Identification and Characterization of Antigenic Properties of Schistosoma japonicum Heat Shock Protein 90α Derived Peptides
Abstract
:1. Introduction
2. Results
2.1. Design and Preparation of Sjp90α-Derived Peptides
2.2. Effects of Sjp90α-Derived Peptides on Splenocytes
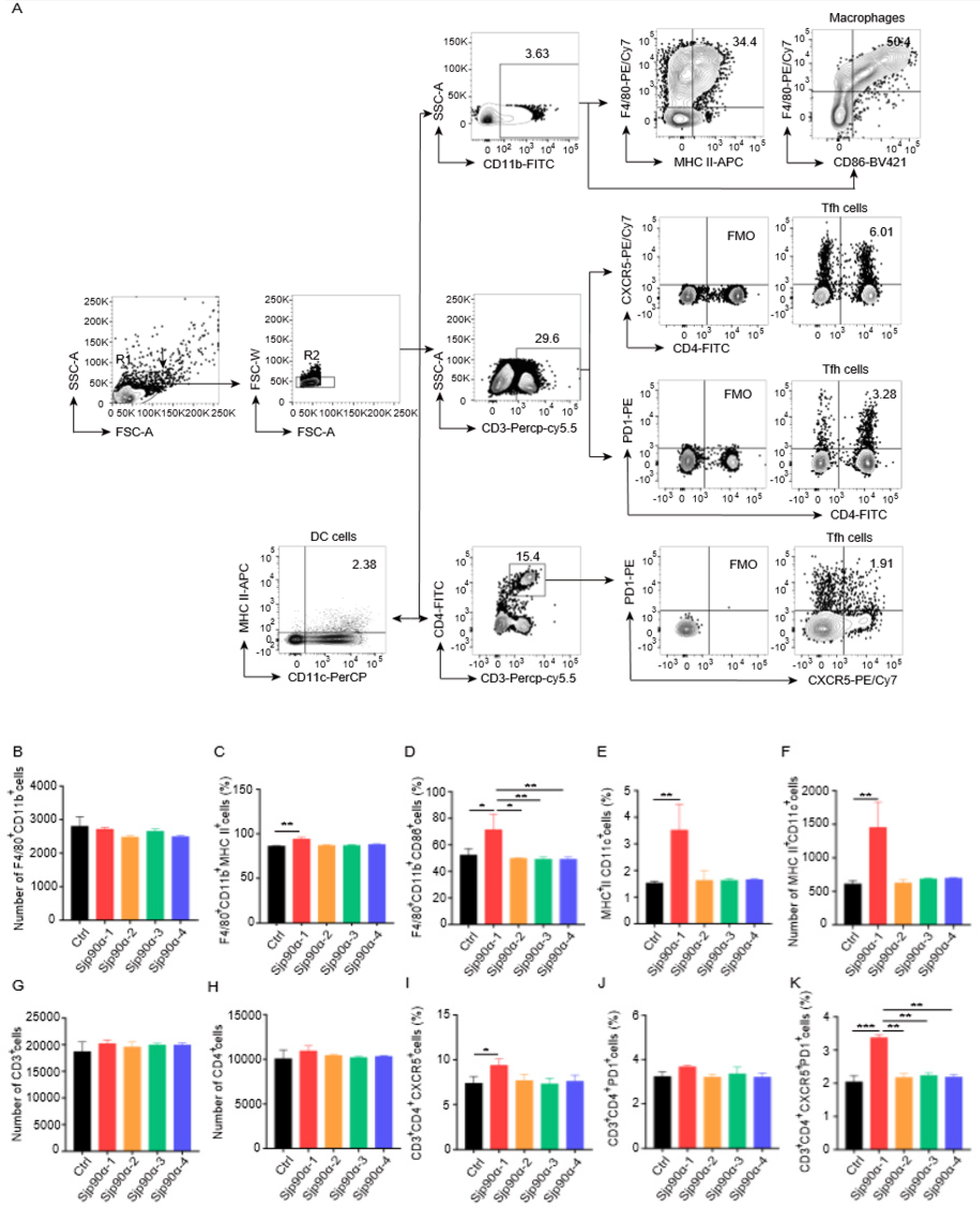
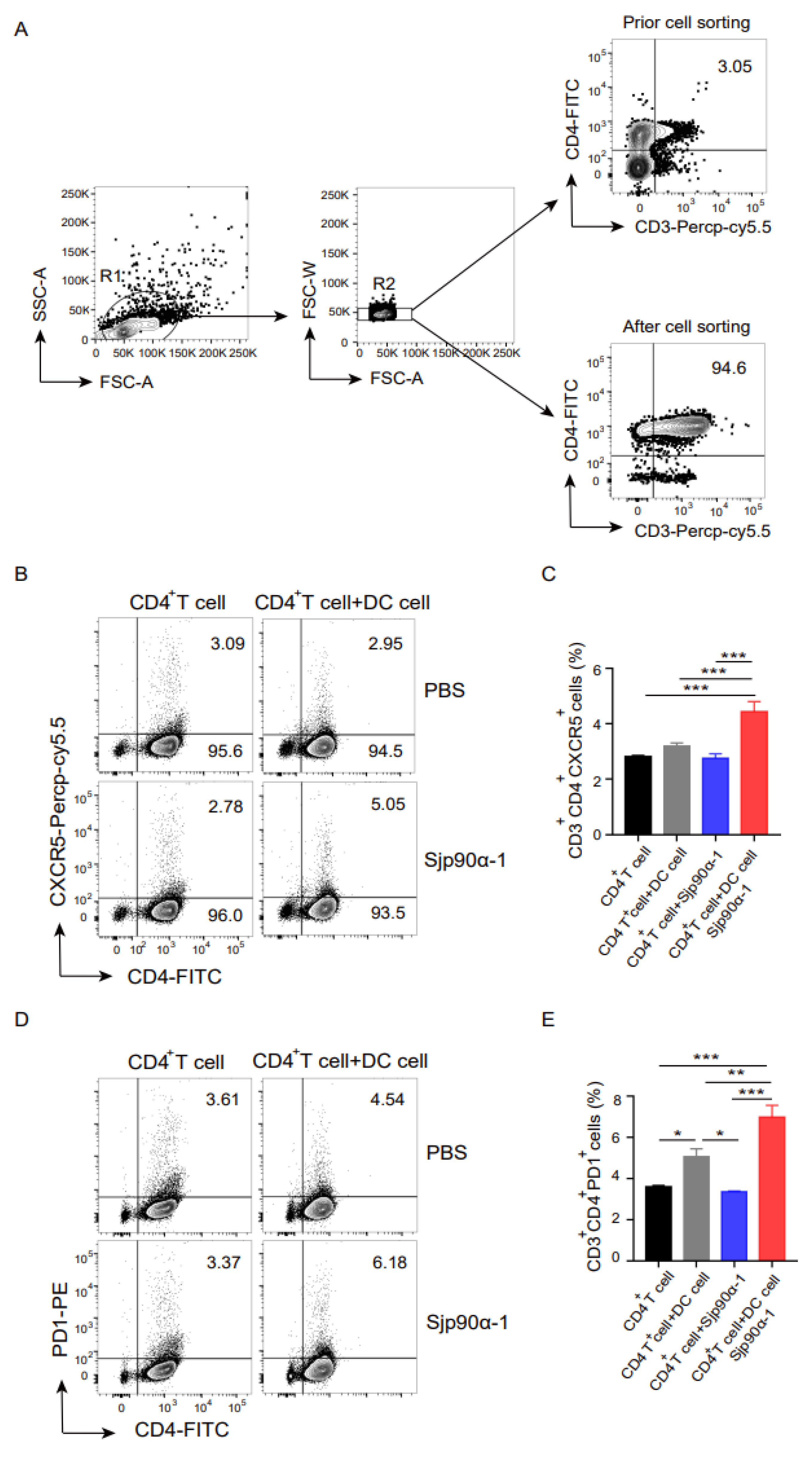
2.3. Sjp90α-1 Peptide-Activated BMDCs Induce CD4+ T-Cell Differentiation into Tfh Cells
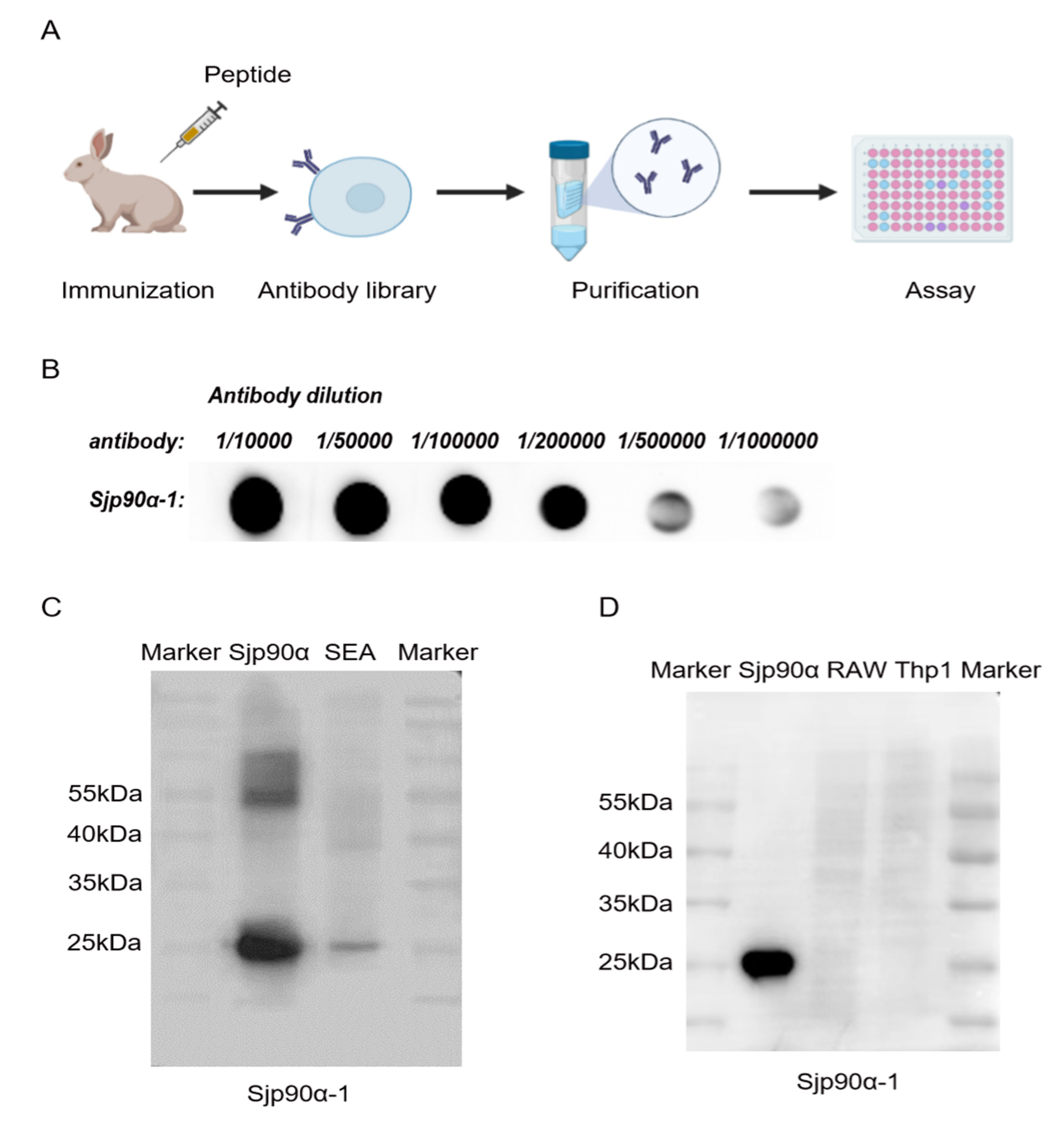
2.4. Preparation and Evaluation of Sjp90α-1-Peptide-based Antibody
2.5. Distribution of Sjp90α in S. japonicum Eggs and Adults
2.6. Dynamics of Sjp90α-1-Peptide-Based Antibody in Host Serum Determined by ELISA
3. Discussion
4. Materials and Methods
4.1. Mice and Infection
4.2. Immunogenic Peptide Designs from Sjp90α and Conjugation to Carrier
4.3. Flow Cytometry
4.4. Collection and Isolation of Serum
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Splenocyte Isolation and the Generation of Bone-Marrow-Derived DCs (BMDCs)
4.7. BMDC/CD4+ T-Cell Cocultures
4.8. Dot Blot Hybridization (DB)
4.9. Preparation of Soluble Egg Antigen (SEA)
4.10. Western Blot
4.11. Immunofluorescence Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Guo, Q.; Fu, Z.; Liu, J.; Lin, J.; Xiao, K.; Sun, P.; Cong, X.; Liu, R.; Hong, Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2021, 213, 105743. [Google Scholar] [CrossRef] [PubMed]
- Verjee, M.A. Schistosomiasis: Still a Cause of Significant Morbidity and Mortality. Res. Rep. Trop. Med. 2019, 10, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, W.J.; Magalhaes, F.D.C.; Elias, A.M.S.; de Castro, V.N.; Favero, V.; Lindholz, C.G.; Oliveira, A.A.; Barbosa, F.S.; Gil, F.; Gomes, M.A.; et al. Evaluation of diagnostic methods for the detection of intestinal schistosomiasis in endemic areas with low parasite loads: Saline gradient, Helmintex, Kato-Katz and rapid urine test. PLoS Negl. Trop. Dis. 2018, 12, e0006232. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Weerakoon, K.G.; Mu, Y.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; McManus, D.P. Comparison of Kato Katz, antibody-based ELISA and droplet digital PCR diagnosis of schistosomiasis japonica: Lessons learnt from a setting of low infection intensity. PLoS Negl. Trop. Dis. 2019, 13, e0007228. [Google Scholar] [CrossRef] [PubMed]
