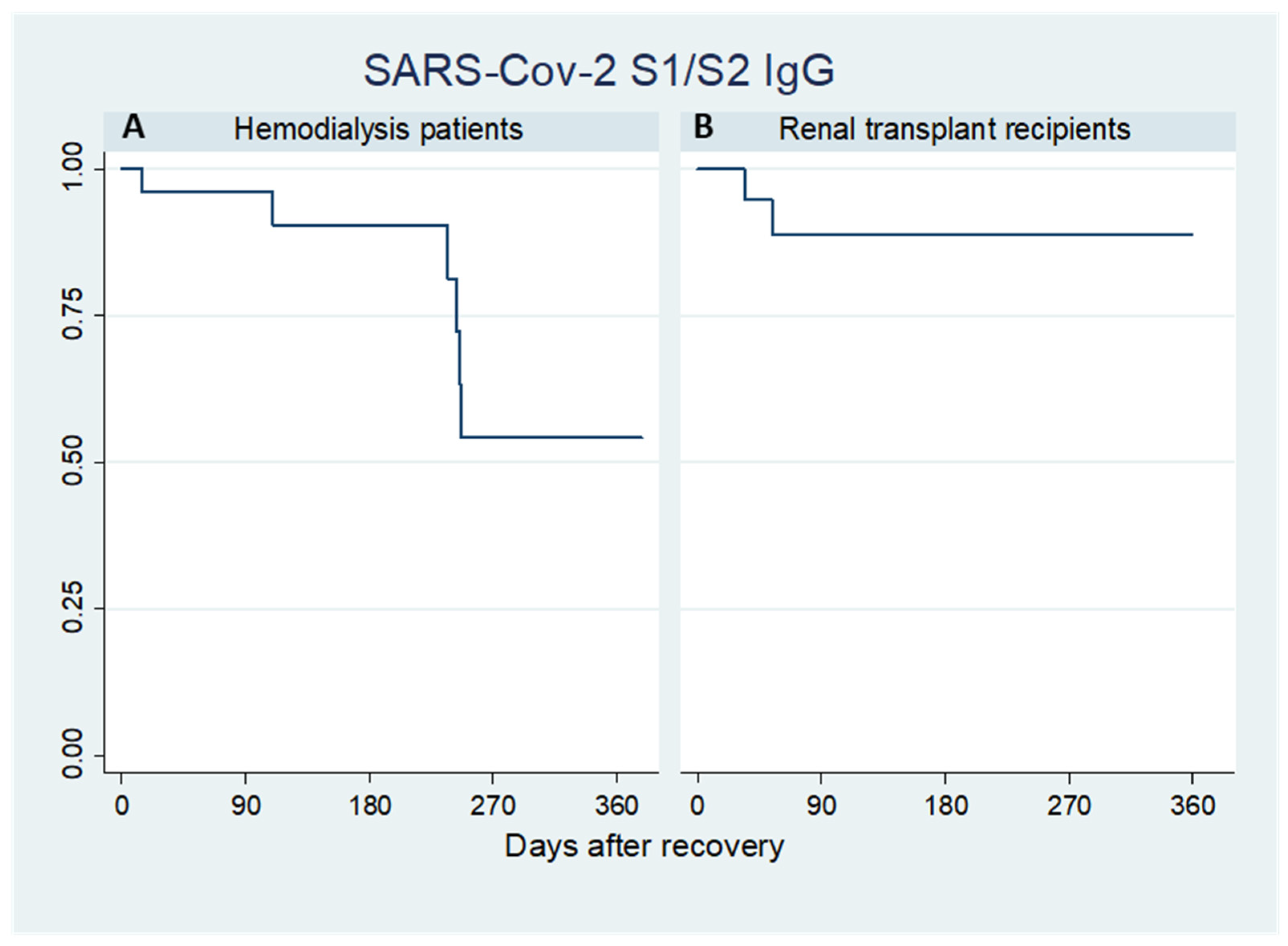



| TOTAL (n = 47) | Hemodialysis Patients (n = 26) | Renal Transplant Recipients (n = 21) | |
|---|---|---|---|
| Age, years | 60.1 ± 15.2 | 65.5 ± 15.0 * | 53.7 ± 13.1 * |
| Gender, M (%) | 36 (76.6%) | 20 (76.9%) | 16 (76.2%) |
| Dialysis vintage, months | / | 33.1 ± 27.9 | / |
| Transplant age, months | / | / | 75.3 ± 59.32 |
| sCreat at baseline, mg/dL | / | / | 1.5 ± 0.7 |
| sCreat at recovery, mg/dL | / | / | 1.6 ± 0.6 |
| Hemoglobin, mg/dL | 11.9 ± 1.7 | 11.6 ± 1.5 | 12.3 ± 1.8 |
| WBC, 103 cells/µL | 7.1 ± 2.4 | 7.4 ± 2.6 | 6.7 ± 2.0 |
| Platelet count, 103 cells/µL | 211 ± 70 | 206 ± 67 | 218 ± 75 |
| CRP, mg/dL | 4.7 ± 7.2 | 6.3 ± 8.7 * | 2.4 ± 2.7 * |
| LDH, mg/dL | 210 ± 53 | 211 ± 56 | 209 ± 50 |
| ALT, mg/dL | 14.7 ± 10.4 | 12.4 ± 9.9 | 18.0 ± 10.3 |
| Degree of respiratory distress | |||
| None/mild | 28 (59.6%) | 17 (65.4%) | 11 (52.4%) |
| Severe | 19 (40.4%) | 9 (34.6%) | 10 (47.6%) |
| Median, AU/mL | Range, AU/mL | IQR, AU/mL | ||
|---|---|---|---|---|
| Hemodialysis patients | T1: 0–30 days (n = 13) | 89.2 | 3.8–369.0 | 37.5–243.0 |
| T2: 31–90 days (n = 14) | 102.0 | 7.9–400.0 | 36.4–221.0 | |
| T3: 91–180 days (n = 9) | 117.5 | 11.5–400.0 | 58.8–400.0 | |
| T4: 181–300 days (n = 12) | 28.5 | 3.8–149.2 | 9.3–50.1 | |
| Renal transplant recipients | T1: 0–30 days (n = 17) | 66.2 | 22.2–258.3 | 33.6–137.3 |