- Hinz, R.; Schwarz, N.G.; Hahn, A.; Frickmann, H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell. Probes 2017, 31, 2–21. [Google Scholar] [CrossRef]
- Hong, Y.; Peng, J.; Jiang, W.; Fu, Z.; Liu, J.; Shi, Y.; Li, X.; Lin, J. Proteomic analysis of schistosoma japonicum schistosomulum proteins that are differentially expressed among hosts differing in their susceptibility to the infection. Mol. Cell. Proteom. 2011, 10, M110.006098. [Google Scholar] [CrossRef] [Green Version]
- De Marco Verissimo, C.; Potriquet, J.; You, H.; McManus, D.P.; Mulvenna, J.; Jones, M.K. Qualitative and quantitative proteomic analyses of Schistosoma japonicum eggs and egg-derived secretory-excretory proteins. Parasites Vectors 2019, 12, 173. [Google Scholar] [CrossRef]
- Hu, W.; Yan, Q.; Shen, D.K.; Liu, F.; Zhu, Z.D.; Song, H.D.; Xu, X.R.; Wang, Z.J.; Rong, Y.P.; Zeng, L.C.; et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat. Genet. 2003, 35, 139–147. [Google Scholar] [CrossRef]
- Liu, F.; Cui, S.J.; Hu, W.; Feng, Z.; Wang, Z.Q.; Han, Z.G. Excretory/secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum. Mol. Cell. Proteom. 2009, 8, 1236–1251. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ji, M.; Li, C.; Du, X.; Hu, W.; McManus, D.P.; You, H. A Biological and Immunological Characterization of Schistosoma Japonicum Heat Shock Proteins 40 and 90alpha. Int. J. Mol. Sci. 2020, 21, 4034. [Google Scholar] [CrossRef]
- Perera, D.J.; Ndao, M. Promising Technologies in the Field of Helminth Vaccines. Front. Immunol. 2021, 12, 711650. [Google Scholar] [CrossRef]
- Patronov, A.; Doytchinova, I. T-cell epitope vaccine design by immunoinformatics. Open Biol. 2013, 3, 120139. [Google Scholar] [CrossRef] [Green Version]
- Korber, B.; LaBute, M.; Yusim, K. Immunoinformatics comes of age. PLoS Comput. Biol. 2006, 2, e71. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Gordon, C.A.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; Marsh, J.M.; McManus, D.P.; Cai, P. Identification of a linear B-cell epitope on the Schistosoma japonicum saposin protein, SjSAP4: Potential as a component of a multi-epitope diagnostic assay. PLoS Negl. Trop. Dis. 2022, 16, e0010619. [Google Scholar] [CrossRef]
- Silverman, J.M.; Reiner, N.E. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front. Cell Infect. Microbiol. 2011, 1, 26. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.P.; Paciello, M.O.; Aviz Teixeira, W.P.; Rivas, A.V.; Agular, R.W.S.; Cangussu, A.S.R.; Barbosa, L.C.B.; Marchetto, R.; Giunchetti, R.C.; Viana, K.F. Immunogenicity of HLA-DR1 and HLA-A2 peptides derived from Leishmania major Gp63 in golden hamsters. Parasite Immunol. 2020, 42, e12780. [Google Scholar] [CrossRef]
- Alloatti, A.; Kotsias, F.; Magalhaes, J.G.; Amigorena, S. Dendritic cell maturation and cross-presentation: Timing matters! Immunol. Rev. 2016, 272, 97–108. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, J.; Chen, H.; Nie, H.; Miller, H.; Gong, Q.; Liu, C. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front. Immunol. 2020, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Fairfax, K.; Nascimento, M.; Huang, S.C.; Everts, B.; Pearce, E.J. Th2 responses in schistosomiasis. Semin. Immunopathol. 2012, 34, 863–871. [Google Scholar] [CrossRef]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Ann. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Bordag, N.; Keller, S. Alpha-helical transmembrane peptides: A “divide and conquer” approach to membrane proteins. Chem. Phys. Lipids 2010, 163, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Tsunei, A.; Kusabuka, H.; Ogaki, E.; Tachibana, M.; Okada, N. Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold. Cells 2020, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Jiang, Y.; Pan, W.; Liu, H.; Yin, J.; Shen, Y.; Cao, J. T follicular helper cells in patients with acute schistosomiasis. Parasites Vectors 2016, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Guan, F.; Sun, L.; Zhang, Y.; Zhang, X.; Lu, S.; Liu, W. B cells induced by Schistosoma japonicum infection display diverse regulatory phenotypes and modulate CD4(+) T cell response. Parasites Vectors 2020, 13, 147. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yang, X.; Li, Y.; Zhu, J.; Zhou, S.; Xu, Z.; He, L.; Xue, X.; Zhang, W.; Dong, X.; et al. Follicular helper T cells promote liver pathology in mice during Schistosoma japonicum infection. PLoS Pathog. 2014, 10, e1004097. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Zhang, Y.; Song, X.; Xu, L.; Xu, Z.; Zhou, S.; Zhu, J.; Jin, X.; Liu, F.; et al. Distribution of Peripheral Memory T Follicular Helper Cells in Patients with Schistosomiasis Japonica. PLoS Negl. Trop. Dis. 2015, 9, e0004015. [Google Scholar] [CrossRef]
- Wilson, R.A.; Jones, M.K. Fifty years of the schistosome tegument: Discoveries, controversies, and outstanding questions. Int. J. Parasitol. 2021, 51, 1213–1232. [Google Scholar] [CrossRef]
- Lundy, S.K.; Lukacs, N.W. Chronic schistosome infection leads to modulation of granuloma formation and systemic immune suppression. Front. Immunol. 2013, 4, 39. [Google Scholar] [CrossRef]
- Burke, M.L.; Jones, M.K.; Gobert, G.N.; Li, Y.S.; Ellis, M.K.; McManus, D.P. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009, 31, 163–176. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.J.; Kanamura, H.Y.; Takei, K.; Hirata, R.D.; Valli, L.C.; Nguyen, N.Y.; de Carvalho Rodrigues, I.; de Jesus, A.R.; Hirata, M.H. Synthetic peptides as an antigenic base in an ELISA for laboratory diagnosis of schistosomiasis mansoni. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Astroz, Y.; Santos, A.; Oliveira, G.; Jensen, L.J. Analysis of Predicted Host-Parasite Interactomes Reveals Commonalities and Specificities Related to Parasitic Lifestyle and Tissues Tropism. Front. Immunol. 2019, 10, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, S.; Ziv, E.; Lantner, F.; Schechter, I. Regulation of HSP70 gene expression during the life cycle of the parasitic helminth Schistosoma mansoni. Eur. J. Biochem. 1993, 212, 589–596. [Google Scholar] [CrossRef]
- Carmena, D.; Benito, A.; Eraso, E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: An update. Acta Trop. 2006, 98, 74–86. [Google Scholar] [CrossRef]
- Armiento, V.; Spanopoulou, A.; Kapurniotu, A. Peptide-Based Molecular Strategies To Interfere with Protein Misfolding, Aggregation, and Cell Degeneration. Angew. Chem. Int. Ed. Engl. 2020, 59, 3372–3384. [Google Scholar] [CrossRef] [Green Version]