| T2: 31–90 days (n = 17) | 69.9 | 3.8–400.0 | 27.2–129.1 | |
| T3: 91–180 days (n = 5) | 78.2 | 4.9–364.0 | 15.2–107.1 | |
| T4: 181–300 days (n = 2) | 22.7 | 15.7–27.4 | 18.0–26.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappuccilli, M.; Bruno, P.F.; Spazzoli, A.; Righini, M.; Flachi, M.; Semprini, S.; Grumiro, L.; Marino, M.M.; Schiavone, P.; Fabbri, E.; et al. Persistence of Antibody Responses to the SARS-CoV-2 in Dialysis Patients and Renal Transplant Recipients Recovered from COVID-19. Pathogens 2021, 10, 1289. https://doi.org/10.3390/pathogens10101289
Cappuccilli M, Bruno PF, Spazzoli A, Righini M, Flachi M, Semprini S, Grumiro L, Marino MM, Schiavone P, Fabbri E, et al. Persistence of Antibody Responses to the SARS-CoV-2 in Dialysis Patients and Renal Transplant Recipients Recovered from COVID-19. Pathogens. 2021; 10(10):1289. https://doi.org/10.3390/pathogens10101289
Chicago/Turabian StyleCappuccilli, Maria, Paolo Ferdinando Bruno, Alessandra Spazzoli, Matteo Righini, Marta Flachi, Simona Semprini, Laura Grumiro, Maria Michela Marino, Pasqua Schiavone, Elisabetta Fabbri, and et al. 2021. "Persistence of Antibody Responses to the SARS-CoV-2 in Dialysis Patients and Renal Transplant Recipients Recovered from COVID-19" Pathogens 10, no. 10: 1289. https://doi.org/10.3390/pathogens10101289