- Trier, N.H.; Houen, G. Peptide Antibodies in Clinical Laboratory Diagnostics. Adv. Clin. Chem. 2017, 81, 43–96. [Google Scholar] [CrossRef]
- Bolhassani, A.; Agi, E. Heat shock proteins in infection. Clin. Chim. Acta 2019, 498, 90–100. [Google Scholar] [CrossRef]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol. Immuno.l 2020, 17, 587–599. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Semmling, V.; Franken, L.; Wagner, H.; Kurts, C. Chemokines: A new dendritic cell signal for T cell activation. Front. Immunol. 2011, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.R.; Chroust, Z.D.; Onyoni, F.; Soong, L. Pattern Recognition Receptors in Innate Immunity to Obligate Intracellular Bacteria. Zoonoses 2021, 1, 10. [Google Scholar] [CrossRef]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef]
- Ahrens, V.M.; Bellmann-Sickert, K.; Beck-Sickinger, A.G. Peptides and peptide conjugates: Therapeutics on the upward path. Future Med. Chem. 2012, 4, 1567–1586. [Google Scholar] [CrossRef]
- Ayithan, N.; Tang, L.; Tan, S.K.; Chen, D.; Wallin, J.J.; Fletcher, S.P.; Kottilil, S.; Poonia, B. Follicular Helper T (TFH) Cell Targeting by TLR8 Signaling For Improving HBsAg-Specific B Cell Response In Chronic Hepatitis B Patients. Front. Immunol. 2021, 12, 735913. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Liu, D.; Li, J.; Zhang, X.; Chen, X.; Hou, S.; Peng, L.; Xu, C.; Liu, W.; et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013, 496, 523–527. [Google Scholar] [CrossRef]
- Winkelmann, F.; Gesell Salazar, M.; Hentschker, C.; Michalik, S.; Machacek, T.; Scharf, C.; Reisinger, E.C.; Volker, U.; Sombetzki, M. Comparative proteome analysis of the tegument of male and female adult Schistosoma mansoni. Sci. Rep. 2022, 12, 7569. [Google Scholar] [CrossRef]
- Gobert, G.N.; McManus, D.P.; McMullan, G.; Creevey, C.J.; Carson, J.; Jones, M.K.; Nawaratna, S.S.K.; Weerakoon, K.G.; You, H. Adult schistosomes have an epithelial bacterial population distinct from the surrounding mammalian host blood. PLoS ONE 2022, 17, e0263188. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, Y.; Han, Y.; Han, H.; Peng, J.; Qiu, C.; Yang, J.; Lu, K.; Fu, Z.; Lin, J. Proteomic analysis of tegument-exposed proteins of female and male Schistosoma japonicum worms. J. Proteome Res. 2013, 12, 5260–5270. [Google Scholar] [CrossRef] [PubMed]
- Aguoru, N.A.; Kirk, R.S.; Walker, A.J. Molecular insights into the heat shock proteins of the human parasitic blood fluke Schistosoma mansoni. Parasites Vectors 2022, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Woodruff, J.; Pathak, P.K.; Matts, R.L.; Deng, J. Crystal structure of the middle and C-terminal domains of Hsp90alpha labeled with a coumarin derivative reveals a potential allosteric binding site as a drug target. Acta Crystallogr D Struct Biol 2022, 78, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, X.; Chang, H.; Kong, Y.; Ni, Y.; Liu, R.; Zhang, X.; Hu, Y.; Yang, Z.; Hou, M.; et al. Rescue of maternal immune activation-induced behavioral abnormalities in adult mouse offspring by pathogen-activated maternal Treg cells. Nat. Neurosci. 2021, 24, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef]
- Chang, H.; He, K.Y.; Li, C.; Ni, Y.Y.; Li, M.N.; Chen, L.; Hou, M.; Zhou, Z.; Xu, Z.P.; Ji, M.J. P21 activated kinase-1 (PAK1) in macrophages is required for promotion of Th17 cell response during helminth infection. J. Cell Mol. Med. 2020, 24, 14325–14338. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Zhu, X.; Xu, X.; Chang, H.; Ni, Y.; Li, C.; He, K.; Chen, L.; Chen, L.; Hou, M.; et al. Identification and Characterization of Antigenic Properties of Schistosoma japonicum Heat Shock Protein 90α Derived Peptides. Pathogens 2022, 11, 1238. https://doi.org/10.3390/pathogens11111238
Shen C, Zhu X, Xu X, Chang H, Ni Y, Li C, He K, Chen L, Chen L, Hou M, et al. Identification and Characterization of Antigenic Properties of Schistosoma japonicum Heat Shock Protein 90α Derived Peptides. Pathogens. 2022; 11(11):1238. https://doi.org/10.3390/pathogens11111238
Chicago/Turabian StyleShen, Chunxiang, Xinyi Zhu, Xuejun Xu, Hao Chang, Yangyue Ni, Chen Li, Kaiyue He, Lin Chen, Lu Chen, Min Hou, and et al. 2022. "Identification and Characterization of Antigenic Properties of Schistosoma japonicum Heat Shock Protein 90α Derived Peptides" Pathogens 11, no. 11: 1238. https://doi.org/10.3390/pathogens11111238






